Abstract
Alopecia areata (AA) is a common autoimmune disease characterized by non-scarring hair loss. Previous studies have demonstrated an association between AA and physiological/psychological stress. In this study, we investigated the effects of heat treatment, a physiological stress, on AA development in C3H/HeJ mice. Whereas this strain of mice are predisposed to AA at low incidence by 18 months of age, we observed a significant increase in the incidence of hair loss in heat-treated 8-month-old C3H/HeJ mice compared with sham-treated mice. Histological analysis detected mononuclear cell infiltration in anagen hair follicles, a characteristic of AA, in heat-treated mouse skin. As expected, increased expression of induced HSPA1A/B (formerly called HSP70i) was detected in skin samples from heat-treated mice. Importantly, increased HSPA1A/B expression was also detected in skin samples from C3H/HeJ mice that developed AA spontaneously. Our results suggest that induction of HSPA1A/B may precipitate the development of AA in C3H/HeJ mice. For future studies, the C3H/HeJ mice with heat treatment may prove a useful model to investigate stress response in AA.
Keywords: Alopecia areata, C3H/HeJ, Heat shock, Stress
Introduction
With a lifetime risk estimated at 1.7%, alopecia areata (AA) is one of the most common organ-specific autoimmune diseases (Safavi et al. 1995). AA is non-scarring, and can affect any hair-bearing region of the body. The most common presentation of AA is isolated, round areas of complete hair loss on the scalp without clinical signs of skin inflammation (Wasserman et al. 2007). Increasing evidence suggests that AA is a T-cell mediated disease brought about by a collapse of the hair follicle’s immune privilege (Paus et al. 2005; King et al. 2008). In affected areas, anagen (growth phase) hair follicles show peri- and intra-follicular lymphocytic infiltrates that include both CD8+ and CD4+ T lymphocytes (Perret et al. 1984; Ranki et al. 1984; Paus et al. 2005), with a predominance of CD8+ cells (Bodemer et al. 2000; Gilhar et al. 2002; Cetin et al. 2009).
Strong evidence suggests that AA is a multigenic disease modified by epigenetic factors. Observations in support of a genetic component include increased familial incidence among affected patients (Hordinsky et al. 1984; van der Steen et al. 1992; Green and Sinclair 2000; Duvic et al. 2001; Martinez-Mir et al. 2007; Ahmed et al. 2008) and an association of AA with several major histocompatibility complex (MHC) class II alleles (Green and Sinclair 2000; Martinez-Mir et al. 2007; Barahmani et al. 2008). Alternatively, environmental factors, such as psychological and physiological stress, have been suggested as a trigger for the onset and/or exacerbation of AA. A significant correlation between a preceding acute stressful life event and the development of AA has been reported in both adults and children (Garcia-Hernandez et al. 1999; Kakourou et al. 2007; Manolache and Benea 2007; Willemsen et al. 2009). In addition, AA has been associated with conditions that may activate cellular stress responses, such as exposure to chemicals (Roselino et al. 1996), increased lipid peroxidation and alterations in the oxidant-antioxidant enzymatic system (Akar et al. 2002; Koca et al. 2005), and recent Epstein–Barr virus infections (Rodriguez and Duvic 2008).
Animal models have greatly advanced our understanding of AA. The C3H/HeJ inbred mice develop spontaneous hair loss that mimics human AA both grossly and histologically, and are the most widely used rodent model in the investigation of the pathogenesis and genetics of AA (Sundberg et al. 1994; McElwee and Hoffmann 2002; Sun et al. 2008). Phenotype typically manifests as multiple patches of spontaneous hair loss on the scalp and dorsal pelage. Histologically, peri- and intra-follicular mononuclear cell infiltrates are present in anagen hair follicles (Sundberg et al. 1994; Sun et al. 2008). The frequency of AA-like phenotype in these mice can reach up to 20% by 18 months of age (Sundberg et al. 1994; McElwee and Hoffmann 2002). Although genetically identical, variability in time of onset, incidence, and extent of AA in the inbred C3H/HeJ mice is indicative of epigenetic factors that may affect susceptibility to AA (McElwee and Hoffmann 2002; McElwee et al. 2003).
