Abstract
Members of the 14-3-3 protein family are involved in many important cellular events, including stress response, survival and apoptosis. Genes of the 14-3-3 family are conserved from plants to humans, and some members are responsive to UV radiation. Here, we report the isolation of the complete cDNA encoding the 14-3-3 epsilon isoform from Paracentrotus lividus sea urchin embryos, referred to as Pl14-3-3ε, and the phylogenetic relationship with other homologues described in different phyla. Pl14-3-3ε mRNA levels were measured by QPCR during development and found to increase from the mesenchyme blastula to the prism stage. In response to UV-B (312 nm) exposure, early stage embryos collected 2 h later showed a 2.3-fold (at 400 J/m2) and a 2.7-fold (at 800 J/m2) increase in Pl14-3-3ε transcript levels compared with controls. The spatial expression of Pl14-3-3ε mRNA, detected by whole mount in situ hybridization in both control and UV-B exposed embryos, harvested at late developmental stages, showed transcripts to be located in the archenteron of gastrula stage and widely distributed in all germ layers, respectively. The Pl14-3-3ε mRNA delocalization parallels the failure in archenteron elongation observed morphologically, as well as the lack of specific endoderm markers, investigated by indirect immuno-fluorescence on whole mount embryos. Results confirm the involvement of 14-3-3ε in the stress response elicited by UV-B and demonstrate, for the first time, its contribution at the transcriptional level in the sea urchin embryo.
Keywords: Stress proteins, Ionising radiations, Gene expression, Morphogenesis, Molecular biomarkers
Introduction
Ultraviolet (UV) radiation is part of the electromagnetic spectrum emitted by the sun. UV-C rays (100–280 nm) are absorbed by the atmospheric ozone layer, while most radiation in the UV-A range (315–400 nm) and 2–5% of UV-B rays (280–315 nm) reach the Earth's surface, their amounts increasing with the thinning of the ozone layer. The intensity of UV rays can vary with latitude, ground reflection, altitude, time of year, time of day, clouding of the sky and air pollution (Smith et al. 1992; Gies et al. 2004). As an example, on a sunny day, at noon, on the Mediterranean coast, the solar UV radiation contains 6% UV-B and 94% UV-A (Diffey 2002). Both UV-A and UV-B have a major influence on human health. It is known that UV-B can have a positive effect, inducing the production of vitamin D in the skin (Holick 1995). However, high UV-B radiation doses lead to direct sunburn and cause severe DNA damage. Specifically, in humans, exposure to solar UV-B radiation can result in acute and chronic health effects on the skin, eyes and immune system, and can accelerate skin aging, cause damage to collagen fibres and lead to cancer (Matsumura and Ananthaswamy 2004; Autier et al. 1995; Hanson et al. 2006; Choi et al. 2005; Loser and Beissert 2009; De la Fuente et al. 2009).
Penetration of UV-B into natural waters can vary considerably, depending on the concentration and optical qualities of dissolved organic matter, phytoplankton or other suspended particles. For example, it has been shown that in shallow marine waters, from 58% (at 1 m depth) to 12% (at 5 m depth) UV-B can penetrate clear tropical waters, whereas from 25% (at 1 m depth) to 0.04% (at 5 m depth) in highly turbid sea waters (Dunne and Brown 1996). This radiation has been shown to affect growth, photosynthesis, nitrogen incorporation and enzyme activity. Planktonic organisms, including embryos/larvae of many species, are more prone to UV-B as they dwell in the top layers of the water column (Hader 2000). Harmful effects of water-penetrating UV-B radiation cause strong impairment of development as well as modifications in cellular protein composition, especially in the modulation of stress proteins levels, misregulation in gene expression and DNA damage (Batel et al. 1998; Lesser et al. 2003; Schröder et al. 2005; Bonaventura et al. 2005, 2006; Holzinger and Lütz 2006; Tedetti and Sempéré 2007; Banaszak and Lesser 2009). A recent report describes a field experiment on sea urchin early embryos placed at different depths in the Gulf of Maine (UVR, 300–400 nm), demonstrating the direct correlation between survivorship, oxidative stress, DNA damage and the optical properties of the water column (Lesser 2010).
A family of stress proteins known to respond to UV-B radiation in humans is the 14-3-3 protein family, originally identified in mammalian brain tissues (Moore and Perez 1967; Petrocelli and Slingerland 2000; Choi et al. 2005). It is a highly conserved family, consisting of 28–30 kDa acidic proteins, found in many organisms from plants to humans, involved in the regulation of many cellular processes such as signal transduction, cell-cycle control, apoptosis, gene expression, stress responses and cancer transformation (reviewed by Morrison 2008). Proteins of the 14-3-3 family are considered chaperones/adaptors, playing important roles in cellular homeostasis (Jeanclos et al. 2001; Tabunoki et al. 2008). Their involvement in stress response has been claimed as evidenced by the increased protein/mRNA levels following exposure to drugs and pesticides. For example, different subsets of 14-3-3 genes were induced after treatment with the fungal toxin fusicoccin in the tomato plant, demonstrating a correlation with the pathogen-associated defense response (Roberts and Bowles 1999). In sponges, the 14-3-3 gene is induced by pesticides (PCB 118), in parallel with the increase in the levels of the heat shock protein 70 transcript, suggesting their role in preventing apoptosis (Wiens et al. 1998). Under physiological conditions 14-3-3 homo- and hetero-dimers (Dougherty and Morrison 2004; Chaudhri et al. 2003) can interact with a wide variety of signalling proteins, including the stress signalling BAD/BAX mitochondrial proteins and the FOXO transcription factor. This interaction is possible only if 14-3-3 proteins are un-phosphorylated and, by sequestering BAD, BAX and FOXO in the cytoplasm, their entrance into the mitochondrion/nucleus is prevented. Following stress, 14-3-3 are phosphorylated by the JNK kinase and cannot bind the pro-apoptotic proteins, which are free to localize to their site of action, promoting apoptosis (reviewed by Aitken 2006; Morrison 2008).
