Abstract
The evolutionary conserved family of heat shock proteins (HSP) is responsible for protecting cells against different types of stress, including oxidative stress. Although the levels of HSPs can be readily measured in blood serum, the levels of HSP70 in patients with different durations of diabetes have not been studied before. We quantified serum HSP70 levels in a healthy control group (n = 36) and two groups of type 2 diabetic patients, defined as newly diagnosed diabetes (n = 36) and patients with diabetes duration of more than 5 years (n = 37). The clinical characteristics and biochemical parameters were evaluated in the studied population. We found that serum HSP70 levels were significantly higher in patients with diabetes when compared with controls (p < 0.001) and it was higher in patients with disease for more than 5 years than in newly diagnosed patients (p < 0.001). Serum HSP70 was inversely correlated with fasting blood sugar in patients with diabetes for more than 5 years (r = −0.500, p = 0.002), positively correlated with the history of hypertension in newly diagnosed patients (p < 0.001), and positively correlated with age in patients with diabetes (r = 0.531, p = 0.001). Serum level of HSP70 is significantly higher in patients with diabetes and correlates with the duration of disease. Higher HSP70 in prolonged diabetes versus newly diagnosed diabetes may be an indicator of metabolic derangement in the course of diabetes.
Keywords: HSP70, Type2 diabetes, Diabetes duration
Introduction
Heat shock proteins (HSP) are a family of stress-responsive proteins that modulate cell function and contribute to protein homeostasis (Asea 2008). The major functions of HSP are protection against apoptotic stimuli, assistance in de novo folding of nascent polypeptides, and prevention of protein misfolding and aggregation (Atalay et al. 2009; Mayer and Bukau 2005). Both excessive and impaired HSP72 expression may disturb cell proliferation and function (Atalay et al. 2009). Increased levels of HSP70 in human monocytes are observed in response to in vitro heat stress (Jafarnejad et al. 2008; Vince et al. 2009).
The molecular mechanisms underlying hyperglycemia-induced effects on inflammation and vascular complications are thought to involve the action of reactive oxygen species within the cell nucleus (Wright et al. 2006). The underlying rationale for our study was that diabetes, and the concomitant oxidative stress associated, may induce a heat shock response. It is widely accepted that the levels of HSP70 expression increase under stressful conditions (Ireland et al. 2007; Pandey et al. 2009; Soti et al. 2005). In addition, serum HSP70 concentrations are positively correlated with markers of inflammation, such as C-reactive proteins, monocyte count, and TNF-α (Mayer and Bukau 2005; Njemini et al. 2004). Moreover, reduced radical scavenging activity in type 2 diabetes leads to overt inflammation and impaired HSP70 function (Pandey et al. 2009).
To date, only a limited number of studies investigated the serum levels of HSP70 in diabetes (Gruden et al. 2009; Hunter-Lavin et al. 2004; Oglesbee et al. 2005). However, these studies did not explore the variation of HSP70 levels with disease progression. Here, we determined the levels of HSP70 in two groups of diabetic patients (newly diagnosed patients, and those with disease for more than 5 years).
Methods
Assembly of cohorts
We performed a cross-sectional survey of an established cohort of 73 patients with type 2 diabetes who were attending the diabetes clinic of Vali-Asr hospital, affiliated to Tehran University of Medical Science, as well as 36 healthy controls. The patients were divided into two groups according to the duration of diabetes: patients with known diabetes for more than 5 years; and patients who were diagnosed de novo. Newly diagnosed patients were identified within 6 months and were not on any treatments. To investigate the effect of age, we stratified the patients into older and younger than 50 years old. Diabetes was diagnosed according to the criteria of American Diabetes Association (“Diagnosis and classification of diabetes mellitus” 2009). Participants of the control group and the diabetic patients of the other two groups were matched for age, sex, and body mass index (BMI). Exclusion criteria were smoking, pregnancy, proteinuria, renal involvement (creatinine >1.5 mg/dl or GFR <70 cc/min), glomerulonephritis, congestive heart failure, and hospital admission in recent months. None of the participants had overt diabetic complication. Demographic and anthropometric data including age, sex duration of diabetes, height, weight in light clothing, and blood pressure in sitting position were recorded. Blood pressure was re measured twice after 5 min and averaged. The BMI (kg/m2) was calculated according to Quetelet formula.
