Abstract
Many brain protective strategies have been tested over short survival intervals, but few have been examined for long term benefit. The inducible member of the Heat shock protein 70 (Hsp70) family, Heat shock protein 72 (Hsp72), has been widely found to reduce ischemic injury. Here we assessed outcome in Hsp72 transgenic overexpressing mice and wild type littermates for one month following transient focal ischemia. Hsp 72 reduced infarct area lost and improved behavioral outcome on rotarod and foot fault at one month. Thus protection by Hsp72 overexpression is long lasting, and includes improved recovery of motor function over one month.
Keywords: Hsp72, focal ischemia, long term, behavior, rotarod
Introduction
Heat shock protein 72 (Hsp72) is the stress inducible cytosolic member of the best studied family of chaperones, the Hsp70 family. Molecular chaperones or heat shock proteins (HSPs) occur in all organisms and are critical for many aspects of cellular function. The Hsp70 family regulates both apoptotic and necrotic cell death [7, 14, 18] blocking apoptosis at several different steps in the pathway. Its acute neuronal protective effects after ischemia are well documented [4, 7, 17], but its long term effects have not been well studied. Using transient middle cerebral artery occlusion (MCAO) in transgenic Hsp72 overexpressing mice [13], we investigated protection morphologically and behaviorally through 1 month survival.
Since MCAO causes focal motor deficits, we used rotarod and footfault. The rotarod test is useful to assess motor coordination and balance after cerebral ischemia in rodents [3] while assessment of locomotion and coordination can be performed using the foot fault test [9, 12] after MCAO.
Materials and Methods
Transient Focal Ischemia
All experiments using animals were performed in accordance with a protocol approved by the Stanford University animal care and use committee, and in keeping with the National Institutes of Health guide. Hsp72 transgenic mice (Hsp) originally created by Dillmann and colleagues [13] were used after backbreeding into C57B/6. Heterozygote transgenic mice were bred with WT mice (C57B/6) and heterozygote Tg mice and WT littermates were studied. The genotypes were established by polymerase chain reaction analysis of DNA extracted from the tails. Male adult Hsp Tg and WT mice weighing 23 to 32 grams were used.
Mice were randomly assigned to either MCAO or Sham (no ischemia but subjected to anesthesia, neck incision, and external carotid artery ligation). The study included 20 wild type (WT) mice subjected to MCAO, 17 Hsp mice subjected to MCAO, 11 WT mice in the Sham group, and 10 Hsp mice in the Sham group. Five mice from the WT MCAO group and three from the Hsp MCAO group died before the end of the experiment and were excluded from analysis. Transient focal ischemia was induced by the intraluminal suture method as previously described [8]. Animals were anesthetized with isoflurane (3% induce, 1.5–2% maintenance) and 60% nitrous oxide in oxygen with spontaneous respiration via face mask. Respiratory rate and rectal temperature were monitored. Under an operating microscope, the left common carotid artery, internal carotid artery, and the external carotid artery were exposed through a midline neck incision. The proximal portions of the left common carotid artery and portions of the external carotid artery were ligated, and a 6-0 silicon-coated nylon suture (Doccol Corporation, E Pennsylvania Ave, Redlands, CA) was introduced into the common carotid artery and advanced about 9 mm beyond the carotid bifurcation to occlude the middle cerebral artery for 60 minutes. Animals were allowed to awaken and return to their cages during MCAO. Mice were reanesthetized to remove the suture, ligate the artery, and close the incision. Rectal temperature was maintained at 37±1.0°C using a Homeothermic blanket control unit (Harvard Apparatus, Holliston, MA).
Behavioral Testing
Rotarod Test
Motor coordination and learning were assessed with the accelerating rotarod (SD Instruments, San Diego, CA). Beginning on the first day of training and continuing every other day for a total of 9 training days each mouse was placed on the 2.75 cm diameter rod with rotation speed increasing from 5 to 10 rpm over 5 min. The time the mouse was able to stay on the rotating rod before falling was determined up to a maximum duration of 300 seconds. The test was repeated three times each day for each mouse, and the scores were averaged for each day. Any mouse not able to achieve at least a 200 second average on the rotarod was excluded from the study, 2 mice of each genotype. Rotarod testing was performed 2 days, 1, 2, 3 and 4 weeks after surgery.