Seven 14-3-3 isoforms (β, γ, ε, ζ, η, σ, τ) are known in mammals (Aitken 2006; Lau et al. 2006); up to 14 were described in the plant Arabidopsis thaliana (Kuromori and Yamamoto 2000), while two isoforms have been identified in yeast, Drosophila melanogaster and Bombyx mori (ε and ζ) (Rosenquist et al. 2001; Tabunoki et al. 2008). At least one isoform has been found in the sponge Geodia cynodium (Wiens et al. 1998), and in the sea urchins Heliocidaris tuberculata, Heliocidaris erythrogramma (ε) (Love et al. 2008). Three isoforms were annotated in the genome of Strongylocentrotus purpuratus, one referred to as ε, and two known as the isoforms 1 and 2 (Fernandez-Guerra et al. 2006; Samanta et al. 2006).
The sea urchin embryo is one of the most used marine invertebrates models for studying apoptosis, cellular stress and biochemical markers of pollution (Russo et al. 2003; Roccheri et al. 2004; Agnello et al. 2007; Robertson et al. 2006; Agnello and Roccheri 2010; Roccheri and Matranga 2009). It offers a suitable model for toxicological and developmental studies as the feeding larva (pluteus) is complete in about 48 h (see Fig. 2, lower panel); the specification of the embryonic territories, including ectoderm, mesoderm and endoderm, begins as early as the 32-cells cleavage stage (Zito and Matranga 2009).
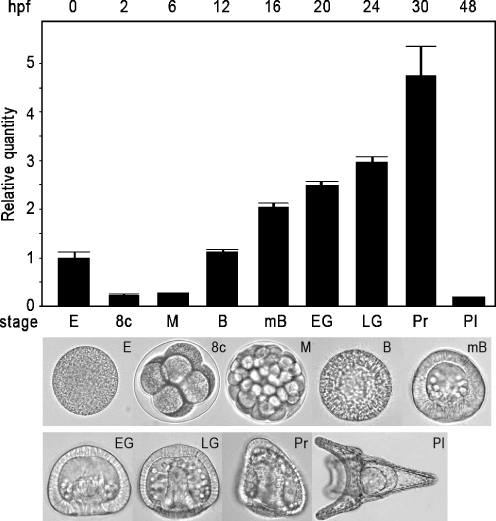
Fig. 2.
Quantitative PCR analysis of the P. lividus 14-3-3ε transcription levels in sea urchin embryos at different developmental stages, showed in the lower panel: E eggs, 8c 8 cells, M morula, B blastula, mB mesenchyme blastula, EG early gastrula, LG late gastrula, Pr prism, Pl plutei, hpf hours post-fertilization. Spz12-1 was used as normalizing endogenous gene; cDNA extracted from eggs was used as reference sample and assumed as 1 in the histogram. Each bar represents the mean of three independent experiments ±SD. Mean values were significantly different according to the one-way ANOVA (P < 0.05), followed by the Tukey's test
The aim of this study was to investigate the temporal and spatial expression of the 14-3-3ε gene in P. lividus sea urchin embryos, during development and in response to UV-B radiation simulated in the laboratory. The complete cDNA encoding Pl14-3-3ε was isolated by reverse transcriptase polymerase chain reaction (RT-PCR) from UV-B-exposed embryos mRNA. Quantitative real-time PCR (QPCR) analysis was used to assess the extent of gene expression; in situ hybridization analysis was performed on whole mount specimens to look at the spatial distribution of 14-3-3ε mRNA in control and in UV-B exposed sea urchin embryos. Results describe the physiological transcription during development and variations after UV-B stress.
Materials and methods
Embryo culture, UV-B experimental exposure and morphological analysis
Gametes were collected from gonads of the sea urchin Paracentrotus lividus harvested in Palermo, Sicily, Italy. Eggs were fertilized and embryos reared at 18°C in Millipore filtered sea water (MFSW) containing antibiotics (50 mg/l streptomycin sulfate and 30 mg/l penicillin), at the dilution of 4,000/ml in glass beckers, with gentle stirring. Exposure procedure was partially modified from what was previously described (Bonaventura et al. 2005, 2006). Briefly, embryos were harvested at the 32 cell stage, about 3 h post-fertilization, dispensed in 90 mm Petri dishes (20 ml) and irradiated with the UV-B lamp (Labortechnik, model VL-6.M.) placed at a distance of 6 cm. Choosing the appropriate irradiation times, 56 and 112 s, embryos received the UV-B doses of 400 and 800 J/m2, respectively. After irradiation embryos were cultured at 18°C in the dark and harvested 2 h later. The morphological analysis of perturbed and control embryos was performed using an inverted microscope (Zeiss Axioscop 2 plus), images were recorded by a digital camera.
RNA extraction and isolation of cDNA by RT-PCR
Total RNA from control and irradiated embryos was extracted according to Russo et al. (2003), precipitated with 2M LiCl (f.c.) overnight at 4°C and quantified by readings at 260 nm using an Eppendorf bio-photometer. Total RNAs (10 μg) from control and exposed embryos were reverse transcribed according to the GIBCO-BRL manufacturer’s instructions. An aliquot of the cDNA obtained (usually 50 ng) was used to perform Polymerase Chain Reaction. The PCR primers were designed on the basis of the specific 14-3-3 gene nucleotidic sequence from Geodia cydonium. Oligonucleotides used for cloning were: forward 5′ CAGTCGCCTACAAGAATGTCGTCGG 3′ and reverse 5′-ACGCAGGAGCTGCATGATGAGAG 3′. PCR was performed by using Pharmacia Taq polymerase under the following conditions: 25 μl of final volume containing 0.8 μM primers, 1× PCR buffer/MgCl2, 0.2 μM dNTP, 2 units of Taq polymerase. (1× cycle: denaturing 94°C for 3 min; 40× cycles: denaturing 94°C for 30 s, annealing 50°C for 45 s, extension 72°C for 30 s; 1× cycle: final extension 72°C for 10 min). The 551 nt amplified fragment was cloned in the TOPO TA vector II (Invitrogen), following the manufacturer protocol. The clone was sequenced using MWG biotech sequencing service. The missing parts at the 5′ and 3′ ends were obtained by primer walking. The compiled sequence was found in the P. lividus 5′ EST library clones obtained from MGE/Genoscope at http://www.molgen.mpg.de/~ag_seaurchin/. The Pl14-3-3ε cDNA sequence deposited at the EMBL database in 2002, accession number: AJ493680, has been updated. Sequences homologies were analyzed using BLAST 2, on the server at National Centre for Biotechnology Information (NCBI) (Altschul et al. 1990).