The research was carried out according to the principles of declaration of Helsinki. The local ethics review committee of Tehran University of Medical Science approved the study protocol. All participants gave written informed consent before participation.
Blood samples
Blood samples were collected after 12 h of fasting, centrifuged, and were kept at −70°C until analysis. Serum creatinine, fasting blood sugar (FBS), total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and HbA1c was measured for all participants. Glucose measurements (intra-assay coefficient of variants [CV] 2.1%, inter-assay CV 2.6%) were carried out using the glucose oxidase method. Cholesterol, HDL-C, LDL-C, and triglycerides were determined using direct enzymatic methods (Parsazmun, Karaj, Iran).
Serum HSP70 analysis
Soluble HSP70 levels were measured using a quantitative sandwich ELISA immunoassay (EKS-700B, Stressgen, USA). The intra- and inter-assay coefficient of variation ranged between 4.5% and 7%. A heat-shocked HeLa cell lysate (Stressgen, USA) was included as positive control. Blood serums were extracted using centrifugation. One milliliter of the special buffer was prepared for each milliliter of serum samples using cold deionized or distilled water. Protease inhibitors were added to the extraction reagents. Serum samples were incubated for 30 min on ice. Serum samples were diluted 1:2 with a special solution available in the kit and transferred to nitrocellulose membrane. The membranes were probed with an antibody against HSP70 (Stressgen, USA).The procedure was followed according to the instructions provided in the kit’s brochure.
We should clarify that the manufacturer has not recommended this kit for plasma analysis. In order to check the validity of EKS-700B kit for our plasma samples, we performed serial dilutions of our positive controls and demonstrated that HSP70 detection is within in the linear range for the amounts presented in our samples (range, 0.1–3 ng/ml). Also, the detection limit was ≤0.03 ng/ml, which was lower than the levels present in our samples.
Statistical analysis
The statistical package SPSS 16 for windows (Chicago, IL, USA), was used for analysis.
Kolmogorov–Smirnov test was employed to test the normality of the variables in each group. Variables distributed normally are presented as mean ± standard error of mean (SEM). Variables with skewed distribution are presented as median [interquintile range]. For comparison of serum HSP70 levels among the groups, non-parametric tests including Kruskal–Wallis test and Mann–Whitney U test were employed, as appropriate. The Pearson’s correlation test was employed to test the association of HSP70 with FBS, HbA1C, triglycerides, cholesterol, HDL-C, and LDL-C within each group. For this analysis, serum HSP70 was transformed into logarithmic scale to change its distribution to normal. Kendall’s tau-b test was employed to investigate the correlation between HSP70 and history of hypertension.
