Abstract
Bovine respiratory disease complex (BRD), a major economic concern to the beef cattle industry all over the world, is triggered by physical, biological and psychological stresses. It is becoming noticeable that the key to reducing BRD appears to be centered at reducing the response to stress. The aims of the present study were to detect individual variations in the stress response of newly received young calves through their leukocyte heat shock protein (Hsp) response, selected neutrophil-related gene expression and oxidative stress, and relate them to pulmonary adhesions at slaughter, an indicative sign of clinical and subclinical episodes of BRD at an early age. Differential expression patterns of Hsp60 and Hsp70A1A were revealed in newly received calves 1 h, 5 h and 1 day after arrival, distinguishing between stress-responsive and non-stress-responsive individuals. Plasma cortisol was also indicative of stress-responsive and non-stress-responsive individuals, 1 h and 5 h after arrival. At the longer term, β-glycan levels were highest 7 days after arrival and significantly correlated with an adhesion-free phenotype at slaughter. Oxidative stress responses, measured through the oxidation products of the exogenous linoleoyl tyrosine (LT) marker, revealed that hydroperoxidation and epoxidation of membranes may readily occur. Based on the LT oxidation products and levels of β-glycan, we present a discriminant analysis model, according to which vulnerable individuals may be predicted at near 100% probability 7 days after arrival. Since clinical signs of BRD may often go undetected in feedlot calves, such a model, after its examination in large-scale experiments, may be a reliable tool for an early prediction of subclinical signs of BRD.
Keywords: Relocation stress, BRD, Pulmonary adhesions, Hsp, Cortisol, MMP-9, l-selectin, β-glycan, LT marker
Introduction
Stress is generally considered to suppress the immune system and may lead to an increase in the occurrence of disease in the presence of a pathogen (Salak-Johnson and McGlone, 2007). Nearly all immune cell classes possess receptors for the stress-related glucocorticoids and the neurohormones epinephrine and norepinephrine (Reiche et al., 2004). For example, neutrophils (phagocytic innate immune cells) are known target of the stress response and exhibit abundant glucocorticoid receptors (Preisler et al., 2000). Thus, glucocorticoids may interfere with the normal function of neutrophils by altering their numbers and the expression of genes that are key regulators of immune functions (Weber et al., 2001; 2004; Buckham Sporer et al., 2007).
Bovine respiratory disease complex (BRD) is the most costly disease in feedlot cattle all over the world due to prevention and treatment costs, morbidity, mortality and deleterious effects on production (Snowder et al., 2007; Thompson et al., 2006). Functional maturity of the bovine respiratory system is not achieved before 1 year of age (Lekeux et al., 1984). Therefore, regardless of immunological (nondeveloped adaptive immunity) and management considerations, respiratory disease occurs more frequently and is more severe in young than in mature cattle.
BRD represents a disease model of multifactorial etiology that involves physical (e.g. transportation), biological and psychological stresses. Whereas short-distance transportation is only part of the overall stress that newly received calves are confronted with, transportation to long distances has been associated with alterations in immune function (Blecha et al., 1984; Buckham Sporer et al., 2007; Gupta et al., 2007; Yagi et al., 2004) and development of BRD when combined with infectious agents (Yates, 1982).
Some of the infectious agents are normally present in the upper respiratory tract of healthy animals. They may convert to pathogenic status when the animals are subjected to any of a wide variety of stressors (Hodgson et al., 2005). The conversion to pathogenic status is a direct consequence of the release of a variety of products: hormones, neurochemicals and neuropeptides in response to stress. These products lead to pathogenicity in two ways: (i) by directly impairing immune function (Blecha et al., 1984; Buckham Sporer et al., 2007; Gupta et al., 2007; Yagi et al., 2004) and (ii) by serving as an environmental stimulus to the resident or acquired microbes to convert to higher virulence state and initiate growth and pathogenic processes (Freestone et al., 2008).
Previous studies indicate that not all similarly stressed individuals were equally likely to develop disease (Cobb and Steptoe 1996). Exposure to viral agents that cause upper respiratory disease induced clinical disease in some individuals but not in others. Moreover, the severity of the clinical symptoms among those who develop illness can vary substantially (Aich et al., 2007). This suggests that some variability exists in the biological vulnerability, and the key to reducing BRD appears to be centered at reducing the response to stress.
Being the mediator between stress stimuli and disease susceptibility, differential stress responses may be the key to distinguish between potentially resistant and vulnerable individuals. More specifically, identifying the stress response to relocation of young calves at physiological and molecular levels may help to create a multilayer phenotype of the BRD-susceptible individual.
Physiologically, two main stress pathways, the hypothalamus–pituitary–adrenal (HPA) and the sympathoadrenal (SA) axis, whose response functions are central to survival, are also central to stress-immune axis activation that may promote disease susceptibility (Hickey et al., 2003). While plasma cortisol is a common measure of transportation stress, the sensitivity of adrenaline and noradrenaline to animal handling during sampling (Minton 1994) may mislead in the interpretation of the outcome.
At the cellular level, organisms cope with various kinds of stresses by a rapid, specific and massive synthesis of a set of proteins termed heat shock proteins (Hsp) (Craig and Lindquist, 1988; Welch, 1990). Recently, it has been reported that elevated levels of intracellular Hsp60 may confer a tissue-dependent cytoprotection in 2-h transported piglets (Zhu et al., 2009). Studies during the last decade indicated that extracellular Hsp, being released from stressed cells, promoted proinflammatory cytokine production and local inflammation through activation of the innate immune system. Their mode of action was shown to be similar to that of lipopolysaccharide (LPS) (Campisi et al., 2003).
The immature antioxidant defense system of neonatal calves may turn them susceptible to oxidative damage (Inanami et al., 1999). Indeed, accumulating circumstantial evidence correlate BRD with oxidative stress (Chirase et al., 2001; Hutcheson and Cole, 1985). In a more recent study, Chirase et al. (2004) reported a significant decrease of serum total antioxidant capacity in calves 1 day posttransportation and related it to an increase in serum malondialdehyde and repeated episodes of BRD.