In this study, we investigated the effects of heat treatment, a physiological stress, on AA development in C3H/HeJ mice. We also examined expression of HSPA1A/B in heat-treated mice as well as mice that developed AA spontaneously.
Materials and methods
Mice and treatment
All animal care and use procedures were approved by the University of Miami Institutional Animal Care and Use Committee (IACUC). Retired C3H/HeJ breeders were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were given rodent chow and water ad libitum. To ensure that heat was applied evenly, we pre-treated mouse skin with petrolatum at the site, and subsequently administered heat using an insulated copper cylinder and tubing connected to a precision circulating water bath. The copper cylinder and tubing are wrapped with insulation material so that the actual temperature of the bottom of the copper cylinder is less than 0.1°C lower than the set temperature of the water bath. Eight-month-old mice were heated on the entire trunk, including dorsal and ventral areas, with 48.5°C circulating water for 20 min daily for 12 consecutive days, and 18-month-old mice were heated at the same temperature and duration once a week for 6 weeks as previously described (Jimenez et al. 2008). Control mice were sham-treated with room temperature water. Both groups were monitored for hair loss throughout the course of treatment. Eight-month-old C3H/HeJ mice were randomized into two groups: 35 mice received heat treatment while 40 received sham treatment. To determine the statistical significance, an unpaired Student t test was conducted after the last treatment to evaluate AA frequency between the two groups.
Histological analysis
Skin samples from the trunk of heat- and sham-treated mice, as well as areas of hair loss in mice with spontaneous AA, were collected and fixed in 10% formalin. Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin. The stained slides were subsequently evaluated using an Axio Observer D1 microscope (Carl Zeiss Microimaging, Thornwood, NY).
Western blot analysis
Protein extraction and Western blot analysis were carried out as previously described (Garcia et al. 2006). Briefly, skin samples (1 cm2) were excised from mice, and subcutaneous fat was removed. Skin samples were homogenized in lysis buffer (75 mM of HEPES, 0.5% of Triton X-100, 50 μg/ml of aprotinin and 100 mM of PMSF) for 1 min. Tissue lysates were cleared by centrifugation and subjected to SDS-PAGE and Western blot analysis. Inducible HSP70 (HSPA1A/B, formerly called HSP70i) was detected using a mouse monoclonal antibody (Assay Designs, Ann Arbor, MI) and secondary antibodies conjugated with alkaline phosphatase and color reaction. Images of the blots were captured and quantitated with a Bio-Rad digital imaging system and Quantity One software (Bio-Rad Laboratories, Hercules, CA). The relative adjusted intensities (ODu/mm2) were plotted.
Results
Incidence of AA in heat- and sham-treated C3H/HeJ mice
All C3H/HeJ mice had a normal appearing hair coat upon arrival to our facility. In the first experiment, we investigated whether heat treatment, as an external stressor, increased the incidence of AA. Eight-month-old mice were heated on the trunk with 48.5°C circulating water for 20 min daily for 12 consecutive days (Jimenez et al. 2008). Control mice were sham-treated with room temperature water. At the end of treatment, eight of 35 (23%) heat-treated 8-month-old mice developed AA, whereas only three of 40 (7.5%) sham-treated mice developed AA (Fig.1a). The increase in the incidence of AA in the heat-treated group was statistically significant (p < 0.0001). We also heat- or sham-treated 18-month-old mice once a week for 6 weeks (Jimenez et al. 2008). Similar hair loss pattern was observed in heat-treated mice and mice that developed AA spontaneously (Fig. 1c and d).
Fig. 1.
a Development of alopecia areata in 8-month-old C3H/HeJ mice upon heat treatment once daily for 12 consecutive days. Number of heat-treated mice = 35; number of sham-treated mice = 40. The percentages of mice with AA are shown for heat- and sham-treated mice (p < 0.0001). b–d Heat-induced alopecia areata in an 18-month-old C3H/HeJ mouse after six weekly heat treatment on the trunk (c) compared with a sham-treated mouse (b) and a mouse that developed AA spontaneously (d)
Among the mice that developed alopecia after heat treatment, alopecia was observed in the trunk area that was treated with heat (Fig. 1c). Additionally, 50% of these mice developed alopecia in areas not exposed to heat (data not shown). Among the three 8-month-old mice that developed alopecia after sham treatment, hair loss was observed in the ventral and dorsal skin and scalp (data not shown). AA in heat-treated mice was confirmed histologically by the presence of mononuclear cell infiltrates in anagen hair follicles, similar to AA that developed spontaneously (Fig. 2c-f). Age- and location-matched sham-treated skin sections from mice that did not develop AA lacked inflammatory infiltrates (Fig. 2a and b). These results suggest that AA ensued from heat treatment is similar to AA that developed spontaneously in C3H/HeJ mice. In addition, these results demonstrated that heat treatment may significantly increase the incidence of AA in genetically susceptible C3H/HeJ mice, as well as induce AA in much younger mice (eight months old versus 18 months old).