Results
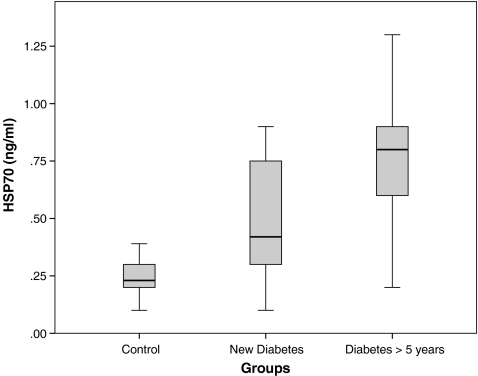
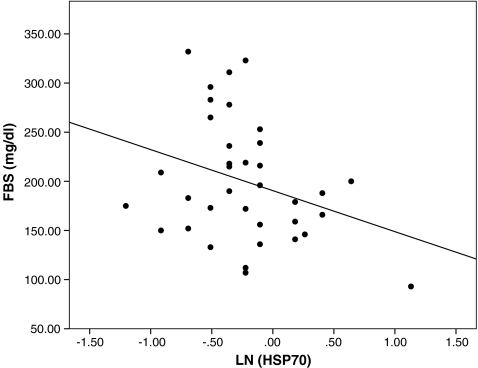
The characteristics of the participants are presented in Table 1. There were no significant differences between groups with respect to age, sex, BMI, systolic and diastolic blood pressure, creatinine, cholesterol, and HDL-C. Serum triglycerides and FBS were significantly higher in diabetic patients than healthy controls (p < 0.001). HbA1C was not significantly different between newly diagnosed patients and those with diabetes duration of more than 5 years. History of hypertension was significantly higher in patients with diabetes duration >5 years than newly diagnosed patients (p = 0.049). Serum HSP70 levels in patients with diabetes was higher than in controls (0.70 [0.59–0.81] vs. 0.23 [0.22–0.30] ng/ml; p < 0.001). Likewise, HSP70 levels were higher in patients with long-standing diabetes than newly diagnosed ones (0.80 [0.70–1.05] vs. 0.42 [0.41–0.64]; p < 0.001; Fig. 1). Logarithmic transformed concentrations of serum HSP70 was inversely correlated (r = −0.500, p < 0.01) with FBS in patients with known disease (Fig. 2). This association remained significant (r = −0.530, p < 0.001) after adjustment for age, sex, and BMI. We did not find any association between serum HSP70 levels and HbA1c in patients with diabetes. There was a significant positive correlation between logarithmic-transformed serum HSP70 levels and age (r = 0.20, p < 0.001), BMI (r = 0.25, p < 0.001), or systolic blood pressure (r = 0.24, p < 0.05) in all studied population. Also, there was a significant positive correlation between serum HSP70 levels and history of hypertension in newly diagnosed patients (r = 0.286, p < 0.01).
Table 1.
Characteristics of participants in the three groups
| Healthy control (n = 36) | Diabetes | ||
|---|---|---|---|
| New case (n = 36) | >5 years duration (n = 37) | ||
| Gender (females, %) | 18 (50.0%) | 18 (50.0%) | 18 (50.0%) |
| Age (year) | 48.92 ± 1.33 | 49.17 ± 1.58 | 49.51 ± 1.59 |
| Body mass index (kg/m2) | 27.40 ± 0.53 | 28.34 ± 0.74 | 28.81 ± 0.86 |
| Duration of diabetes (years) | – | – | 7.00 [6.46–9.96] |
| Systolic blood pressure (mmHg) | 120.0 [116.2–124.0] | 120.0 [119.7–130.8] | 120.0 [119.7–137.3] |
| Diastolic blood pressure (mmHg) | 80.0 [76.3–81.2] | 80.0 [76.4–83.7] | 80.0 [74.7–82.1] |
| History of hypertension (n, %) | 0 | 7 (19.4%) | 15 (40.5%) |
| History of ischemic heart disease (n, %) | 0 | 0 | 6 (16.2%) |
| Fasting blood sugar (mg/dL) | 92.0 [88.2–93.1] | 172.0 [171.8–202.6] | 190.0 [181.9–234.3] |
| HbA1c (%) | – | 7.80 [7.5–8.85] | 8.20 [7.4–9.07] |
| Creatinine (mg/dL) | 0.89 ± 0.03 | 0.91 ± 0.03 | 0.96 ± 0.03 |
| Triglyceride (mg/dL) | 139.0 [125.7–165.6] | 181.0 [164.8–262.7] | 219.0 [186.8–234.9] |
| Cholesterol (mg/dL) | 200.36 ± 7.44 | 201.72 ± 6.47 | 192.43 ± 7.75 |
| HDL-C (mg/dL) | 45.78 ± 2.01 | 42.56 ± 1.61 | 42.46 ± 1.95 |
| LDL-C (mg/dL) | 106.14 ± 4.21 | 116.63 ± 4.32 | 89.95 ± 4.47 |
| HSP70 (ng/ml) | 0.23 [0.221–0.302] | 0.48 [0.42–0.64] | 0.80 [0.76–1.05] |
Continuous variables are expressed as mean ± standard error of mean (SEM) or median [interquartile range]
Fig. 1.