Clinical BRD occurs most commonly during the first 3–6 weeks after arrival at the feedlot (Radostits et al., 2000). To date, the main clinical signs of BRD are subjective and depend on the stage and extent of the disease process. However, clinical signs of BRD may often go undetected in feedlot calves (Wittum et al., 1996). This reflection of diagnostic oversight emphasizes the need of an objective, accurate and reliable tool for an early prediction of the risk to develop BRD by newly received calves. Such detection would be of great significance, since early treatment of calves tending to develop BRD might improve their welfare and prevent the appearance of typical pulmonary lesions and adhesions that adversely affect their performance (Gardner et al., 1999; Thompson et al., 2006).
In light of the above, the objective of the current study was to detect individual stress responses to relocation among young calves, in the blood tissue, through their leukocyte Hsp response, neutrophil-related gene expression and oxidative stress, as possible keys to predict individuals that are prone to develop BRD after arrival.
Materials and methods
Animals and experimental design
This study was divided into two experiments and conducted at the beef cattle unit in Newe Ya'ar Research Center. Twenty-five Holstein–Friesian bull calves were included in the study. At the age of 4–7 days, calves were 2-h truck transported from their original dairy farm at Bet Dagan to the nursery in Newe Ya'ar. From the day of arrival, calves were placed together in a 180-m2 roof-covered yard and predominantly consumed milk replacer (125 g/l; Top 440, Kofolk, Petach-Tikva, Israel) until the age of 60 days, while having also free access to suckling total mixed ration (Brosh et al., 1992) and water. Milk replacer was supplied to calves using computer-controlled suckling machine, equipped with a real-time algorithm embedded in a software application (Gavish, Givat Brenner, Israel), to allow individual milk allocations.
Experiment 1
This experiment was conducted to follow the short-term effect of relocation on the levels of Hsp. Twelve calves were included in this experiment. Prior to transportation and 1 h, 5 h and 1 day after arrival, blood was sampled for the isolation of RNA.
Experiment 2
This experiment was designed to follow the effect of relocation of 13 young Holstein–Friesian calves on the expression pattern of selected genes with significant role in the innate immunity. Levels of matrix metalloproteinase-9 (MMP-9), l-selectin and β-glycan were followed pretransportation and 1, 4 and 7 days after arrival.
Evaluation of oxidative stress in these individuals was carried out using the linoleoyl tyrosine (LT) marker (see below), but with an additional sampling point—10 days after arrival. After weaning, calves were held in a feedlot and fed common fattening ration. At the age of 12 months, they were transferred to the slaughterhouse where they were analyzed for the existence of pulmonary adhesions.
Phenotypes of clinical and subclinical episodes of BRD
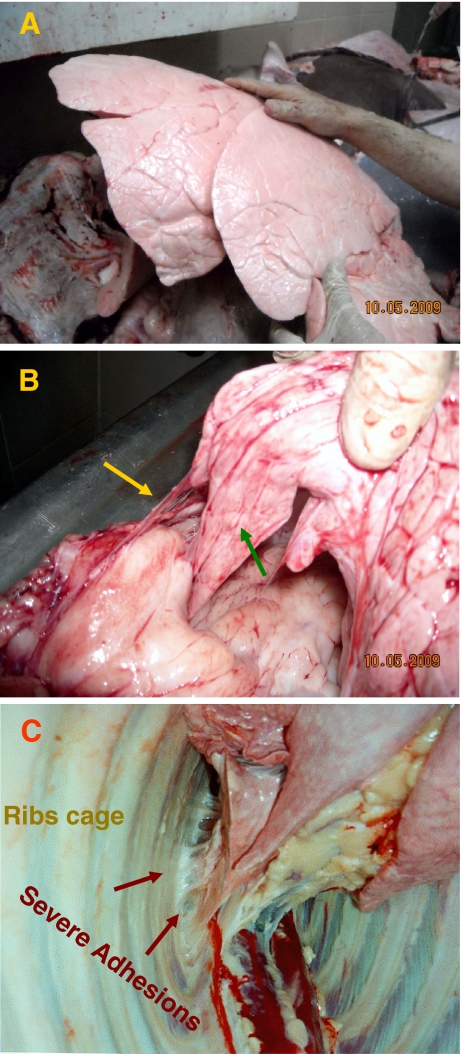
Susceptible individuals can be readily identified as animals showing severe lower tract respiratory symptoms indicative of BRD. However, clinical signs of BRD may often go undetected in feedlot calves. Hence, resistant individuals are more difficult to identify; unaffected animals may simply be individuals that were not exposed to the stressor or to the infectious agents, or could be those that were actually infected but did not develop clinical symptoms. To overcome this, we made use of a unique Israel Kosher slaughter classification into three categories with respect to lung adhesions: adhesion-free (so-called Glatt-Kosher), slight adhesions (Kosher)—representative of no BRD episodes—and severe adhesions (non-Kosher) (Fig. 1).
Fig. 1.
Kosher status of the meat is mainly determined by the appearance of pulmonary adhesions. These phenotypes are indicative of clinical and subclinical episodes of BRD at young age. Panel a represents a Glatt-Kosher status in which no adhesions are observed, whereas in panels b and c, a non-Kosher state, characterized by severe pulmonary adhesions (marked by arrows), is shown
Blood sampling, RNA isolation and reverse transcriptase–polymerase chain reaction
Blood was sampled from the jugular vein of calves, using evacuated tubes (Greiner Bio-One GmbH, Kremsmunster, Austria) containing acid citrate dextrose (ACD) or EDTA as anticoagulant.
Blood samples collected into ACD-containing tubes were used to analyze neutrophil-related gene expression. Blood samples collected into EDTA-containing tubes were used for the analysis of Hsp, cortisol and evaluation of oxidative stress by the exogenous LT-marker (see below).
The blood was centrifuged at 1,000 × g, 4°C, to separate the cells from the plasma. The buffy coat was transferred to a new chilled Eppendorf tube. Remaining red blood cells were removed by red blood cell lysis buffer (Roche Pharmaceuticals, Petach Tikva, Israel, cat # 1-814-389). Leukocytes were then washed with cold PBS and immediately used for RNA isolation.