Foot Fault Test
The foot fault test (FFT) (model# 1028, Columbus Instrument, Columbus, Ohio), used is similar to the one originally described by Hernandez and Schallert [9, 12]. The mouse is placed on a horizontal ladder with the rungs separated by 3 cm and placed above a horizontal sensor plate for automatic recording of faults, events in which a paw falls or slips between the bars. Prior to MCAO, each mouse was trained on the task with three trials per day every third day for a total of 3 training days prior to surgery. On the second training day the sensor plate was electrified so the mice received a foot shock for each foot fault on the third trial. After MCAO, mice were tested on days 2, 8, and 4 weeks; the results of the 3 trials each day were averaged.
Determination of brain infarct area lost at 28 days
After removing and photographing the intact brains, brain area loss was determined using cresyl violet staining to assess remaining area on four 50 µm coronal sections cut on a vibratome (Leica VT 1000S, Heidelberger, Nussloch, Germany) and located at rostral 1.54 mm, 0.26 mm, and caudal −1.02, and −2.30 mm to bregma [15] as previously described [16]. Sections were mounted on gelatin-coated microscope slides, incubated in 1.0% cresyl violet acetate (Sigma, St. Louis, Mo) for 8 min then dehydrated sequentially in 70%, 95%, 100% ethanol, and xylenes baths at room temperature. Percent brain loss was calculated as the area of the contralateral hemisphere minus the ipsilateral hemisphere/contralateral hemisphere × 100, averaged for the sections.
Statistics
The data represented by the graphs are mean ± SE, or all individual values are shown. Assessment of genotype, treatment, and interaction effects were determined by two way ANOVA for each timepoint of the behavioral data. Effect of infarct area and genotype at 28 days was analyzed by two way ANOVA. We used a repeated-measure ANOVA model to assess the genotype effect on behavioral performance from 1–4 weeks after MCAO. For comparisons of two groups a t-test was used. Significant difference was P < 0.05.
Results
Effect on Rotarod Performance
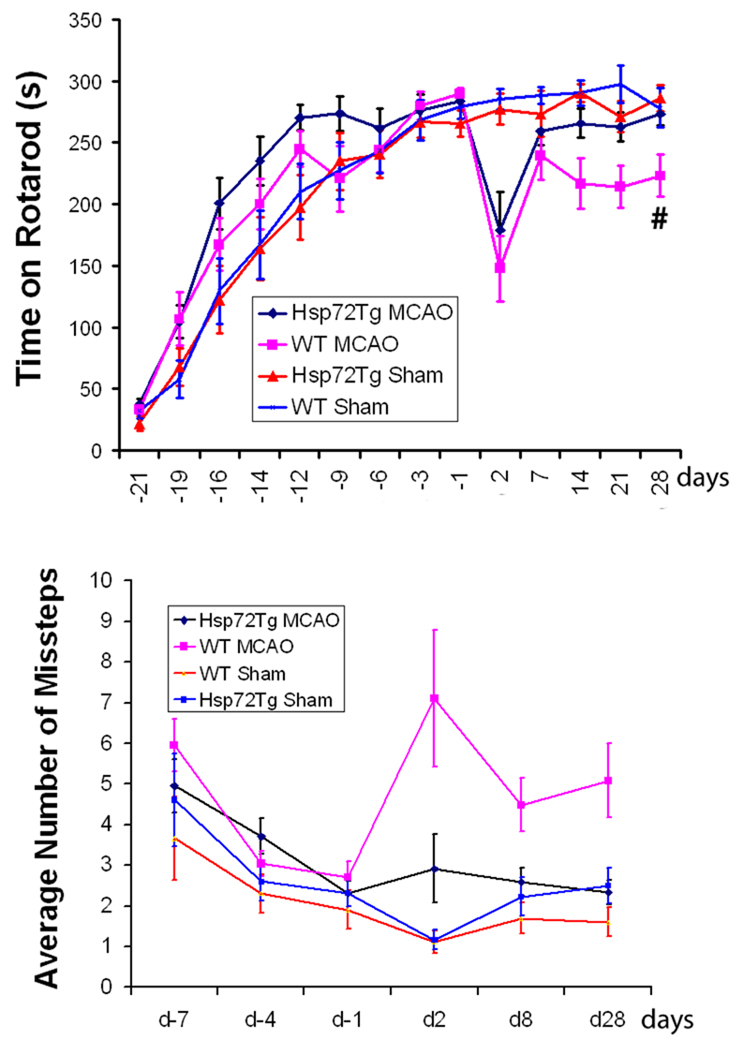
Both genotypes of mice, WT and Hsp72, were able to learn the rotarod task and by the 9th training day performance reached a plateau (Figure 1A). Immediately prior to surgery there is no genotype difference, rotarod times were similar for both strains. Sham mice had similar rotarod performance immediately before and after sham surgery and through 1 month of post-testing, demonstrating that the mice achieved maximum performance prior to surgery and sham surgery did not affect their ability to perform the rotarod task even at 2 days after anesthesia and sham surgery. Immediately after MCAO surgery there was a similar marked drop in performance in both Hsp72 and WT mice subjected to MCAO. With increasing recovery, the Hsp72 mice had greater recovery after one week, with performance reaching that of sham by 28 days, while the WT mice still had a significant deficit in performance which appeared stable by 28 days. The time on the rotarod in Hsp72 MCAO mice