Sequence comparisons
The 14-3-3ε sequences, selected from ten species at NCBI, were utilized for the alignment. Multiple alignments were performed with ClustalW (Thompson et al. 1994). Alignments were examined and adjusted manually. A phylogenetic tree was generated by the Neighbour Joining method choosing a Blosum matrix in agreement with alignment (Saitou and Nei 1987).
Northern blotting
We used the procedure previously described (Russo et al. 2003). Briefly, total RNA (20 μg) isolated from control and UV-B irradiated embryos as described above were run on 1.5% agarose gel, under denaturing conditions (formamide 50%, MOPS 1×, formaldeide 5.5%). RNA was blotted to a Hybond nylon membrane by capillary transfer in 20× SSC, and UV-crosslinked (30 s at 254 nm). Hybridization conditions were chosen according to the DIG-Nucleic Acid Detection protocol (Roche), using an antisense DIG-labelled RNA probe, obtained by in vitro run-off transcribed by Sp6 polymerase. The nylon membrane, containing total RNA, was hybridized with the 551nt Pl14-3-3ε DIG labelled antisense RNA probe. Hybridization was performed in denaturing hybridization solution containing 50% formamide, at 50°C. The stringency wash was carried out in 0.2× SSC-0.1% SDS at 50°C.
Quantitative real-time PCR
Quantification measurements of gene expression were performed as described by the manual of Applied Biosystems Step One Plus real time PCR, a Comparative Threshold Cycle Method, using SYBR Green chemistry (Livak and Schmittgen 2001). Spz12-1 mRNA was used as the internal endogenous reference gene (Minokawa et al. 2004). cDNAs were synthesized according to Invitrogen Superscript II RNase H reverse transcriptase protocol. The QPCR was run as follow: 1× cycle denaturing 95°C for 10′ for DNA polymerase activation; 38× cycles: melting 95°C for 15″, annealing/extension 60°C for 60″. Oligonucleotides used to amplify the epsilon 14-3-3 gene were: forward 5′TCAAGGCGTGCAGCATTGCATAC3′ and reverse 5′TTCTCGATCAATCCTCTGTAGT3′. The amplicon length was 116 nt. Statistical analyses of values were performed by one-way ANOVA analysis of variance test, followed by the multiple comparison test of Tukey's, using the Origin 8.1 statistical program, and level of significance was set to P < 0.05.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Kiyomoto et al. 2007). All the pre-hybridization and hybridization steps were carried out in 96-well plates, using 30–40 embryos per well. Hybridization was carried out with 500 ng/ml single strand sense or anti-sense 14-3-3 DIG- labelled RNA probes overnight at 65°C, for 18 h. After washings, embryos were mounted on glass slides and observed using a Zeiss Axioscop 2 plus inverted microscope; images were recorded by a digital camera. Hybridization with sense probe showed no specific signal.
Whole-mount indirect immuno-fluorescence
Control and UV-B treated embryos were fixed in 4% paraformaldehyde in MFSW for 1 h at r.t. and stored in 100% MeOH at −20°C until use. The indirect immuno-fluorescence was performed using the monoclonal antibody (McAb) 5c7, specific for the endoderm marker Endo 1 (Wessel and McClay 1985), a kind gift from Prof. D.R. McClay. Briefly, embryos were rehydratated with 0.1% Tween in TBS (TBST) and incubated with the McAb, diluted 1:5 in TBST overnight at 4°C. After extensive washes, embryos were incubated for 1 h with TRITC-conjugated rabbit anti-mouse IgG (Sigma Chemical Co., St. Louis, MO, USA), diluted 1:200 in TBST. Embryos were observed under an inverted microscope (Zeiss Axioscop 2 plus) equipped for epifluorescence and the images were recorded by a digital camera.
Results
Characterization of P. lividus 14-3-3ε cDNA
We isolated the complete cDNA encoding the 14-3-3ε of the sea urchin P. lividus having a nucleotidic sequence of 762 nt in length. Deduced protein sequence was analyzed by the BLAST program, and identified significant similarities with ε isoforms found in many organisms. In fact, the isolated cDNA showed 74% of nucleotidic identity with 14-3-3ε isoforms of H. erithrogramma and H. tuberculata sea urchin species, 72% with S. purpuratus, 68% with B. mori, 65% with Culex pipiens and Drosophila sp. Blasted Pl14-3-3ε aminoacidic sequence showed percentage of identity with isoforms from other organisms as follow: 64% with H. tuberculata, H. erithrogramma and S. purpuratus, 61% with the plant A. thaliana, 60% with the D. melanogaster, B. mori and C. pipiens; 59% with human, mouse, bovine, Xenopus and chicken. The percentage of identity decreased if Pl14-3-3ε protein was compared with other isoforms (beta, gamma, delta, etc.). Thus, Pl14-3-3ε belongs to the 14-3-3 family, whose members are characterized by signature patterns of two highly conserved regions: the first is a peptide of 11 residues located in the N-terminal section [RA]-N-L-[LIV]-S-[VG]-[GA]-Y-[KN]-N-[IVA]; the second, a 20-amino acid region located in the C-terminal section Y-K-[DE]-[SG]-T-L-I-[IML]-Q-L-[LF]-[RHC]-D-N-[LF]-T-[LS]-W-[TANS]-[SAD]. In Pl14-3-3ε, 85% identity is found for the first region and 91% for the second.