Total RNA was extracted from leukocytes using High Pure RNA Isolation Kit (Roche), according to the manufacturer's recommendation. To remove genomic DNA contamination, samples were treated with DNase (Epicenter, Madison, Wisconsin, USA, cat # DB0711k) according to the manufacturer's recommendation. The concentration of RNA as well as its quality was measured by Nano-Drop (ND-1000). Quality of total RNA was additionally estimated by nondenaturating agarose gel. The RNA was stored in −80°C or immediately utilized for reverse transcriptase–polymerase chain reaction (RT-PCR) using the Verso cDNA Kit (Thermo Fisher Scientific: Abgene House, Epsom, UK, Inc., cat # AB1453/A). The T-Personal PCR machine (Biometra Goettingen, Germany) was programmed as follows: 42°C for 60 min for the RT step followed by 95°C for 2 min and the amplification steps of 94°C for 2 min, 60°C for 40 s and 72°C for 1:30 min. A master mix was prepared and aliquoted to test tubes, each of which was amplified for 30 cycles. The RT-PCR reactions were performed using the pairs of primers shown in Table 1. The primers for Hsp60 and Hsp70A1A were designed using the software “Primer 3”. To amplify MMP-9, l-selectin, β-glycan and β-actin, we used primers as in Buckham Sporer et al. (2007). All primers were synthesized by Sigma-Aldrich (Rehovot, Israel). PCR products were run on agarose gel, and intensity of cDNA bands was quantified by densitometry software (TINA; Raytest Isotope Messgerate Gmbh, Staubenhardt, Germany). The stable expression of β-actin across treatments enabled its use as an endogenous control to measure relative expression of tested genes.
Table 1.
PCR primer pairs used to amplify the tested genes
| Gene | Forward sequence | Reverse sequence | Length (bp) |
|---|---|---|---|
| Hsp60 | CCAGTGGAAATCAGGAGAGG | TGAGCATTGGCAATTTCAAG | 392 |
| Hsp70A1A | GAGGCGGACAAGAAGAAGGT | CTTGCATAGCTGATGGCTGA | 340 |
| β-glycan | TTGTTGGGTGACTCGT | AAGGATTTAAAACTGTGGTT | 309 |
| MMP-9 | CAGACCTTTGAGGGCGAACT | TCGTCGAAGTGGGCATCTC | 296 |
| l-selectin | CCCAACAACAGGAAGAGTAAG | TGCCAGCCAAATGATAAA | 711 |
| β-actin | AAGGCCAACCGTGAGAAGATG | TGCGGTGGACGATGGAG | 781 |
Cortisol analysis
The plasma derived from EDTA anticoagulated blood was used to determine circulating cortisol concentration by the Elecsys cortisol assay (Roche, cat # 11875116). Samples were treated according to manufacturer instructions and run on the Elecsys 1010 analytical apparatus.
Evaluation of oxidative stress
Oxidative stress of relocated calves was evaluated by the exogenous LT marker. The designed LT marker consists of the amino acid tyrosine (Tyr) covalently bound to the polyunsaturated fatty acid linoleic acid (LA), forming N-linoleoyl tyrosine (Szuchman et al., 2006). This molecule contains both a lipophilic subunit (the LA), which can enter into the lipophilic tissues and membranes, and hydrophilic subunits, the carboxylic group as well as the hydroxyl phenol. The LA and Tyr molecules are known to be easily oxidized under oxidative stress and to generate specific oxidized products, depending on the type of reactive oxygen species/reactive nitrogen species (ROS/RNS) present in vivo. Blood samples (1 ml) from pre- and posttransported calves were collected in tubes containing 8 μl of LT marker [from a stock solution of 20 mM marker dissolved in dimethyl sulfoxide (DMSO)] and 5 μl of heptadecanoyl tyrosine as internal standard (from a stock solution of 20 mM dissolved in DMSO). Blood was incubated in a water bath at 37°C for 1 h, after which it was extracted with 8 ml of hexane/2-propanol (3:2 v/v), containing 10 ppm butylated hydroxyl anisol. The organic phase was collected and evaporated under nitrogen, and samples were kept under nitrogen at −20°C until analysis.
LT was analyzed by liquid chromatography/mass spectrometry (LC/MS), as described in Szuchman et al. (2006). Briefly, the LC/MS was equipped with a high-performance liquid chromatography (HPLC) model 2790 (Waters, MA, USA) and a Waters photodiode array detector (Model 996), connected to an MS (Waters Micromass Quattro Ultima MS, UK). The HPLC column was a 3.5-μm C18 ODS XTerra (Waters) and the eluents were a gradient of solution A (0.1% acetic acid in acetonitrile) and solution B (0.1% acetic acid in double distilled water (DDW)) as follows: starting with 40% A, changing to 60% A for 2 min, and then to 80% A for 10 min. Finally, the column was washed with a solution of 98% A. MS/MS analysis of the oxidized products was performed in scan mode using electrospray-negative ions. The source temperature of the MS was set at 150°C, with a cone gas flow of 24 l/h and a desolvation gas flow of 500 l/h. Peak spectra were monitored between 30 and 600 m/z. Collision-induced dissociation MS was performed, using collision energy of 25–30 eV and a capillary voltage of 3–3.5 kV. Multiple-reaction monitoring was performed under the same conditions. A calibration curve of LT was run in each analysis set.
Statistical analysis
Three one-tailed two-sample t tests were performed to test for differences in post/pretransportation ratio of Hsp60 and Hsp70A1A, followed by a Bonferroni correction to adjust all P values to control for group-wide type I error (Rice 1989). Thus, only P < 0.017 (0.05/3) was considered significant.
Repeated-measures analysis of variance was performed to test for differences in the expression pattern of MMP-9, l-selectin and β-glycan. Differences in mRNA abundance of these genes in relation to time after arrival were further tested by Bonferroni multiple comparison. Four one-tailed two-sample t tests were performed to test for differences in oxidation products of the LT marker among Kosher and non-Kosher individuals, followed by a Bonferroni correction to adjust all P values to control for group-wide type I error. Thus, only P < 0.013 (0.05/4) was considered significant.