Box plot demonstrating the higher serum level of HSP-70 (ng/ml) in newly diagnosed diabetes vs. control group (p < 0.01) and in patients with diabetes duration >5 years compared with newly diagnosed diabetic patients (p < 0.001)
Fig. 2.
Scatter plot demonstrating a significant correlation (r = −0.50, p = 0.002) between the log-transformed serum HSP70 levels (ng/ml) and fasting blood sugar (FBS) in patients with diabetes duration of more than 5 years
Discussion
Our data showed that circulating levels of HSP70 are increased in patients with diabetes and are associated with the duration of the disease. Our results support the hypothesis that HSP70 may play an important role in determining the biological characteristics of long-standing diabetes. In addition, serum HSP70 levels correlated inversely with FBS, and correlated positively with age in patients with longer disease duration.
Clinical studies on the levels of HSP70 in diabetes are limited. There were two reports of serum HSP70 level in type 1 diabetic patients (Gruden et al. 2009; Oglesbee et al. 2005). In a case–control study conducted in type 1 diabetics, increased level of HSP72 was observed in diabetic ketoacidosis which was significantly decreased after treatment (Oglesbee et al. 2005). However, another case–control study reported immeasurable serum HSP70 level in type 1 diabetic patients with and without microvascular complications (Gruden et al. 2009).
Our results are clearly supported by other studies in type 2 diabetes. In a previous study, serum HSP70 levels were found to be higher in non-insulin treated type 2 diabetes subjects in comparison with insulin treated ones (Hunter-Lavin et al. 2004). A cross-sectional study showed increased level of HSP70 in mononuclear cells of type 2 diabetic patients versus normal subjects (Yabunaka et al. 1995). Similarly, a case–control study which measured oxidative stress markers in patients suffering from type 2 diabetes-induced nephropathy and healthy controls, revealed higher HSP70 concentration in lymphocytes of uremic diabetic patients than in non-uremic ones (Calabrese et al. 2007).
Oxidative stress contributes to chronic complications of diabetes (Wei et al. 2009). In addition, the increased levels of HSP70 with duration of diabetes further support a role for continued stress in the course of the disease. However, some studies indicate that despite stress induction of HSPs, there is no daily fluctuation in HSP70 levels in the serum. Thus, day time stress, such as increased temperature, does not play a significant role in HSP70 induction (Fortes and Whitham 2009).
In diabetes, the levels of HSPs are higher in some tissues and lower in other tissues. Defects in heat shock response are observed in diabetic wounds (Atalay et al. 2009). There is a delayed induction of HSP72 in diabetes. However, chronic open wounds in diabetic mice resulted in a more potent HSP70 induction than in a normally healing wound (Atalay et al. 2009). Ubiquitination of insulin-like growth factor-1 receptor is suppressed by HSP10 and HSP60, which results in insulin-like growth factor-1 receptor signaling in cardiac muscle (Shan et al. 2003). Both chaperones play important roles in cardiac protection. HSP60 expression is impaired in the heart of diabetic animals (Shan et al. 2003; Oksala et al. 2006), and this may contribute to diabetic cardiomyopathy. Hyperthermia-induced HSP72 expression is impaired in streptozotocin-induced diabetic heart which is associated with insulin deficiency in these tissues (Shinohara et al. 2006). Lower levels of HSP expression in insulin sensitive tissues, such as muscle and heart, have been reported (Atalay et al. 2004; Hooper 2007). Atalay and coworkers recently reported impaired rise in HSP70 protein in exercising diabetic animals, when there was an increased mRNA expression in these subjects (Lappalainen et al. 2008). However, increased levels of HSP70 and HSP60 have been reported in the kidney (Atalay et al. 2009; Oksala et al. 2007) and liver (Oksala et al. 2006) of diabetic animals, respectively.