Fig. 2.
Histological analysis of skin sections stained with hematoxylin and eosin from a C3H/HeJ sham-treated mouse (a, b), a mouse that developed AA upon heat treatment (c, d), and a mouse that developed AA spontaneously (e, f). Arrows point to mononuclear cell infiltrates in anagen hair follicles in mice that showed AA (d, f). Panels b, d and f are images photographed at higher magnification
HSPA1A/B expression in C3H/HeJ mice
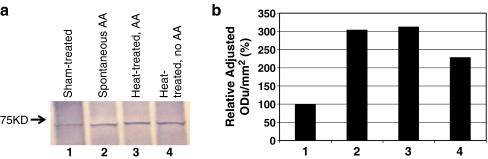
In the second experiment, we compared HSPA1A/B (inducible HSP70, HSP70i) expression in heat-treated mice with and without AA with sham-treated control mice with no AA, as well as mice that developed AA spontaneously. HSPA1A and HSPA1B are two of the most heat-inducible HSPs (Kampinga et al. 2009). Since we cannot predict whether an 8-month-old non-phenotypic mouse will develop AA spontaneously as it ages, we employed 18-month-old mice, at which time the incidence of AA-like phenotype peaks in C3H/HeJ mice (Sundberg et al. 1994; McElwee and Hoffmann 2002). In this experiment, HSPA1A/B was used as a molecular marker for the cellular stress response induced by heat treatment. Western blot analysis was carried out to detect HSPA1A/B expression in skin samples from heat- and sham-treated mice, as well as mice with spontaneous AA (Fig. 3). Sham-treated mouse skin exhibited a low baseline level of HSPA1A/B expression, while skin samples from mice with spontaneous AA and from heat-treated mice with AA showed a threefold increased expression of HSPA1A/B (Fig. 3a and b). Interestingly, skin samples from heat-treated mice that did not develop AA also showed increased expression of HSPA1A/B than sham-treated mice (Fig. 3a and b). This is consistent with our previous observation that increased HSPA1A/B expression was detected by immunohistochemistry throughout the hair follicles after heat treatment of skin at 48.5°C for 20 min compared with control (Jimenez et al. 2008). These results suggest that increased HSPA1A/B expression may predispose genetically susceptible mice to AA development.
Fig. 3.
Western blot analysis of HSPA1A/B expression in skin samples from C3H/HeJ mice (a) and quantification (b). Lane 1 sham-treated, lane 2 spontaneous AA, lane 3 heat-treated AA, lane 4 heat-treated without AA phenotype
Discussion
In this study, we investigated whether heat shock, a physiological stress, plays a role in the development of AA in C3H/HeJ mice, a strain predisposed to AA at advanced age (18 months). We demonstrated that heat treatment significantly increased the incidence of AA in C3H/HeJ mice at 8 months of age. In addition, we showed that expression of HSPA1A/B, a major HSP induced by heat shock, was up-regulated in heat-treated mice as well as mice that developed AA spontaneously.