We used Pearson correlation coefficient (r) to test the correlation between (1) pulmonary adhesions (Kosher vs. non-Kosher status) and MMP-9, l-selectin and β-glycan expression; (2) pulmonary adhesions and oxidative products of the LT marker. Discriminant analysis (DA, Wilks method) was performed to determine the accuracy of LT oxidation products (i.e. epoxide, hydroperoxide and 526.8) and β-glycan in distinguishing Kosher from non-Kosher animals. These four measured variables were included independently in DA to determine which of the variables best classified Kosher and non-Kosher animals; that is, maximizing the number of true positives and minimizing the number of false positives. In a preliminary analysis, we found that the best discriminant function was achieved 7 days after arrival. Therefore, we only present the results of this analysis. DA strictly requires that at least twice as many samples as variables are studied. In this time window, we had data of 11 animals for the four variables. Statistical analyses were performed using SPSS for Windows (version 17.0).
Results
Short-term effects of relocation—Hsp expression and circulating cortisol
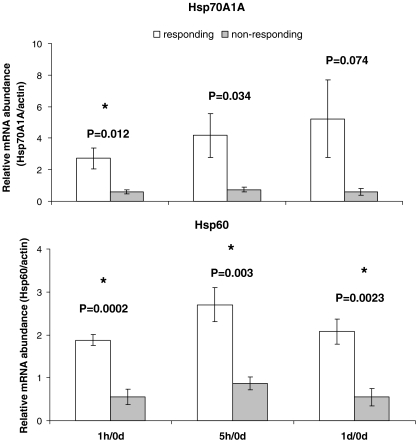
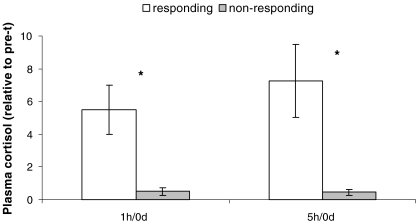
The relative abundance of Hsp70A1A and Hsp60 in white blood cells, normalized to β-actin, is shown in Fig. 2. As seen, differential responses to relocation were observed among young calves. To distinguish individuals that responded to relocation by elevating Hsp from nonresponding individuals, we calculated the average of the total post/pretransportation ratio of Hsp for each individual. Individuals with proportions of <1.5 were considered nonresponsive, while individuals having a post/pretransportation ratio >1.6 were considered responsive. While in 5 of the 12 calves examined, no change in the relative Hsp gene expression was detected after arrival, the relative levels of Hsp in the other 7 calves was elevated 1 h, 5 h and 1 day after arrival (Hsp70A1A: P = 0.012, P = 0.034, P = 0.074 for 1 h, 5 h and 1 day after arrival, respectively; Hsp60: P = 0.0002, P = 0.003, P = 0.0023 for 1 h, 5 h and 1 day after arrival, respectively). Due to statistical concerns, because of repeated two-tailed t tests, we performed a Bonferroni correction. Accordingly, the degree of significance was set to 1.7% instead of 5%. Under these stringent statistical conditions, higher relative abundance of Hsp70A1A was significant only 1 h after arrival. In contrast, the post/pretransportation ratio of Hsp60 was higher in the responsive individuals at all time points (Fig. 2). As for the Hsp, classification into responders (seven individuals) and nonresponder individuals (five individuals) was achieved also through the relative concentrations of circulating cortisol. Cortisol values were 0.91 ± 0.15, 4.99 ± 0.95 and 6.59 ± 1.23 ng/ml in the responders, pretransportation, 1 h and 5 h after arrival, respectively, and 0.82 ± 0.14, 0.42 ± 0.12 and 0.37 ± 0.12 ng/ml in nonresponders. Relative to pretransportation, these values were 11- and 16.6-fold (P < 0.01) higher in responding individuals, 1 h and 5 h after arrival, respectively (Fig. 3).
Fig. 2.
Variations in the relative mRNA abundance of Hsp70A1Aand Hsp60 (relative to pretransportation; 0d) in leukocytes of relocated Holstein–Friesian calves. Data are presented as means ± SEM. P values above bars indicate significant differences between individuals with elevated (responding) and non-elevated (nonresponding) Hsp levels. Asterisks denote significant differences after Bonferroni correction
Fig. 3.
Variations in the levels of circulating cortisol (relative to pretransportation; pre-t). Data are presented as means ± SD. Asterisks denote significant differences after Bonferroni correction (P < 0.01)
Long-term effects of relocation on neutrophil-related gene expression and oxidative stress
Among the 13 calves that were included in experiment 2, six were scored Kosher at the abattoir while seven were defined non-Kosher. These definitions were based on the existence of pulmonary adhesions, which are indicative of clinical and subclinical BRD episodes at young age (Fig. 1).
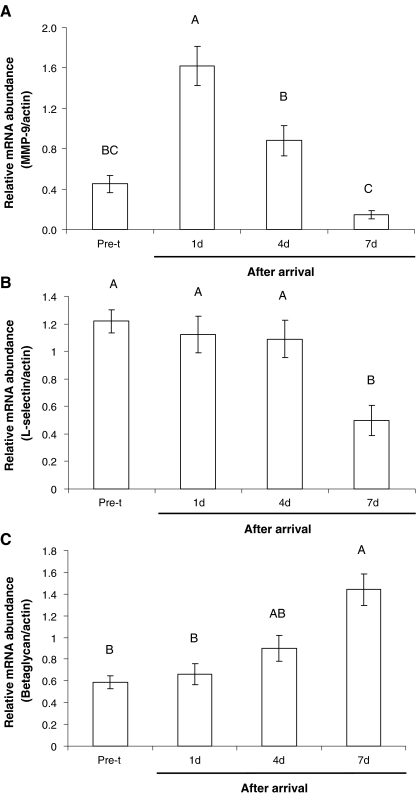
The relative mRNA abundance of MMP-9, l-selectin and β-glycan of all 13 calves is shown in Fig 4. As seen, MMP-9 increased significantly 1 day after arrival but returned to levels similar to those of pretransportation at 4 and 7 days after arrival (F3,24 = 22.32, P < 0.001). The relative mRNA abundance of l-selectin remained unchanged 1 and 4 days after arrival but decreased significantly thereafter (F3,30 = 29.75, P < 0.001). In marked contrast with the above-mentioned genes, the relative abundance of β-glycan mRNA continuously increased after arrival being significantly higher 7 days posttransportation (F3,30 = 13.09, P < 0.001). In spite of the significant changes in the levels of the three genes, only the increase of β-glycan 7 days after arrival differed between Kosher and non-Kosher individuals (t9 = 3.25, P = 0.01), being higher in the former calves.