It has been reported that the 14-3-3 family has a high sequence conservation among isoforms; in agreement, we found a high degree of identity when comparing P. lividus 14-3-3ε with homologs from different phyla. Serine residues, which are typical phosphorylation sites usually important for interaction with signaling intracellular partners, lie in conserved positions in all organisms (Morrison 2008). Accordingly, by comparing the predicted amino acid sequence of sea urchin, plant, insects and a few vertebrates, we found that out of the 15 total Ser present in Pl14-3-3ε, six are conserved in all organisms analyzed in this study (Ser-56, Ser-74, Ser-160, Ser-190, Ser-227, Ser-231), and three only in sea urchins (Ser-48, Ser-151, Ser-157). This is in agreement with the notion of a certain variability in the position of Ser phosphorylation sites in all isoforms (Aitken 2006). ClustalW alignment and the NJ phylogenetic tree were performed with representative 14-3-3ε isoforms from distant species (from plants to humans) (Fig. 1). The alignment showed a high number of identical residues and conservative substitutions among sea urchins, insects, mammals and plants (Fig. 1a). Based on the alignment, we generated a NJ tree (Fig. 1b) which showed different classes of vertebrates cluster separately from invertebrates and that, among invertebrates, one branch is divided into two parts, one for the sea urchins group and one for A. thaliana.
Fig. 1.
a Multiple sequence alignment of 14-3-3ε isoforms from different species. Identical amino acids are shaded in black, conservative amino acids substitutions are shaded in grey. b Phylogenetic tree derived from a. Branch lengths are proportional to evolutionary distance showing the divergence among different species of sea urchin and other organisms. The scale bar indicates an evolutionary distance of 0.1aa substitutions per position in the sequence. The Genbank accession number are: Paracentrotus lividus (AJ493680), H. tuberculata (ABX45047), Strongylocentrotus purpuratus (Glean 3_03825), A. thaliana (Q541X6), Bombyx mori (NP_001091764.1), D. melanogaster (P92177), Xenopus laevis (O57468), Homo sapiens (P62258), Mus musculus (P62259), Bos taurus (P62261), Gallus gallus (Q5ZMT0.1)
Differential expression of P. lividus 14-3-3ε mRNA in control and UV-B exposed embryos
We performed QPCR experiments with cDNA samples obtained by reverse transcription from total RNA extracted at different developmental stages of sea urchin embryos: unfertilized eggs, eight cells, morula, blastula, mesenchyme blastula, early gastrula, late gastrula, prism and pluteus (see Fig. 2, lower panel). Quantitative measurements were performed using a comparative threshold cycle method, in which the Spz12-1 cDNA PCR product was used as an endogenous control gene, which was assumed to be constant during development. The egg cDNA was used as reference sample and was assumed as 1 in arbitrary units. The histogram in Fig. 2 shows the Pl14-3-3ε transcript levels during sea urchin development: the relative quantity fold increases ranged from 2 to 4.75 at the blastula and prism stages, respectively. Lower levels were detected between eight-cell stage and morula, as well as at the pluteus stage. Therefore, it seems that during the period between fertilization and morula, no mRNA synthesis is required, as the transcripts detected in the unfertilized egg are probably used during the first developmental stages.
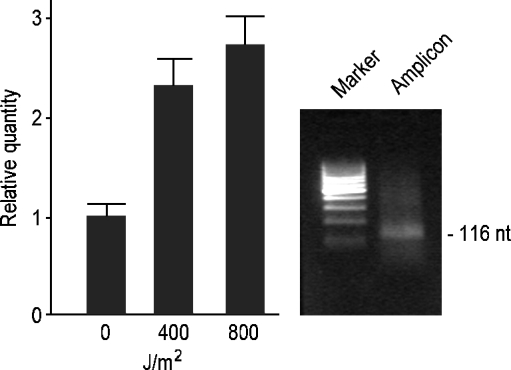
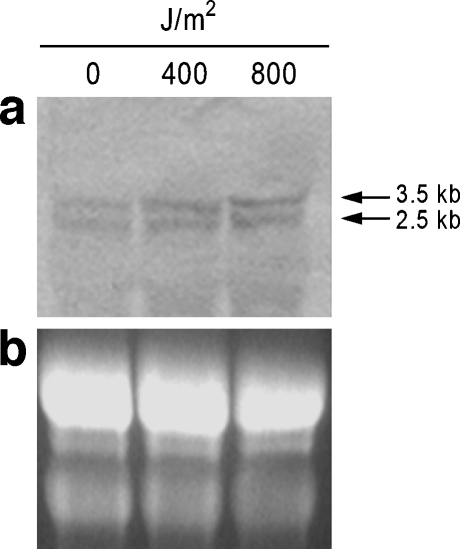
To determine if UV-B radiation had an effect on the Pl14-3-3ε gene, mRNA expression levels were investigated by QPCR in control and UV-B exposed embryos at the doses of 400 and 800 J/m2. The doses were chosen on the basis that in previous studies most embryos displayed abnormal morphologies and expressed the hsp70 stress protein in a dose-dependent manner (Bonaventura et al. 2006). We selected the 32 cell stage for UV-B exposure as Pl14-3-3ε transcript levels appear lower than other stages (Fig. 2). Moreover, the 32 cell stage is a crucial period during development and represents an ideal stage to study stress effects. The histogram in Fig. 3 summarizes results of three independent QPCR analyses and is representative of two different UV-B exposure experiments. In particular, we found a 2.3-fold increase in 400 J/m2 UV-B exposed embryos and a 2.7-fold increase in 800 J/m2 UV-B exposed embryos, compared with the controls. By Northern blot analysis at high stringency conditions, two transcripts were detected with approximate lengths of 3.5 and 2.5 kb (Fig. 4a). The ribosomal RNA bands (26S and 18S), corresponding to about 4 and 2 kb are shown in the agarose gel in Fig. 4b.