The use of inbred rodent models such as the C3H/HeJ mice and the Dundee experimental bald rats (DEBR) has greatly facilitated our understanding of AA (Michie et al. 1991; Oliver et al. 1991; Sundberg et al. 1994; Sun et al. 2008). Among several strains of mice, the C3H/HeJ mice show the highest frequency of spontaneous AA, and are the most commonly used mouse strain for the study of AA (Sundberg et al. 1994; Sun et al. 2008). Penetrance of AA in this strain remains low, with the highest frequency of spontaneous AA reaching 20% by 18 months of age (Sundberg et al. 1994; McElwee and Hoffmann 2002). However, full-thickness skin grafts from affected mice to non-phenotypic C3H/HeJ mice transfers the AA-like phenotype in host skin 7∼10 weeks after grafting at a much higher incidence, 15 of 15 mice (100%) in one study and 43 of 50 mice (86%) in another study (McElwee et al. 1998; 2003). Such grafting-induced onset of AA provides investigators with the ability to control onset of the disease in a large number of animals. It has facilitated investigations into the role of non-genetic factors that may affect AA susceptibility, such as gonadal steroid hormones and diet (McElwee et al. 2001; McElwee et al. 2003). In the current study, we applied heat treatment to C3H/HeJ mice to analyze how stress plays a role on the development of AA. We showed that with heat treatment, C3H/HeJ mice can develop AA at a much earlier age, 8 months instead of 18 months, and at a higher incidence. Therefore, in addition to grafting, heat treatment may provide another approach to obtain C3H/HeJ mice with AA phenotype at a younger age, in a larger number. Additionally, C3H/HeJ mice with heat treatment may also be a useful tool to study stress response in AA.
There have been a number of studies investigating the role of stress in AA development. Correlations between a preceding acute stressful life event and the development of AA have been reported in both adults and children (Garcia-Hernandez et al. 1999; Kakourou et al. 2007; Manolache and Benea 2007; Willemsen et al. 2009). However, it is not an easy task to reliably distinguish “stress” as a genuine disease trigger from disease-induced distress, given the tremendous psychosocial burden in AA patients (Gulec et al. 2004; Tucker 2009). Studies in mice have shown that psychoemotional stress can indeed negatively impact hair growth, and the C3H/HeJ mice predisposed to AA have a significantly blunted systemic hypothalamic–pituitary–adrenal (HPA) response to acute physiological stress, as well as a defective adaptation to repeated psychological stress (Paus and Arck 2009; Zhang et al. 2009). However, the altered HPA activity was likely a consequence of the immune response associated with AA. In this study, the increased incidence of AA in 8-month-old C3H/HeJ mice after heat treatment suggests that heat stress may precipitate AA development. Whereas the underlying mechanism remains unclear, recent association of the MHC class I chain-related gene A (MICA) antigen with AA in humans provides insight into how stress may trigger AA development in predisposed individuals. The stress-induced MICA molecule has been associated with several other autoimmune diseases such as diabetes mellitus and psoriasis (Barahmani et al. 2006), and it was identified as a potential candidate gene and part of an extended HLA haplotype that may contribute to susceptibility of AA in humans (Barahmani et al. 2006). MICA is expressed on the surface of epithelial cells of the gastric and intestinal mucosa (Stephens 2001), endothelial cells, fibroblasts, monocytes (Zwirner et al. 1999), and keratinocytes within the epidermis and hair follicles (Tay et al. 2000). It is also a ligand for receptor NKG2D found on natural killer (NK) cells, γδ T cells and CD8+ T lymphocytes (Bauer et al. 1999). The interaction of the NKG2D receptor with MICA has been reported to enhance NK and T-cell responses (Bauer et al. 1999). Thus, the immunogenic function of MICA provides some implications for the role of cellular stress in triggering autoimmunity in predisposed humans.