Fig. 4.
The effect of relocation on the expression pattern of the neutrophil-related genes MMP-9 (a), l-selectin (b) and β-glycan (c) in young Holstein–Friesian calves. Data are presented as means ± SEM. Different letters above bars indicate significant differences between treatments (Bonferroni multiple comparison, P < 0.05). Pre-t pretransportation
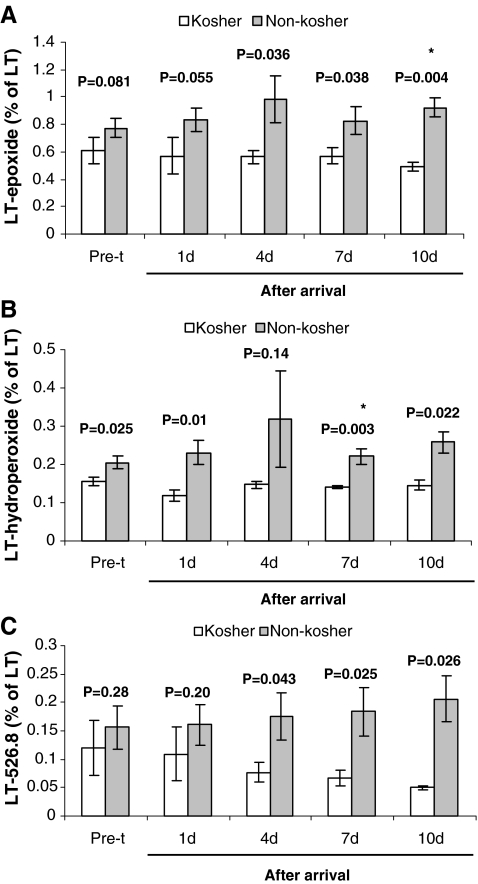
Three different types of oxidized LT products were detected by LC/MS/MS in our experimental system, pre- and posttransportation. These products, which abundance was referred to the initial LT concentration, were epoxidation of the linoleic acid subunit (LT-epoxide), an oxidation of the linoleic acid to hydroperoxide (LT-hydroperoxide) and an unidentified yet molecule with the m/z of 526.8 (LT-526.8). All three oxidized LT products were higher in the non-Kosher individuals pretransportation and after arrival, and with the exception of LT-526.8 pretransportation and 1 day after arrival, LT-epoxide pretransportation and 1 day after arrival and LT-hydroperoxide 4 days after arrival, were significantly elevated in non-Kosher individuals. However, under the stringent statistical conditions of Bonferroni correction, in which significance was set to 1.3% instead of 5%, significance was revealed only 10 days after arrival for LT-epoxide and 7 days after arrival for LT-hydroperoxide (Fig. 5). Interestingly, testing the percentage of LT-526.8 (from the initial LT) as a function of experimental day revealed a negative correlation for Kosher (r = −0.98 P = 0.004) and positive correlation for non-Kosher individuals (r = 0.99 P = 0.001) (Fig. 5c). This is in contrast to the other oxidized LT products, in which their proportion of the LT remained relatively constant in Kosher individuals pre- and posttransportation.
Fig. 5.
Capability of the oxidation products of LT marker, LT-epoxide (a), LT-hydroperoxide (b) and LT-526.8 (c) in young relocated Holstein–Friesian calves to predict pulmonary adhesions (non-Kosher) at slaughter at the age of 12 months. Data are presented as means ± SEM. P values above bars indicate significant differences between Kosher and non-Kosher individuals in the percentage of LT oxidation products, either pre- or posttransportation. Asterisks denote significant differences after Bonferroni correction. Pre-t pretransportation
Discriminant classification using four variables [the three LT oxidation products (epoxide, hydroperoxide and 526.8) and β-glycan)] correctly identified five (near 100%) of the Kosher animals and six (near 100%) of the non-Kosher animals (Wilks λ = 0.13; χ2 = 14.30; df = 4, P = 0.006). The canonical discriminate function was: Y = 37.696 × LT-hydroperoxide + 3.673 × LT-526.8 + 0.4 × LT-epoxide − 2.298 × β-glycan − 4.47. The DA function was strongly related to hydroperoxide (best predictor) than to LT-526.8 and epoxide, whereas the contribution of β-glycan was the lowest. The Kosher animals had a group centroid located in the negative region (−2.57), whereas in the non-Kosher animals, it is located in the positive region (2.14) on the discriminant function.
Discussion
Stress perturbs the animals' homeostasis by inducing changes in the activity of the SA and HPA axis. Consequently, the elevated secretion of catecholamines and glucocorticoids from the medulla and cortex of the adrenal gland, respectively, although being central to survival, also activates the stress-immune axis that in turn may promote disease susceptibility (Hickey et al., 2003). This is reflected in impairment of the immune function (Haddad et al., 2002; Reiche et al., 2004; Salak-Johnson and McGlone, 2007) and pathogenicity enhancement of resident or acquired microbes (Freestone et al., 2008).
It is generally recognized that stress is a major factor leading to development of BRD in cattle. BRD is an opportunistic disease in that the respiratory symptoms can be caused by many different infectious agents. Some of these infectious agents are normally present in the upper respiratory tract of healthy animals. They convert to pathogenic status when the animals are subjected to any of a wide variety of physical, biological and psychological stressors.
Reduced immune response allows primary viral infection (Reiche et al, 2004), and this additional stress together with increased microbial pathogenicity opens the way to secondary bacterial infection (Pasteurella haemolytica, Pasteurella multocida, Haemophilus somnus and Mycoplasma pneumoniae) and to presentation of severe BRD symptoms.