Fig. 3.
Quantitative PCR analysis of Pl14-3-3ε mRNAs in control and UV-B exposed (400 and 800 J/m2) sea urchin cleavage embryos. Relative levels are expressed in arbitrary units as fold increase compared with the control sample (0 J/m2) assumed as 1 in the histogram, using the endogenous gene Spz12-1 for normalization. The amplicon band size (116 nt) was visualized on 2% agarose gel, using as reference the low-range DNA ladder marker by MBI Fermentas. Each bar represents the mean of three independent experiments ±SD. Mean values were significantly different according to the one-way ANOVA (P < 0.05), followed by the Tukey's Test
Fig. 4.
Northern blot analysis of Pl-14-3-3ε gene expression in normal embryos and UV-B exposed embryos (400 J/m2 and 800 J/m2). Total RNAs were loaded on a denaturing agarose gel (b), transferred to a nylon membrane (a), and hybridized with a 551 bp DIG-labelled antisense RNA probe, transcribed from Pl14-3-3ε cDNA. Positions of the two transcripts are indicated on the right. In b are visible ribosomal RNAs of known length (about 4 and 2 kb)
Spatial expression of Pl14-3-3ε in control and UV-B exposed embryos
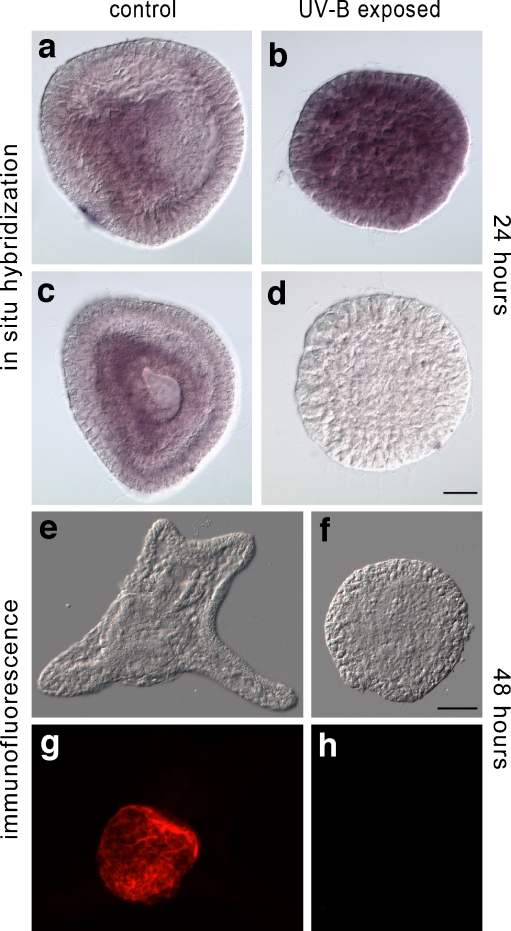
Given the abnormal morphologies obtained after UV-B irradiation, which produced embryos almost lacking a skeleton and with an under-differentiated endoderm (Bonaventura et al. 2006) and the specific territorial localization of the H. tuberculata 14-3-3ε in the invaginating archenteron (Love et al. 2008), it was interesting to determine if the Pl14-3-3ε transcript underwent spatial variations in embryos exposed to UV-B. For this purpose, 32 cells embryos were exposed to 400 J/m2 UV-B and harvested 24 h after irradiation, with the aim of letting them reach the gastrula stage, namely when embryonic territories are well defined. Fixed embryos were hybridized with the Pl14-3-3ε 551nt antisense DIG labelled RNA probe. In Fig. 5, the specific labelling is localized in the invaginating archenteron of the control gastrula embryos (Fig. 5a, c). By contrast, in UV-B exposed embryos, which developed abnormally with disorganized territories, the Pl14.3.3ε mRNA is spread around the whole embryo (Fig. 5b). To confirm that endoderm cells do not differentiate, as suggested by the lack of a normal tripartite gut in 48-h-treated embryos (this paper, Bonaventura et al. 2006), we looked for the presence of Endo1 protein (Wessel and McClay 1985), a late endoderm marker specifically expressed in midgut and hindgut. As shown in Fig. 5 UV-B exposed embryos, characterized by extreme abnormal morphology (f, h), do not express Endo1 protein (h), which by contrast, is detected in controls (g) by whole mount indirect immuno-fluorescence.
Fig. 5.