The cellular stress response is characterized by an elevation of heat shock proteins (HSPs) (Park et al. 2005; Voellmy 2006). HSPs are a family of stress-responsive proteins that deal with thermal and other proteotoxic stresses including ultraviolet light, heavy metal, inflammation, and oxidative stress (Kampinga et al. 2009). The heat shock response is triggered by the accumulation of nonnative proteins in stressed cells, resulting in an increased synthesis of HSPs, which facilitates the subsequent refolding of denatured proteins by acting as chaperones and blocking caspase-dependent apoptosis (Milani et al. 2002; Voellmy 2006). Through active secretion or cell death, HSPs are released into the extracellular spaces, where they may influence both the innate immune response as well as the adaptive immune response. HSPs conjugated to antigenic peptides are taken up by antigen presenting cells such as dendritic cells (DCs), and the peptides may then be transferred to MHC class I molecules, leading to T-cell activation through CD8+ T lymphocytes (Calderwood et al. 2007). HSPs can also act independently from associated peptides, stimulating the innate immune system (Milani et al. 2002). HSPA (HSP70), in particular, may efficiently prime circulating T cells, evidenced by their promotion of DC function and, together with antigen, triggering autoimmune diseases such as diabetes mellitus (Milani et al. 2002; Millar et al. 2003). In addition, HSPA has been shown to induce the expression of NKG2D ligand MICA on DCs, and activate NK cells to kill target cells expressing MICA in an NKG2D-dependent manner (Elsner et al. 2010). Furthermore, HSPA-augmented IFN-gamma release was abrogated by antibody against MICA (Qiao et al. 2008). Thus, extracellular HSPA released during either stress or inflammatory cell death may serve as a critical link between NK and DCs in mounting immune responses against infections, cancers, and self-antigens (Qiao et al. 2008). HSPA may also play a role in the development of vitiligo, a T-cell mediated autoimmune disease characterized by skin depigmentation. HSPA was shown to be released by stressed melanocytes in culture, which may mediate DC activation that led to killing of stressed melanocytes (Kroll et al. 2005).
Whereas increased HSPA expression has been associated with various autoimmune diseases such as vitiligo and autoimmune diabetes mellitus (Millar et al. 2003; Kroll et al. 2005; Denman et al. 2008), our study is the first to demonstrate a significant association between HSPA and AA. Interestingly, in our study, HSPA1A/B expression was increased in all mice with AA and heat-treated mice. Thus, induction of HSPA1A/B by heat treatment suggests a mechanism by which cellular stress may increase C3H/HeJ mice susceptibility to AA. Furthermore, a correlation between increased HSPA1A/B expression in heat-treated mice with AA and mice with spontaneous AA could imply that HSPA1A/B may be a predisposing factor for AA development. Future studies are required to determine the nature of HSPA1A/B involvement in human AA development.
Acknowledgments
We gratefully acknowledge the Locks of Love Foundation (J.J.J.) and the Brian V. Jegasothy M.D. Basic Science Research Award (T.C.W.) for their support in this investigation. T.C.W. is supported by a Career Development Award from NIH/NIAMS (AR-050487). The authors would like to thank Jie Li, M.D., Ph.D., Ling Tang, Ph.D., Brenda Roberts, Ph.D., and Carmen I. Perez for assistance.
Conflict of interest The authors state no conflict of interest.
Abbreviations
- AA
Alopecia areata
- DC
Dendritic cell
- DEBR
Dundee experimental bald rats
- HPA
Hypothalamic–pituitary–adrenal
- HSP
Heat shock protein
- HSPA
HSP70
- HSPA1A/B
Inducible HSP70, HSP70i
- MICA
Major histocompatibility complex class I chain-related A
- NK
Natural killer
References
- Ahmed AM, Barahmani N, Duvic M, Registry National Alopecia Areata. Familial alopecia areata and chronic thrombocytopenia. J Am Acad Dermatol. 2008;58(5 Suppl 1):S75–S77. doi: 10.1016/j.jaad.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Akar A, Arca E, Erbil H, Akay C, Sayal A, Gur AR. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85–90. doi: 10.1016/S0923-1811(02)00015-4. [DOI] [PubMed] [Google Scholar]
- Barahmani N, Andrade M, Slusser JP, Zhang Q, Duvic M. Major histocompatibility complex class I chain-related gene A polymorphisms and extended haplotypes are associated with familial alopecia areata. J Invest Dermatol. 2006;126:74–78. doi: 10.1038/sj.jid.5700009. [DOI] [PubMed] [Google Scholar]