In light of the above, we suggest that the key to reducing BRD appears to be centered at reducing the response to stress. This suggestion may further be supported by the following observations: (i) not all similarly stressed individuals are equally likely to develop disease (Cobb and Steptoe 1996); (ii) dominant animals may have enhanced immune activation, whereas in subordinates, the same immune component in response to the same stressor may be suppressed. This could explain why individual animals within a group respond differently to stressors and to disease challenges (Salak-Johnson and McGlone, 2007). (iii) Exposure to viral agents that cause upper respiratory disease-induced clinical disease in some individuals but not in others. Moreover, the severity of the clinical symptoms among those who develop illness can vary substantially (Aich et al., 2007).
In the current study, we examined whether young Holstein–Friesian calves would differentially respond to relocation by means of increased relative mRNA abundance of Hsp60 and Hsp70A1A. These genes were chosen because it is now well established that when secreted from the cells, they serve as danger signals to the innate immune system (Chen et al., 1999; Asea et al., 2002; Williams and Ireland, 2008). The findings shown herein demonstrate that young calves differ in their short-term responses to relocation; while in some individuals, there is increased relative mRNA abundance of intracellular Hsp60, and to a lesser extent of Hsp70A1A, 1 h, 5 h and 1 day after arrival, in other individuals these responses were barely detectable. Distinction between responders and nonresponders was revealed also according to the analysis of circulating cortisol, a widely acceptable indicator of stress response.
Altered expression of Hsp has been extensively documented in association with a broad variety of diseases including fever, inflammation, infection and tissue trauma (Morimoto and Santoro, 1998). Concerning the proven cytoprotective role of intracellular Hsp, a central question to be raised is whether the expression of Hsp in each of these pathologies reflects adaptation to the pathophysiological state in question or indicates a nonoptimal adjustment of the cellular environment. A more specific definition of the question, with regard to relocation and development of BRD, would be whether responding calves, in terms of elevated expression of intracellular Hsp, might be considered resistant or susceptible to BRD. A similar peradventure comes from studies in transported piglets, which are susceptible to acute heart failure and sudden death syndrome. Bao et al. (2008) reported an attenuation of four cardiac Hsp members, in response to 6 h road transportation. In accordance with this study, Zhu et al. (2009) found more than 60% reduction in cardiac Hsp60 of piglets, following 2 h transportation. Most interestingly, the authors report a tissue-specific Hsp response; in addition to the decrease in cardiac Hsp, a significant increase and nonchanged levels of Hsp60 were detected for the stomach, liver and kidney, respectively.
To add more value to the unrevealed issue of the relation between Hsp induction and susceptibility/resistance to BRD, the immunomodulatory role of Hsp should also be taken into account. The intracellular Hsp machinery confers cellular protection either through the role of Hsp as cellular chaperones (Lindquist and Craig, 1988) or by down-regulating transcription of proinflammatory cytokines (Morimoto and Santoro, 1998). Yet, during the last decade, the area of extracellular Hsp has been evolving. Whereas the usual view is that Hsp are released from necrotic cells in a nonphysiological controlled process, eliciting innate and adaptive proinflammatory responses (Basu et al., 2000), evidence has emerged that Hsp can be released from viable cells into the extracellular environment under physiological conditions and regulate proinflammatory disease processes (Pockley 2003). In the context of the current study, it is yet to be determined whether the changes in intracellular Hsp reflect changes of extracellular Hsp in young relocated calves. Two scenarios are likely to occur with the two types of calves, Hsp responsive and nonresponsive, described herein: (i) in Hsp responsive individuals, extracellular Hsp would potentially act as a danger signal, thereby targeting enhanced immune response that might cause lung injuries and adhesions and trigger reduced productivity, but be beneficial when pathogens are present; (ii) non-Hsp-responsive calves may escape lung injuries and adhesions caused by the proinflammatory activity but be prone to develop BRD dependent on presence of pathogens. In this case the phenotype at the abattoir may be Glatt-Kosher. To gain more insight into the expected scenario, the effect of relocation on extracellular Hsp concentrations, the consequent proinflammatory response and the BRD episodes and pulmonary damage (adhesions) should be linked together.
Early detection of subclinical signs prior to the development of BRD is a crucial step in the preventive management of BRD, as it might enable the adoption of better animal husbandry techniques and lead to significant improvements in animal health and productivity. Young relocated Holstein–Friesian calves are characterized by undeveloped adaptive immune system, and thus rely mostly on their innate immunity to defend from pathogenic challenges. Neutrophils, crucial phagocytes for the acute defense of the lungs, respond to transportation stress by altering their numbers, migration capacity and function (Murata et al., 1987; Yagi et al., 2004). A large bulk of studies demonstrated that exogenously administrated or endogenously secreted glucocorticoids modulate the expression of neutrophil genes that regulate apoptosis, adhesion and inflammation (Chang et al., 2004; Burton et al., 2005; Weber et al., 2001, 2006). In a recent study, Buckham Sporer et al. (2007) showed that changes in concentrations of plasma cortisol might mediate the effect of transportation stress on altered expression of neutrophils. Using 9-month-old Belgian Blue × Friesian bull calves, the authors tested the effect of 9 h road transportation on the expression of candidate genes known to participate in neutrophil-mediated defense and inflammation in the lung. Given that neutrophil genes, important for the regulation of apoptosis, tissue remodeling, margination and antibacterial functions, were affected by transportation, the authors concluded that the major effect was on proinflammatory and antibacterial activities.
In the current study, we followed the effect of relocation stress on the relative mRNA abundance of MMP-9, l-selectin and β-glycan, genes that are relevant to the inflammatory activity of neutrophils, 1, 4 and 7 days after arrival. Our hope was to reveal correlation between the changes in the levels of these genes, after arrival, at an early age, and the existence of lung adhesions at slaughter. Such correlation would help in early prediction of vulnerable individuals, prone to develop BRD. The general trend was a decrease of the proinflammatory genes, MMP-9 and l-selectin, and an increase of β-glycan, 7 days after arrival. MMP-9 is a protease known to increase proinflammatory activity of neutrophils by degrading tissue extracellular matrix, thus assisting them in migrating from the blood to the infected site. MMP-9 has been implicated in acute lung injuries (Fligiel et al., 2006).