Spatial expression of Pl14-3-3ε by whole mount in situ hybridization with 551 bp DIG-antisense Pl-14-3-3ε RNA (a–c) and negative sense probe (d), in control (a, c) and 400 J/m2 UV-B exposed embryos (b, d), 24 h post-fertilization. Early gastrula control embryo, lateral view(a); late gastrula control embryo, vegetal pole view (c). Bright field (e, f) and indirect immuno-fluorescence (g, h) with McAb specific for Endo1 of control (g) and 400 J/m2 UV-B exposed embryo (h), 48 h post-fertilization. Bar = 20 μm
Discussion
In this paper we report the complete cDNA encoding of a member of the 14-3-3 family of proteins that play important roles in cellular homeostasis and stress responses. The 762 bp long nucleotidic sequence for the epsilon isoform has been characterized in P. lividus sea urchin embryos, and is referred to as Pl14-3-3ε. It encodes a 254 residues-long protein with a high percentage of identity (59–64%) with similar proteins from different phyla. Interestingly, ε is the only 14-3-3 isoform isolated from sea urchin embryos of different species to be investigated so far, including P. lividus (this article), H. tuberculata, H. erithrogramma (Love et al. 2008). Three isoforms were also annotated in the genome of S. purpuratus (Sea Urchin Genome Sequencing et al. 2006; Samanta et al. 2006). The ε isoform has been described as a “living fossil”, suggesting the hypothesis that it might be a functionally conserved copy of an ancestral gene and that other isoforms have arisen from more recent duplication events (Wang and Shakes 1996). Other embryonic ε isoforms have been isolated from Xenopus (Lau et al. 2006), B. mori (Tabunoki et al. 2008) and Drosophila (Acevedo et al. 2007). The presence of the protein has been shown to be essential for embryonic hatching in Drosophila (Acevedo et al. 2007). In addition, functional knock-down experiments demonstrated that Xenopus embryos lacking ε protein (and to a major extent τ protein) displayed unique defects in gastrulation (exogastrula) and axial patterning (Lau et al. 2006).
We found two Pl14-3-3ε transcripts of about 2.5 and 3.5 kb, detected both in control and UV-B exposed embryos, that are indicative of possible mechanisms of alternative splicing. This is in agreement with literature reports on the presence of multiple mRNA differing in the 5′/3′ UTR lengths, but encoding the same protein, as found in the case of 14-3-3ζ and β isoforms of human keratinocytes (Leffers et al. 1993). The same holds true for the six ζ and the two ε transcript variants found in human cells (MGC Project Team 2004) and the two transcripts encoding for θ isoform in mouse male germ line cells (Perego and Berruti 1997). It has been reported that the size of the 14-3-3 mRNA isoform is extremely variable, ranging from 1.7 to 4.4 kb (Perego and Berruti 1997; Leffers et al. 1993; Love et al. 2008). In H. tuberculata and H. erythrogramma sea urchins, ε isoform transcripts have a length of 4.4 kb (Love et al. 2008).
It has been described that different 14-3-3 isoforms have distinct sub-cellular and tissue distributions in many organisms, as well as temporal changes in their expression during development (reviewed by MacKintosh 2004; Aitken 2006). In Xenopus, expression analysis revealed that 14-3-3β, ε, γ, τ have a spatial specific location in late embryos (Lau et al. 2006). Here, we confirmed previous results obtained in H. tuberculata and H. erythrogramma by Love et al. (2008), who described the spatial expression of 14-3-3ε mRNA restricted to the archenteron at the gastrula stage. The new finding is that, in response to UV-B stress, Pl14-3-3ε is up-regulated and ectopically expressed in all embryonic territories. The latter correlates with defects in the archenteron morphogenesis and the lack of a specific territorial marker. To explain the fact that UV-B radiation up-regulates the Pl14-3-3ε gene, and coincidentally deregulates its appropriate site-specific expression, we can hypothesize that a de-repression of the Pl14-3-3ε gene is occurring. Similar misregulation mechanisms associated with defects in morphogenesis have been reported, as for example in the case of the nodal gene, whose expression is extended ectopically after treatment with nichel which causes radialization in P. lividus embryos (Duboc et al. 2010).
In conclusion, we have demonstrated a direct dose-dependent relationship between UV-B exposure of sea urchin embryos and Pl14-3-3ε mRNA levels, suggesting its implication in the regulative cascade activated in the stress response. To the best of our knowledge this is the first time that a UV-B induced 14-3-3ε transcriptional regulative mechanism has been demonstrated. In the past other authors demonstrated increased protein levels after exposure to UV-B of human skin (Choi et al. 2005) and melanoma cells (Petrocelli and Slingerland 2000), suggesting a role for 14-3-3 in photo-aging processes and cell cycle regulation, respectively. As previously described, upon stress 14-3-3 proteins are phosphorylated by the JNK kinase and are thus unable to bind BAD, BAX and FOXO, which are then able to translocate to mitochondria and nuclei, exerting their pro-apoptotic functions (Morrison 2008). The increase in Pl14-3-3ε mRNA detected after UV-B stress could result in higher 14-3-3 protein levels (possibly not phosphorylated) which could then bind a large quantity of the above mentioned factors, determining cellular survival. This hypothesis accords with our previous reports describing HSP70 increased levels in response to UV-B and suggesting its role in protecting cells from apoptosis (Bonaventura et al. 2005, 2006). However, as at the moment we have no information on the levels of phosphorylated or de-phosphorylated Pl 14-3-3ε, because of the lack in cross-reactivity of available antibodies, further investigation is needed to verify the suggested hypothesis. On the basis of studies described here we propose the use of sea urchin embryos for examining the role of 14-3-3 in cell stress response pathways. Finally, 14-3-3ε could be used as a valuable molecular biomarker to identify the dangerous effects of sunlight occurring in marine organisms living in shallow waters.
Acknowledgements
We thank the Marie Curie Ph.D. student K. Karakostis, for his initial useful support to QPCR experiments. This research was supported in part by: EU-UV-TOX Project Contract EVK3-CT-1999-00005, ASI MoMA Project Contract N°1/014/06/0 and EU-ITN Biomintec Project, Contract N°215507.