- Barahmani N, Andrade M, Slusser JP, Wei Q, Hordinsky M, Price VH, Christiano A, Norris D, Reveille J, Duvic M. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240–243. doi: 10.1038/sj.jid.5700973. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Bodemer C, Peuchmaur M, Fraitaig S, Chatenoud L, Brousse N, Prost Y. Role of cytotoxic T cells in chronic alopecia areata. J Invest Dermatol. 2000;114:112–116. doi: 10.1046/j.1523-1747.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- Cetin ED, Savk E, Uslu M, Eskin M, Karul A. Investigation of the inflammatory mechanisms in alopecia areata. Am J Dermatopathol. 2009;31:53–60. doi: 10.1097/DAD.0b013e318185a66e. [DOI] [PubMed] [Google Scholar]
- Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patino JA, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M, Nelson A, Andrade M. The genetics of alopecia areata. Clin Dermatol. 2001;19:135–139. doi: 10.1016/S0738-081X(00)00124-3. [DOI] [PubMed] [Google Scholar]
- Elsner L, Flügge PF, Lozano J, Muppala V, Eiz-Vesper B, Demiroglu SY, Malzahn D, Herrmann T, Brunner E, Bickeböller H, Multhoff G, Walter L, Dressel R (2010) The endogenous danger signals HSP70 and MICA cooperate in the activation of cytotoxic effector functions of NK cells. J Cell Mol Med in press [DOI] [PMC free article] [PubMed]
- Garcia JT, Ferracci F, Jackson MW, Joseph SS, Pattis I, Plano LR, Fischer W, Plano GV. Measurement of effector protein injection by type III and type IV secretion systems by using a 13-residue phosphorylatable glycogen synthase kinase tag. Infect Immun. 2006;74:5645–5657. doi: 10.1128/IAI.00690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625–632. doi: 10.1111/j.1346-8138.1999.tb02063.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Mediation of alopecia areata by CD4+ and CD8+ T lymphocytes: transfer to human scalp explants on Prkdc(scid) mice. Arch Dermatol. 2002;138:916–922. doi: 10.1001/archderm.138.7.916. [DOI] [PubMed] [Google Scholar]
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213–218. doi: 10.1046/j.1440-0960.2000.00439.x. [DOI] [PubMed] [Google Scholar]
- Gulec AT, Tanriverdi N, Duru C, Saray Y, Akcali C. The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int J Dermatol. 2004;43:352–356. doi: 10.1111/j.1365-4632.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- Hordinsky MK, Hallgren H, Nelson D, Filipovich AH. Familial alopecia areata. HLA antigens and autoantibody formation in an American family. Arch Dermatol. 1984;120:464–468. doi: 10.1001/archderm.120.4.464. [DOI] [PubMed] [Google Scholar]
- Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, Connelly EA, Chen Q, Zou J, Goldenberg C, Voellmy R. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13:31–38. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakourou T, Karachristou K, Chrousos G. A case series of alopecia areata in children: impact of personal and family history of stress and autoimmunity. J Eur Acad Dermatol Venereol. 2007;21:356–359. doi: 10.1111/j.1468-3083.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LE, Jr, McElwee KJ, Sundberg JP. Alopecia areata. Curr Dir Autoimmun. 2008;10:280–312. doi: 10.1159/000131749. [DOI] [PubMed] [Google Scholar]
- Koca R, Armutcu F, Altinyazar C, Gurel A. Evaluation of lipid peroxidation, oxidant/antioxidant status, and serum nitric oxide levels in alopecia areata. Med Sci Monit. 2005;11:CR296–299. [PubMed] [Google Scholar]
- Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124:798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21:921–928. doi: 10.1111/j.1468-3083.2006.02106.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Mir A, Zlotogorski A, Gordon D, Petukhova L, Mo J, Gilliam TC, Londono D, Haynes C, Ott J, Hordinsky M, Nanova K, Norris D, Price V, Duvic M, Christiano AM. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316–328. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee KJ, Hoffmann R. Alopecia areata—animal models. Clin Exp Dermatol. 2002;27:410–417. doi: 10.1046/j.1365-2230.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, King LE, Jr, Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Silva K, Beamer WG, King LE, Jr, Sundberg JP. Melanocyte and gonad activity as potential severity modifying factors in C3H/HeJ mouse alopecia areata. Exp Dermatol. 2001;10:420–429. doi: 10.1034/j.1600-0625.2001.100605.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Niiyama S, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, Hoffmann R. Dietary soy oil content and soy-derived phytoestrogen genistein increase resistance to alopecia areata onset in C3H/HeJ mice. Exp Dermatol. 2003;12:30–36. doi: 10.1034/j.1600-0625.2003.120104.x. [DOI] [PubMed] [Google Scholar]