Our findings are supported by observations suggesting that serum HSP70 may originate in the vascular endothelium, a tissue which is not particularly sensitive to insulin activity (Asea 2008; Csermely 2008). Moreover, the splanchnic bed of the mesentery is the origin of the rise in HSP70 with exercise (Febbraio et al. 2002). Therefore, our findings are consistent with the notion that inflammation from advanced diabetic state increases HSP70 in non-insulin-sensitive diabetic tissues like endothelium. Diabetes is also associated with impaired nitric oxide (NO) release from endothelial cells (Bhatia et al. 2003). Reduced NO release from endothelial cells have an oxidizing effect which increase HSP70 expression (Hooper and Hooper 2004). Nevertheless, most studies on effects of diabetes on serum HSP70 level are confounded by in vitro studies and it is still unclear what the precise in vivo mechanisms are. Future studies may elucidate the origin of serum HSP70 in diabetic patients.
In type 2 diabetic monkeys serum HSP70 levels are decreased in comparison with control animals (Kavanagh et al. 2009). The diabetic monkeys were both older and heavier in this study. It is shown that aging leads to a diminished heat shock response, thereby resulting in deterioration of stress adaptation (Njemini et al. 2004; Soti et al. 2005). Age-dependent waning of HSP response have been shown by many studies (Calderwood et al. 2009; Mayer and Bukau 2005; Njemini et al. 2004; Njemini et al. 2003; Yabunaka et al. 1995). However, we found a significant positive correlation between HSP70 level and age (r = 0.20, p < 0.001) in all studied population
Our data also demonstrate a significant negative correlation between FBS and HSP70 in patients with known disease, which is consistent with previous studies (Oglesbee et al. 2005; Patti et al. 2003). A negative correlation between FBS and serum HSP70 level was also reported in diabetic monkeys (Kavanagh et al. 2009). This reduction may be due to decreased release of HSP70 from hepatosplanic circulation (Patti et al. 2003). One hypothesis is that insulin resistance may contribute to decreased level of HSP70 (Hooper 2007; Hooper and Hooper 2009). Insulin potentiates HSP70 expression in heart (Li et al. 2006) and this may explain the negative correlation between HSP70 and FBS in patients with known disease. In addition, we did not find any association between serum HSP70 level and HbA1c in patients with diabetes. This shows the acute response of serum HSP70 levels to hyperglycemia, independent of HbA1c as the indicator of average long-term serum sugar level.
Genetic studies identified HSP70 mutations in patients with diabetes. In a case–control study conducted in a Tunisian population, mutated HSP70 gene was associated with an increased risk of diabetes and obesity (Bouassida et al. 2004). This may be another explanation for the increased levels of HSP70 in prolonged diabetes. We propose that mutated malfunctioning HSP70 proteins will result into a vicious cycle of stress reduction and accelerated HSP70 expression.
We also observed a positive correlation between serum HSP70 levels and systolic blood pressure in all studied groups and the history of hypertension in newly diagnosed patients. HSP70 response in essential hypertension and atherosclerosis have been previously investigated (Njemini et al. 2003; Pockley et al. 2009; Zhang et al. 2008). Higher circulating levels of HSP70 predict the future development of cardiovascular disease in established hypertension (Pockley et al. 2003). Increased HSP70 concentration was reported in transient pregnancy hypertension (Molvarec et al. 2006). Also, there is a strong genetic interaction between HSP70 family polymorphisms and the risk of essential hypertension (Li et al. 2009).