References
- Acevedo SF, Tsigkari KK, Grammenoudi S, Skoulakis EM. In vivo functional specificity and homeostasis of Drosophila 14-3-3 proteins. Genetics. 2007;177:239–253. doi: 10.1534/genetics.107.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello M, Roccheri MC. Apoptosis: focus on sea urchin development. Apoptosis. 2010;15:322–330. doi: 10.1007/s10495-009-0420-0. [DOI] [PubMed] [Google Scholar]
- Agnello M, Filosto S, Scudiero R, Rinaldi AM, Roccheri MC. Cadmium induces apoptotic response in sea urchin embryos. Cell Stress Chaperones. 2007;12:44–50. doi: 10.1379/CSC-229R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Autier P, Dore JF, Schifflers E, et al. Melanoma and use of sunscreens: an aortic case control study in Germany, Belgium and France. Int J Cancer. 1995;61:749–755. doi: 10.1002/ijc.2910610602. [DOI] [PubMed] [Google Scholar]
- Banaszak AT, Lesser MP. Effects of solar ultraviolet radiation on coral reef organisms. Photochem Photobiol Sci. 2009;8:1276–1294. doi: 10.1039/b902763g. [DOI] [PubMed] [Google Scholar]
- Batel R, Fafandjel M, Blumbach B, Schröder HC, Hassanein HM, Müller IM, Müller WE. Expression of the human XPB/ERCC-3 excision repair gene-homolog in the sponge Geodia cydonium after exposure to ultraviolet radiation. Mutat Res. 1998;409:123–133. doi: 10.1016/s0921-8777(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Bonaventura R, Poma V, Costa C, Matranga V. UV-B radiation prevents skeleton growth and stimulates the expression of stress markers in sea urchin embryos. Biochem Biophys Res Commun. 2005;328:150–157. doi: 10.1016/j.bbrc.2004.12.161. [DOI] [PubMed] [Google Scholar]
- Bonaventura R, Poma V, Russo R, Zito F, Matranga V. Effects of UV-B radiation on development and hsp70 expression in sea urchin cleavage embryos. Mar Biol. 2006;149:79–86. doi: 10.1007/s00227-005-0213-0. [DOI] [Google Scholar]
- Chaudhri M, Scarabel M, Aitken A. Mammalian and yeast 14-3-3 isoforms form distinct patternsof dimers in vivo. Biochem Biophys Res Commun. 2003;300:679–685. doi: 10.1016/S0006-291X(02)02902-9. [DOI] [PubMed] [Google Scholar]
- Choi KC, Lee S, Kwak SY, Kim MS, Choi HK, Kim KH, Chung JH, Park SH. Increased expression of 14-3-3 varepsilon protein in intrinsically aged and photoaged human skin in vivo. Mech Ageing Dev. 2005;126:629–636. doi: 10.1016/j.mad.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fuente H, Lamana A, Mittelbrunn M, Perez-Gala S, Gonzalez S, García-Diez A, Vega M, Sanchez-Madrid F. Identification of genes responsive to solar simulated UV radiation in human monocyte-derived dendritic cells. PLoS ONE. 2009;4(8):e6735. doi: 10.1371/journal.pone.0006735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/S1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117:1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lapraz F, Saudemont A, Bessodes N, Mekpoh F, Haillot E, Quirin M, Lepage T. Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo. Development. 2010;137:223–235. doi: 10.1242/dev.042531. [DOI] [PubMed] [Google Scholar]
- Dunne RP, Brown BE. Penetration of solar UVB radiation in shallow tropical waters and its potential biologicaleffects on coral reefs; results from the central Indian Ocean and Andaman Sea. Mar Ecol Prog Ser. 1996;144:109–118. doi: 10.3354/meps144109. [DOI] [Google Scholar]
- Fernandez-Guerra A, et al. The genomic repertoire for cell cycle control and DNA metabolism in S. purpuratus. Dev Biol. 2006;300:238–251. doi: 10.1016/j.ydbio.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Gies P, Roy C, Javorniczky J, Henderson S, Lemus-Deschamps L, Driscoll C. Global Solar UV Index: Australian measurements, forecasts and comparison with the UK. Photochem Photobiol. 2004;79:32–39. doi: 10.1562/0031-8655(2004)79<32:GSUIAM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hader DP. Effects of solar UV-B radiation on aquatic ecosystems. Adv Space Res. 2000;26:2029–2040. doi: 10.1016/S0273-1177(00)00170-8. [DOI] [PubMed] [Google Scholar]
- Hanson KM, Gratton E, Bardeen CJ. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic Biol Med. 2006;41:1205–1212. doi: 10.1016/j.freeradbiomed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- Holzinger A, Lütz C. Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron. 2006;37:190–207. doi: 10.1016/j.micron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3 eta interacts with the nicotinic acetylcholine receptor a4 subunit. J Biol Chem. 2001;276:28281–28290. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- Kiyomoto M, Zito F, Costa C, Poma V, Sciarrino S, Matranga V. Skeletogenesis by transfated secondary mesenchyme cells is dependent on extracellular matrix-ectoderm interactions in Paracentrotus lividus sea urchin embryos. Dev Growth Differ. 2007;49:731–741. doi: 10.1111/j.1440-169X.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Kuromori T, Yamamoto M. Members of Arabidopsis 14-3-3 gene family trans-complement two types of defects in fission yeast. Plant Sci. 2000;158:155–161. doi: 10.1016/S0168-9452(00)00320-4. [DOI] [PubMed] [Google Scholar]
- Lau JMC, Wu C, Muslin AJ. Differential role of 14-3-3 family members in Xenopus development. Dev Dyn. 2006;235:1761–1776. doi: 10.1002/dvdy.20816. [DOI] [PubMed] [Google Scholar]