- Michie HJ, Jahoda CA, Oliver RF, Johnson BE. The DEBR rat: an animal model of human alopecia areata. Br J Dermatol. 1991;125:94–100. doi: 10.1111/j.1365-2133.1991.tb06054.x. [DOI] [PubMed] [Google Scholar]
- Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, et al. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperthermia. 2002;18:563–575. doi: 10.1080/02656730210166140. [DOI] [PubMed] [Google Scholar]
- Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- Oliver RF, Jahoda CA, Horne KA, Michie HJ, Poulton T, Johnson BE. The DEBR rat model for alopecia areata. J Invest Dermatol. 1991;96:97S. doi: 10.1111/1523-1747.ep12472251. [DOI] [PubMed] [Google Scholar]
- Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PubMed] [Google Scholar]
- Paus R, Arck P. Neuroendocrine perspectives in alopecia areata: does stress play a role? J Invest Dermatol. 2009;129:1324–1326. doi: 10.1038/jid.2009.111. [DOI] [PubMed] [Google Scholar]
- Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26–30. [PubMed] [Google Scholar]
- Qiao Y, Liu B, Li Z. Activation of NK cells by extracellular heat shock protein 70 through induction of NKG2D ligands on dendritic cells. Cancer Immun. 2008;8:12. [PMC free article] [PubMed] [Google Scholar]
- Ranki A, Kianto U, Kanerva L, Tolvanen E, Johansson E. Immunohistochemical and electron microscopic characterization of the cellular infiltrate in alopecia (areata, totalis, and universalis) J Invest Dermatol. 1984;83:7–11. doi: 10.1111/1523-1747.ep12261627. [DOI] [PubMed] [Google Scholar]
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein–Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137–139. doi: 10.1016/j.jaad.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Roselino AM, Almeida AM, Hippolito MA, Cerqueira BC, Maffei CM, Menezes JB, Vieira RE, Assis SL, Ali SA. Clinical-epidemiologic study of alopecia areata. Int J Dermatol. 1996;35:181–184. doi: 10.1111/j.1365-4362.1996.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–633. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- Stephens HA. MICA and MICB genes: can the enigma of their polymorphism be resolved? Trends Immunol. 2001;22:378–385. doi: 10.1016/S1471-4906(01)01960-3. [DOI] [PubMed] [Google Scholar]
- Sun J, Silva KA, McElwee KJ, King LE, Jr, Sundberg JP. The C3H/HeJ mouse and DEBR rat models for alopecia areata: review of preclinical drug screening approaches and results. Exp Dermatol. 2008;17:793–805. doi: 10.1111/j.1600-0625.2008.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP, Cordy WR, King LE., Jr Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–856. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- Tay GK, Hui J, Gaudieri S, Schmitt-Egenolf M, Martinez OP, Leelayuwat C, Williamson JF, Eiermann TH, Dawkins RL. PERB11 (MIC): a polymorphic MHC gene is expressed in skin and single nucleotide polymorphisms are associated with psoriasis. Clin Exp Immunol. 2000;119:553–558. doi: 10.1046/j.1365-2249.2000.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. Bald is beautiful? The psychosocial impact of alopecia areata. J Health Psychol. 2009;14:142–151. doi: 10.1177/1359105308097954. [DOI] [PubMed] [Google Scholar]
- Steen P, Traupe H, Happle R, Boezeman J, Strater R, Hamm H. The genetic risk for alopecia areata in first degree relatives of severely affected patients. An estimate. Acta Derm Venereol. 1992;72:373–375. [PubMed] [Google Scholar]
- Voellmy R (2006) Feedback regulation of the heat shock response. Handb Exp Pharmacol: 43–68. [DOI] [PubMed]
- Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol. 2007;46:121–131. doi: 10.1111/j.1365-4632.2007.03193.x. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Vanderlinden J, Roseeuw D, Haentjens P. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388–393. doi: 10.1016/j.jaad.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yu M, Yu W, Weinberg J, Shapiro J, McElwee KJ. Development of alopecia areata is associated with higher central and peripheral hypothalamic–pituitary–adrenal tone in the skin graft induced C3H/HeJ mouse model. J Invest Dermatol. 2009;129:1527–1538. doi: 10.1038/jid.2008.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirner NW, Dole K, Stastny P. Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum Immunol. 1999;60:323–330. doi: 10.1016/S0198-8859(98)00128-1. [DOI] [PubMed] [Google Scholar]