The principal limitation of the present study is its cross-sectional nature which preclude the determination of the direction of causality. Besides as previously stated, ESK-700B Stressgen is not recommended for serum HSP70 analysis by the manufacturer. In order to use this kit we checked the linearity of HSP70 detection within our sample range (0.1–3 ng/ml). Since this kit could linearly detect the differences between higher and lower values of HSP70 in our sample range, we assumed it to be appropriate for signifying the differences between our participants. There might be some disturbances in HSP70 detection for ranges other than our evaluated range and we confirm that this kit is not generally suitable for detection of serum HSP70. In the present study, we took advantage of a relatively large sample size and the close similarity between the groups in most of the potentially confounding clinical and laboratory variables.
In conclusion, we have shown that HSP70 is increased in the circulation of diabetic patients and correlates positively with the chronicity of disease. This may add to the understanding of the biological characteristics of HSPs in diabetes. Our data also suggest that HSP70 may have the potential to be used as a biomarker in diabetes.
Contributor Information
Manouchehr Nakhjavani, Phone: +98-21-8841791, FAX: +98-21-64432466, Email: nakhjavanim@tums.ac.ir.
Afsaneh Morteza, Email: aafsaneh03@gmail.com.
Leila Khajeali, Email: leila_khajehali@yahoo.com.
Alireza Esteghamati, Email: esteghamati@tums.ac.ir.
Omid Khalilzadeh, Email: khalilzadeh@razi.tums.ac.ir.
Firouzeh Asgarani, Email: f_asgarani@razi.tums.ac.ir.
Tiago F. Outeiro, Email: touteiro@gmail.com
References
- Asea A. Hsp70: a chaperokine. Novartis Found Symp. 2008;291:173–179. doi: 10.1002/9780470754030.ch13. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10:85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalay M, Oksala NKJ, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hnninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97:605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Shukla R, Venkata Madhu S, Kaur Gambhir J, Madhava Prabhu K. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem. 2003;36:557–562. doi: 10.1016/S0009-9120(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Bouassida KZ, Chouchane L, Jellouli K, Cherif S, Haddad S, Gabbouj S, Danguir J. Polymorphism of stress protein HSP70-2 gene in Tunisians: susceptibility implications in type 2 diabetes and obesity. Diabetes Metab. 2004;30:175–180. doi: 10.1016/S1262-3636(07)70104-0. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Mauro M, Stella AM, Castellino P. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P. The biology of extracellular molecular chaperones. Chair's introduction. Novartis Foundation Symposium. 2008;291:1–2. doi: 10.1002/9780470754030.ch1. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes MB, Whitham M. No endogenous circadian rhythm in resting plasma Hsp72 concentration in humans. Cell Stress Chaperones. 2009;14:273–280. doi: 10.1007/s12192-008-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Pinach S, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Cavallo-Perin P. ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med. 2009;266:527–536. doi: 10.1111/j.1365-2796.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Insulin signaling, GSK-3, heat shock proteins and the natural history of type 2 diabetes mellitus: a hypothesis. Metab Syndr Relat Disord. 2007;5:220–230. doi: 10.1089/met.2007.0005. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Is low-heat shock protein 70 a primary or a secondary event in the development of atherosclerosis? Hypertension. 2004;43:e18–e19. doi: 10.1161/01.HYP.0000118134.56524.b1. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress and Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Lavin C, Hudson PR, Mukherjee S, Davies GK, Williams CP, Harvey JN, Child DF, Williams JH. Folate supplementation reduces serum hsp70 levels in patients with type 2 diabetes. Cell Stress Chaperones. 2004;9:344–349. doi: 10.1379/CSC-28R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland HE, Leoni F, Altaie O, Birch CS, Coleman RC, Hunter-Lavin C, Williams JH. Measuring the secretion of heat shock proteins from cells. Methods. 