four weeks after surgery was close to the original 283.5 ± 7.8 seconds before surgery and not different from the sham Hsp72 group. While WT mice were still significantly worse than their presurgery level (289.2 ± 5.1 seconds) and WT sham surgery. Hsp72 mice have better recovery after MCAO by repeated-measure ANOVA for rotarod from week 1, 2, 3 and 4 post surgery (P = 0.0336, Figure 1A). We then specifically took into account both infarct area and genotype with a two-way ANOVA model and found that after the effect of infarct area was accounted for, the genotype effect was no longer significant (P = 0.46 for the main effect of genotype and = 0.81 for the interaction term between genotype and infarct area). This suggests that the primary effect of genotype may be on infarct area.
Figure 1. Figure 1A. Hsp72 mice show better recovery of rotarod performance after MCAO.
Both MCAO mice Hsp72 (n = 14) and WT (n = 15) learned the task and by the 9th training day performance exceeded 270 seconds. Sham treated Hsp72 (n = 10) and WT (n = 11) showed similar rotarod performance before and after surgery, through 4 weeks of post-testing. Immediately after MCAO surgery there was a similar marked drop in performance in two MCAO groups, but recovery was significantly greater in the Hsp72 mice since week one which achieved the same level of performance as sham, while WT mice were still significantly worse than sham. At 4 weeks recovery 2 way ANOVA showed a genotype effect (p = 0.01). Figure 1B. Hsp72 mice show better footfault performance after MCAO. While starting levels of footfault were similar immediately before surgery, after MCAO Hsp72 (n = 14) mice had significantly fewer foot faults than WT MCAO (n = 15) mice after four weeks. Sham Hsp72 (n = 10) and sham WT (n = 11) show similar performance before and after surgery and through 1 month of post-testing. After MCAO, WT MCAO mice had significantly more foot faults on days 2, 8, and 4 weeks after surgery compared to Hsp 72 and both sham groups, repeated-measure ANOVA showed a significant genotype effect for weeks 1 and 4 post surgery (P = 0.0234).
Effect on Foot Fault Performance
After three training days prior to surgery there was no difference between the genotypes on the foot fault task (Figure 1B). Sham mice show similar performance before and after surgery and through 1 month of post-testing. After MCAO, WT mice had significantly increased numbers of foot faults when tested on days 2, 8, and 4 weeks after surgery compared to either Hsp 72 MCAO or either sham group. On day 2 post surgery 2 way ANOVA showed both a treatment (P = 0.003) and a genotype effect (P=0.05). On day 28 there was still a treatment effect (P=0.037) and an interaction effect (P=0.015). Hsp72 MCAO mice had 2.3 ± 0.3 faults, about half as many missteps as the WT MCAO mice (5.1 ± 0.9) on day 28. The foot fault test clearly shows a persistent motor/coordination deficit after MCAO in WT mice, while Hsp72 mice recover to baseline. Hsp72 mice also have a significant genotype effect for foot fault at week 1 and 4 post surgery by repeated measures ANOVA (P = 0.0234, Figure 1B).