Of all three mentioned genes, β-glycan was exclusively found in the current study to be correlated with improved Kosher state at slaughter; we show herein that calves with elevated mRNA abundance of β-glycan 7 days after arrival are characterized by adhesion-free lungs, suggesting beneficial health properties for this gene in young transported calves. β-glycan is a receptor for the potent anti-inflammatory cytokine transforming growth factor β (TGF-β), which plays a pivotal role in the maintenance of immune cell homeostasis. Its anti-inflammatory activity is well exemplified in TGF-β1 newborn null mice that die shortly after weaning as a result of multifocal, inflammatory disease with lymphocyte infiltration into multiple organs (Kulkarni et al., 1993; Shull et al., 1992). TGF-β modulates the proliferation, differentiation and function of all classes of lymphocytes, macrophages, and dendritic cells, thus regulating the innate, non-antigen-specific as well as the antigen-specific immunity (Letterio and Roberts, 1998). Interestingly, it was shown that TGF-β1 suppresses MMP-9 transcription by a region of the MMP-9 promoter containing the nuclear factor-κB site (Ogawa et al., 2004). The inhibitory role of TGF-β1 on MMP-9 was further demonstrated in human monocytes, at the protein level (Nguyen et al., 2005). It is thus tempting to assume that the elevated relative abundance of β-glycan mRNA in young relocated calves may account for the decreased levels of MMP-9, leading to diminished formation of pulmonary adhesions. Indeed, a significant, fourfold increase in MMP-9 was observed 1 day after arrival, while β-glycan mRNA abundance still remained unchanged, but decreased below initial levels upon a significant increase in β-glycan mRNA, 7 days after arrival. Thus, it seems that the β-glycan/MMP-9 ratio among individuals may in part affect the fate of pulmonary adhesion formation.
An important aspect is whether and how Hsp are involved in this scenario. Several circumstantial evidence may link the elevated Hsp expression in some of the relocated calves with lung adhesions observed at slaughter. (i) Elevated expression of intracellular Hsp may reflect an increased release of extracellular Hsp (Campisi et al., 2003). (ii) Toll-like receptor 2 (TLR2) and TLR4 act as cell surface receptors for extracellular Hsp and LPS, and transduce a proinflammatory signal to innate immune cells including neutrophils (Johnson and Fleshner, 2006). (iii) Bovine neutrophils, in addition to their phagocytic and bactericidal properties, play a supportive role in the innate immune response through their ability to produce immunoregulating cytokines when stimulated by LPS (Sohn et al., 2007). (iv) Extracellular Hsp90α assists in the activation of MMP-2 (Eustace and Jay, 2004).
Oxidative stress has been implicated in the pathogenesis of several ruminant diseases including BRD (Miller and Brzezinska-Slebodzinska, 1993; Chirase et al., 2004).
ROS/RNS are products of normal metabolic pathways and are formed during the destruction of invading organisms. An excess of ROS/RNS is associated with changes in the structure and function of biomolecules such as DNA, proteins and lipids (Szuchman et al., 2008). The documented relation between transportation, oxidative stress and BRD makes the identification of imbalances in the oxido/redox system for the prediction of pathological conditions early on in their development very relevant.
In the current study, we used the LT marker to predict such conditions in young relocated calves. Exposure of LT marker to blood samples of calves pretransportation and at various periods after arrival resulted in the formation of three oxidized LT products—LT-epoxide, LT-hydroperoxide and LT-526.8. At least two out of the three LT oxidized products observed are related to linoleic acid oxidation, the epoxide and the hydroperoxide, implying that cell membranes are readily oxidized in young calves, in response to relocation stress. Of all the three products, LT-epoxide appeared at the highest proportions. However, as judged by the discriminant classification, its contribution to the prediction of pulmonary adhesions, 7 days after arrival, was the lowest.
It is noteworthy that adhesion-free calves were characterized by lower percentage of each of the LT-products pre- and posttransportation. This means that individuals prone to develop BRD/pulmonary adhesions may be classified as such early at life and be handled/treated in an appropriate manner.
Summary
Relocation stress is one of the key events in the life history of young calves with respect to the pathogenesis of BRD. However, subclinical signs of BRD may often go undetected but deleteriously affect welfare and productivity of feedlot calves. This is often demonstrated at slaughter, where calves without apparent BRD episodes during growth happen to have pulmonary lesions and adhesions. The overall hypothesis of the current study is that the response to relocation varies among young calves, and the key to reducing subclinical and clinical BRD appears to be centered at reducing the response to stress. The data presented herein show that relocation stress responding vs. nonresponding individuals can be classified according to the pattern of their Hsp response. If this pattern would reflect also changes in extracellular Hsp, it might well explain the enigma of pulmonary damage without apparent BRD episodes.
We present a discriminant analysis model, based on LT oxidative products and β-glycan, according to which vulnerable individuals may be predicted at near 100% probability, 7 days after arrival. However, a large-scale experiment should be carried out to confirm this model.
Acknowledgements
This research was supported by funds from the Israeli Milk Marketing Board and Northern R&D. Contribution No. 555/09 from the ARO, the Volcani Center, Bet Dagan, Israel.