- Leffers H, Madsen P, Rasmussen HH, Honore B, Andersen AH, Walbum E, Vandekerckhove J, Celis JE. Molecular cloning and expression of the transformation sensitive epithelial Marker Stratifin: a member of a protein family that has been involved in the protein kinase C signalling pathway. J Mol Biol. 1993;231:982–998. doi: 10.1006/jmbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Depth-dependent effects of ultraviolet radiation on survivorship, oxidative stress and DNA damage in sea urchin (Strongylocentrotus droebachiensis) embryos from the Gulf of Maine. Photochem Photobiol. 2010;86:382–388. doi: 10.1111/j.1751-1097.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- Lesser MP, Kruse VA, Barry TM. Exposure to ultraviolet radiation causes apoptosis in developing sea urchin embryos. J Exp Biol. 2003;206:4097–4103. doi: 10.1242/jeb.00621. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loser K, Beissert S. Regulation of cutaneous immunity by the environment: an important role for UV irradiation and vitamin D. Int Immunopharmacol. 2009;9:587–589. doi: 10.1016/j.intimp.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Love AC, Lee AE, Andrews ME, Raff RA. Co-option and dissociation in larval origins and evolution: the sea urchin larval gut. Evol Dev. 2008;10:74–88. doi: 10.1111/j.1525-142X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381:329–342. doi: 10.1042/BJ20031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- MGC Project Team The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC) Genome Res. 2004;14:2121–2127. doi: 10.1101/gr.2596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokawa T, Rasta JP, Arenas-Mena C, Franco CB, Davidson EH. Expression patterns of four different regulatory genes that function during sea urchin development. Gene Expr Patterns. 2004;4:449–456. doi: 10.1016/j.modgep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Moore BE, Perez VJ. In: Physiological and biochemical aspects of nervous integration. Carlson FD, editor. Englewood Cliffs: Prentice Hall; 1967. pp. 343–359. [Google Scholar]
- Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2008;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego L, Berruti G. Molecular cloning and tissue-specific expression of the mouse homologue of the rat brain14-3-3 Q protein: characterization of its cellular and developmental pattern of expression in the male germ line. Mol Reprod Dev. 1997;47:370–379. doi: 10.1002/(SICI)1098-2795(199708)47:4<370::AID-MRD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Petrocelli T, Slingerland J. UVB induced cell cycle checkpoints in an early stage human melanoma line WM35. Oncogene. 2000;19:4480–4490. doi: 10.1038/sj.onc.1203808. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Bowles DJ. Fusicoccin, 14-3-3 proteins, and defense responses in tomato plants. Plant Physiol. 1999;119:1243–1250. doi: 10.1104/pp.119.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AJ, Croce J, Carbonneau S, Voronina E, Miranda E, McClay DR, Coffman JA. The genomic underpinnings of apoptosis in Strongylocentrotus purpuratus. Dev Biol. 2006;300(1):321–334. doi: 10.1016/j.ydbio.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Matranga V (2009) Cellular, biochemical and molecular effects of cadmium on marine invertebrates: focus on Paracentrotus lividus sea urchin development. In: Parvau RG (ed) Cadmium in the Environment. Nova Science Publishers Inc., New York. pp 337–366 ISBN: 1607419343 ISBN13: 9781607419341
- Roccheri MC, Agnello M, Bonaventura R, Matranga V. Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem Biophys Res Commun. 2004;321:80–87. doi: 10.1016/j.bbrc.2004.06.108. [DOI] [PubMed] [Google Scholar]
- Rosenquist M, Alsterfjord M, Larsson C, Sommarin M. Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol. 2001;127:142–149. doi: 10.1104/pp.127.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Bonaventura R, Zito F, Schroder HC, Muller I, Muller WE, Matranga V. Stress to cadmium monitored by metallothionein gene induction in Paracentrotus lividus embryos. Cell Stress Chaperones. 2003;8:232–241. doi: 10.1379/1466-1268(2003)008<0232:STCMBM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samanta MP, Tongprasit W, Istrail S, Cameron RA, Tu Q, Davidson EH, Stolc V. The transcriptome of the sea urchin embryo. Science. 2006;314(5801):960–962. doi: 10.1126/science.1131898. [DOI] [PubMed] [Google Scholar]
- Schröder HC, Bella G, Janipour N, Bonaventura R, Russo R, Müller WE, Matranga V. DNA damage and developmental defects after exposure to UV and heavy metals in sea urchin cells and embryos compared to other invertebrates. Prog Mol Subcell Biol. 2005;39:111–137. doi: 10.1007/3-540-27683-1_6. [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. Sodergren E, Weinstock GM, Davidson EH, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314(5801):941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Prézelin BB, Baker KS, Bidigare RR, Boucher NP, Coley T, Karentz D, MacIntyre S, Matlick HA, Menzies D, et al. Ozone depletion: ultraviolet radiation and phytoplankton biology in antarctic waters. Science. 1992;255:952–959. doi: 10.1126/science.1546292. [DOI] [PubMed] [Google Scholar]
- Tabunoki H, Shimada T, Banno Y, Sato R, Kajiwara H, Mita K, Satoh J. Identification of Bombyx mori 14-3-3 orthologs and the interactor Hsp60. Neurosci Res. 2008;61:271–280. doi: 10.1016/j.neures.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Tedetti M, Sempéré R. Penetration of ultraviolet radiation in the marine environment. A review. Photochem Photobiol. 2007;82:389–397. doi: 10.1562/2005-11-09-IR-733. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Shakes DC. Molecular evolution of the 14-3-3 protein family. J Mol Evol. 1996;43(4):384–398. doi: 10.1007/BF02339012. [DOI] [PubMed] [Google Scholar]
- Wessel GM, McClay DR. Sequential expression of germ-layer specific molecules in the sea urchin embryo. Dev Biol. 1985;111:451–463. doi: 10.1016/0012-1606(85)90497-X. [DOI] [PubMed] [Google Scholar]
- Wiens M, et al. Induction of gene expression of the chaperones 14–3–3 and hsp70 by PCB 118 (2, 3′, 4, 4′, 5-pentachloro-bipheyl) in the marine sponge Geodia Cynodium: novel biomarkers for polychlorinated biphenyls. Mar Ecol Prog Ser. 1998;165:247–257. doi: 10.3354/meps165247. [DOI] [Google Scholar]
- Zito F, Matranga V (2009) Secondary mesenchyme cells as potential stem cells of the sea urchin embryo. In: Rinkevich B, Matranga V (eds) Stem cells in marine organisms, Springer, Berlin. pp 187–213 doi:10.1007/978-90-481-2767-2