Long Term Effect on Brain Infarct Area Loss
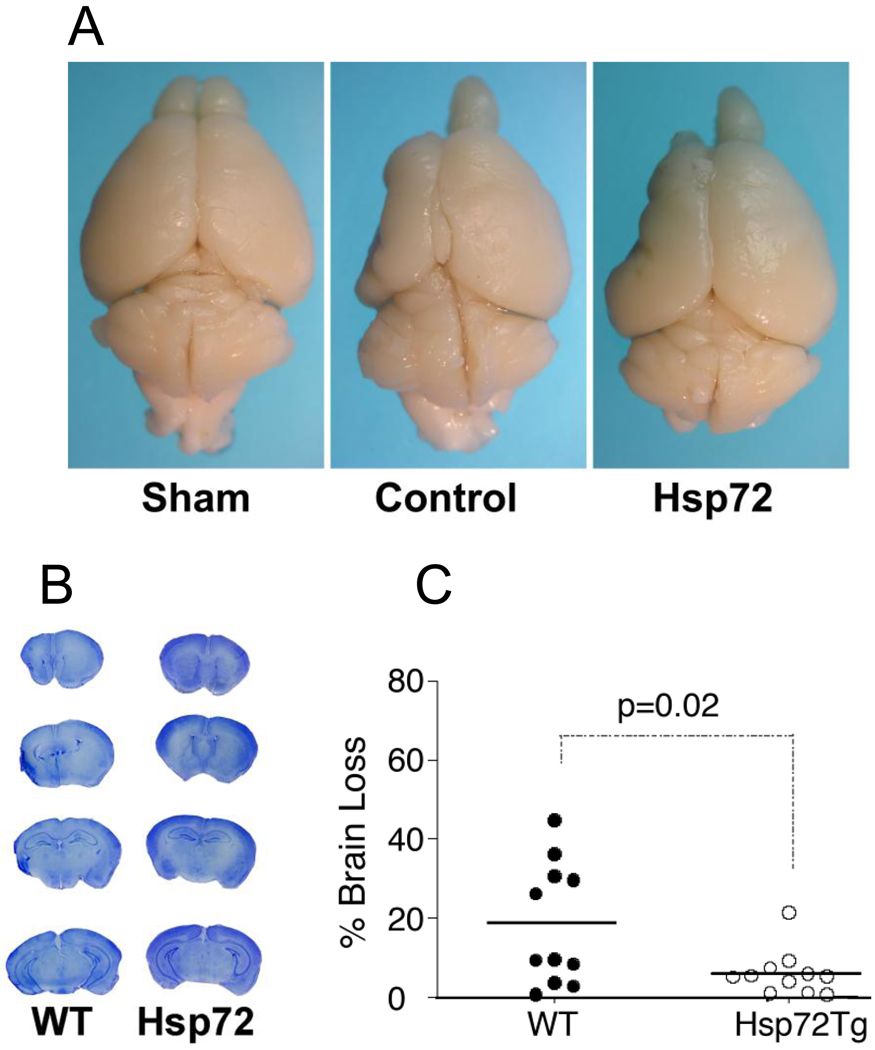
Four weeks after MCAO, WT mice had noticeable brain shrinkage on the ipsilateral side. Although the Hsp72 overexpressing mice exhibited some shrinkage, it was less than WT mice (fig 2A). Brain area loss analysis by cresyl violet staining showed 18 ± 4.6% brain loss in WT mice, but a 6.0 ± 1.7% brain area loss in the Hsp72 overexpressing mice ( Hsp72 compared to WT, P = 0.023; Figure 2B, C).
Fig 2. Hsp72 mice suffer less brain area loss at 4 weeks after MCAO.
2A. Four weeks after MCAO, WT and Hsp72 mice had brain shrinkage on the ipsilateral side compared to sham (fig 2A). The Hsp72 mice exhibited less shrinkage than WT mice. 2 B,C. Brain area loss was quantitated by Cresyl violet staining compared to the contralateral hemisphere (100%). Brain section staining showed more distortion and shrinkage in the ipsilateral hemisphere in WT mice compared to the Hsp72 overexpressing mice (# Hsp72 compared to WT, P = 0.023).
Discussion
Several neuroprotective strategies have been shown to have transient effects. Work beginning with that of Dietrich et al demonstrated that strong protection by mild post-ischemic hypothermia seen at 3 days was reduced at 7 days and absent at 2 months [5]. Intraischemic isoflurane treatment reduced infarct area acutely, but the effect was lost by 14 days [11]. Thus assessment of long term outcome is essential. Here we show that when animals are allowed to survive for 1 month after focal ischemia both histological brain loss and performance on two motor behavioral tasks are significantly improved at 1 month survival in Hsp72 overexpressing mice.