2007;43:176–183. doi: 10.1016/j.ymeth.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ. Investigation of the mechanisms involved in the high-dose and long-term acetyl salicylic acid therapy of type I diabetic rats. J Pharmacol Exp Ther. 2008;324:850–857. doi: 10.1124/jpet.107.130914. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell stress & chaperones. 2009;14:291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen Z, Lappalainen J, Oksala NK, Laaksonen DE, Khanna S, Sen CK, Atalay M (2008) Exercise training and experimental diabetes modulate heat shock protein response in brain. Scand J Med Sci Sports (in press) [DOI] [PubMed]
- Li G, Ali IS, Currie RW. Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1709–1721. doi: 10.1152/ajpheart.00201.2006. [DOI] [PubMed] [Google Scholar]
- Li JX, Tang BP, Sun HP, Feng M, Cheng ZH, Niu WQ. Interacting contribution of the five polymorphisms in three genes of Hsp70 family to essential hypertension in Uygur ethnicity. Cell Stress Chaperones. 2009;14:355–362. doi: 10.1007/s12192-008-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. Hum Hypertens. 2006;20:780–786. doi: 10.1038/sj.jhh.1002060. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5:31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Vanden Abeele M, Vandebosch S, Mets T. The induction of heat shock protein 70 in peripheral mononuclear blood cells in elderly patients: a role for inflammatory markers. Hum Immunol. 2003;64:575–585. doi: 10.1016/s0198-8859(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. J Biomed Biotechnol. 2005;38:900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Oksala NK, Lappalainen J, Laaksonen DE, Khanna S, Kaarniranta K, Sen CK, Atalay M. Alpha-lipoic acid modulates heat shock factor-1 expression in streptozotocin-induced diabetic rat kidney. Antioxid Redox Signal. 2007;9:497–506. doi: 10.1089/ars.2006.1450. [DOI] [PubMed] [Google Scholar]
- Oksala NKJ, Laaksonen DE, Lappalainen J, Khanna S, Nakao C, Hanninen O, Sen CK, Atalay M. Heat shock protein 60 response to exercise in diabetes. Effects of a-lipoic acid supplementation. J Diabetes Complications. 2006;20:257–261. doi: 10.1016/j.jdiacomp.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. 2009;23:23. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009;14:545–553. doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- Shan YX, Yang TL, Mestril R, Wang PH. Hsp10 and Hsp60 suppress ubiquitination of insulin-like growth factor-1 receptor and augment insulin-like growth factor-1 receptor signaling in cardiac muscle: implications on decreased myocardial protection in diabetic cardiomyopathy. J Biol Chem. 2003;278:45492–45498. doi: 10.1074/jbc.M304498200. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Takahashi N, Ooie T, Hara M, Shigematsu S, Nakagawa M, Yonemochi H, Saikawa T, Yoshimatsu H. Phosphatidylinositol 3-kinase-dependent activation of akt, an essential signal for hyperthermia-induced heat-shock protein 72, is attenuated in streptozotocin-induced diabetic heart. Diabetes. 2006;55:1307–1315. doi: 10.2337/db05-0266. [DOI] [PubMed] [Google Scholar]
- Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince RV, Oliver K, Midgley AW, McNaughton LR, Madden LA. In vitro heat shock of human monocytes results in a proportional increase of inducible Hsp70 expression according to the basal content. Amino Acids. 2009;38:1423–1428. doi: 10.1007/s00726-009-0354-4. [DOI] [PubMed] [Google Scholar]
- Wei W, Liu Q, Tan Y, Liu L, Li X, Cai L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin. 2009;33:370–377. doi: 10.3109/03630260903212175. [DOI] [PubMed] [Google Scholar]
- Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabunaka N, Ohtsuka Y, Watanabe I, Noro H, Fujisawa H, Agishi Y. Elevated levels of heat-shock protein 70 (HSP70) in the mononuclear cells of patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;30:143–147. doi: 10.1016/0168-8227(95)01151-X. [DOI] [PubMed] [Google Scholar]
- Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation. 2008;118:2687–2693. doi: 10.1161/CIRCULATIONAHA.108.781856. [DOI] [PubMed] [Google Scholar]