Prior work found neuroprotection with Hsp72 overexpression against a variety of ischemic insults in vivo and in vitro [4, 7] that was present over short survival intervals. One study assessed the effect of post-treatment with Herpes viral vectors encoding either Hsp70 or Hsp27 [2] through 2 months survival. They observed long term protection with Hsp27 but not Hsp70. However, they also saw no acute protection at 24 hr with their Hsp70 vector [1], in contrast to observations from our lab and others, in which Hsp72 overexpression using a different Herpes viral vector was effective in markedly increasing neuronal survival when given either as pretreatment, or post-insult treatment [10, 19]. In all of these tests of gene therapy the use of Herpes viral vectors restricted expression to the small region of the brain into which it was stereotaxicly injected, and induced expression primarily in neurons. In the current study transgenic mice were used in which Hsp72 is overexpressed in all cell types. This likely contributed to both acute protection and increased recovery over 1 month.
One very interesting finding of the present study is the increased ability of the Hsp72 overexpressing mice to recover function over the first month following stroke, Hsp72 mice have a better middle-to-long term recovery by repeated-measure ANOVA, even though the acute behavioral deficit on rotarod was similar between WT and Hsp mice. Hsp72 also reduces infarct area and this is likely a major contribution to ability to recovery since when this is taken into account the genotype effect is no longer significant. Recovery may also reflect greater plasticity and Hsp72 may not only benefit the response to the acute injury, but also improve long term response. One possible mechanism for this could be protection of neural precursor cells, as the recent study by Doeppner showed better survival of doublecortin positive young neurons when Hsp70 protein was administer to mice after MCAO [6]. The cellular and molecular mechanisms underlying this observation deserve further investigation. Of the two tests employed, the rotarod test was more challenging and more sensitive allowing greater discrimination of recovery of performance following stroke.
In conclusion, Hsp72 overexpression improves not only acute histological and behavioral response to stroke, but also benefits motor performance through 1 month of recovery. Since the improved functional recovery starts from a similar deficit in the case of rotarod, further research should now be done on the mechanisms of increased functional recovery.
Research highlights.
Hsp72 is a widely studied chaperone for its protective properties
Transgenic mice overexpressing Hsp72 are protected from brain loss due to stroke
Hsp72 mice recover motor function back to baseline over 28 days, WT do not
Acknowledgement
This work was supported in part by NIH grant RO1 GM49831 to R. Giffard. We thank Dr. Ming Zheng for help with statistics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Badin RA, Lythgoe MF, van der Weerd L, Thomas DL, Gadian DG, Latchman DS. Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb Blood Flow Metab. 2006;26:371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- 2.Badin RA, Modo M, Cheetham M, Thomas DL, Gadian DG, Latchman DS, Lythgoe MF. Protective effect of post-ischaemic viral delivery of heat shock proteins in vivo. J Cereb Blood Flow Metab. 2009;29:254–263. doi: 10.1038/jcbfm.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 6.Doeppner TR, Nagel F, Dietz GP, Weise J, Tonges L, Schwarting S, Bahr M. TAT-Hsp70-mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2009;29:1187–1196. doi: 10.1038/jcbfm.2009.44. [DOI] [PubMed] [Google Scholar]
- 7.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg. 2009;108:280–287. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, Yenari MA. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi M, Kimbro JR, Drummond JC, Cole DJ, Kelly PJ, Patel PM. Isoflurane delays but does not prevent cerebral infarction in rats subjected to focal ischemia. Anesthesiology. 2000;92:1335–1342. doi: 10.1097/00000542-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J.Clin.Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304:505–512. doi: 10.1016/s0006-291x(03)00623-5. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 16.Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. Journal of neuroscience methods. 2004;139:203–207. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 17.van der Weerd L, Lythgoe MF, Badin RA, Valentim LM, Akbar MT, de Belleroche JS, Latchman DS, Gadian DG. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp Neurol. 2005;195:257–266. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Yaglom JA, Ekhterae D, Gabai VL, Sherman MY. Regulation of necrosis of H9c2 myogenic cells upon transient energy deprivation. Rapid deenergization of mitochondria precedes necrosis and is controlled by reactive oxygen species, stress kinase JNK, HSP72 and ARC. J Biol Chem. 2003;278:50483–50496. doi: 10.1074/jbc.M306903200. [DOI] [PubMed] [Google Scholar]
- 19.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy [see comments] Ann.Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]