References
- Aich P, Jalal S, Czuba C, Schatte G, Herzog K, Olson DJH, Ross ARS, Potter AA, Babiuk LA, Griebel P. Comparative approaches to the investigation of responses to stress and viral infection in cattle. OMICS. 2007;11(4):413–434. doi: 10.1089/omi.2007.0023. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008;13:459–466. doi: 10.1007/s12192-008-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Blecha F, Boyles SL, Riley JG. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman Angus feeder calves. J Anim Sci. 1984;59(3):576–583. doi: 10.2527/jas1984.593576x. [DOI] [PubMed] [Google Scholar]
- Brosh A, Lidski I, Nitsan Z. The effect of the fat level in milk replacer on its digestibility and performance of suckling calves. Meshek Habakar VeHahalav Heker Uma'as. 1992;14:25–31. [Google Scholar]
- Buckham Sporer KR, Burton JL, Earley B, Crowe MA. Transportation stress in young bulls alters expression of neutrophil genes important for the regulation of apoptosis, tissue remodeling, margination, and anti-bacterial function. Vet Immun Immunopath. 2007;118:19–29. doi: 10.1016/j.vetimm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Burton JL, Madsen SA, Chang LC, Weber PSD, Buckham KR, Dorp R, Hickey MC, Earley B. Gene expression signatures in neutrophils exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet Immun Immunopath. 2005;105:197–219. doi: 10.1016/j.vetimm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Madsen SA, Toelboell T, Weber PSD, Burton JL. Effects of glucocorticoids on Fas gene expression in bovine blood neutrophils. J Endocrinol. 2004;183:569–583. doi: 10.1677/joe.1.05822. [DOI] [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat shock protein: a danger signal to the innate immune system. J Immunol. 1999;162(6):3212–3219. [PubMed] [Google Scholar]
- Chirase NK, Greene LW, Purdy CW, Loan RW, Briggs RE, McDowell LR.Effect of environmental stressors on ADG, serum retinol and α-tocopherol concentrations, and incidence of bovine respiratory disease of feeder steers (abstr) J Anim Sci 200179suppl 118811204699 [Google Scholar]
- Chirase NK, Greene LW, Purdy CW, Loan RW, Auvermann BW, Parker DB, Walborg EF, Jr, Stevenson DE, Xu Y, Klaunig JE. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am J Vet Res. 2004;65:860–864. doi: 10.2460/ajvr.2004.65.860. [DOI] [PubMed] [Google Scholar]
- Cobb JM, Steptoe A. Psychosocial stress and susceptibility to upper respiratory tract illness in an adult population sample. Psychosom Med. 1996;58:404–412. doi: 10.1097/00006842-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Craig EA, Lindquist S. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Eustace BK, Jay DG. Extracellular roles for the molecular chaperone Hsp90. Cell Cycle. 2004;3:1098–1100. [PubMed] [Google Scholar]
- Fligiel SEG, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. 2006;37:422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Freestone PPE, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Gardner BA, Dolezal HG, Bryant LK, Owens FN, Smith RA. Health of finishing steers: effects on performance, carcass traits and meat tenderness. J Anim Sci. 1999;77:3168–3175. doi: 10.2527/1999.77123168x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Earley B, Crowe MA. Effect of 12-h road transportation on physiological, immunological, and haematological parameters in bulls housed at different space allowances. Vet J. 2007;173:605–616. doi: 10.1016/j.tvjl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuron-immune–endocrine interactions: a role for the hypothalamic–pituitary–adrenal revolving axis. J Neuroimmunol. 2002;133(1–2):1–19. doi: 10.1016/S0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hickey MC, Drennan M, Earley B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 2003;81:2847–2855. doi: 10.2527/2003.81112847x. [DOI] [PubMed] [Google Scholar]
- Hodgson PD, Aich P, Manuja A, Hokamp K, Roche FM, Brinkman FSL, Potter A, Babiuk LA, Griebel PJ. Effect of stress on viral–bacterial synergy in bovine respiratory disease: novel mechanisms to regulate inflammation. Comp Funct Genom. 2005;6:244–250. doi: 10.1002/cfg.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DP, Cole NA. Vitamin E and selenium for yearling feedlot cattle (abstr) Fed Proc. 1985;44:549. [Google Scholar]
- Inanami O, Shiga A, Okada K, Sato R, Miyake Y, Kuwabara M. Lipid peroxides and antioxidants in serum of neonatal calves. Am J Vet Res. 1999;60:452–457. [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukok Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekeux P, Hajer R, Breukink HJ. Effect of somatic growth on pulmonary function values in healthy Friesian cattle. Am J Vet Res. 1984;45:2003–2007. [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGFβ. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Miller JK, Brzezinska-Slebodzinska E. Oxidative stress, antioxidants and animal function. J Dairy Sci. 1993;76:2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- Minton JE. Function of the hypothalamus–pituitary–adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J Anim Sci. 1994;72:1891–1898. doi: 10.2527/1994.7271891x. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Murata H, Takahashi H, Matsumoto H. The effects of road transportation on peripheral blood lymphocyte subpopulations, lymphocyte blastogenesis and neutrophil function in calves. Br Vet J. 1987;143:166–174. doi: 10.1016/0007-1935(87)90008-X. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Knapnougel P, Lesavre P, Bauvois B. Inhibition of matrix metalloproteinase-9 by interferons and TGF-β1 through distinct signalings accounts for reduced monocyte invasiveness. FEBS Lett. 2005;579:5487–5493. doi: 10.1016/j.febslet.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor beta is mediated by a nuclear factor-kappaB site. Biochem J. 2004;381:413–422. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Preisler MT, Weber PSD, Tempelman RJ, Erskine RJ, Hunt H, Burton JL. Glucocorticoid receptor down-regulation in neutrophils of periparturient cows. Am J Vet Res. 2000;61:14–19. doi: 10.2460/ajvr.2000.61.14. [DOI] [PubMed] [Google Scholar]
- Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats and horses. 9. London: W.B. Saunders; 2000. [Google Scholar]
- Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.2307/2409177. [DOI] [PubMed] [Google Scholar]
- Salak-Johnson JL, McGlone JJ. Making sense of apparently conflicting data: stress and immunity in swine and cattle. J Anim Sci. 2007;85(E. Suppl):E81–E88. doi: 10.2527/jas.2006-538. [DOI] [PubMed] [Google Scholar]
- Shull MM, Kier OI, AB PS, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowder GD, Vleck LD, Cundiff LV, Bennett GL, Koohmaraie M, Dikeman ME. Bovine respiratory disease in feedlot cattle: phenotypic, environmental, and genetic correlations with growth, carcass, and longissimus palatability traits. J Anim Sci. 2007;85:1885–1892. doi: 10.2527/jas.2007-0008. [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Paape MJ, Connor EE, Bannerman DD, Fetterer RH, Peters RR. Bacterial lipopolysaccharide stimulates bovine neutrophil production of TNF-α, IL-1β, IL-12 and INF-γ. Vet Res. 2007;38:809–818. doi: 10.1051/vetres:2007033. [DOI] [PubMed] [Google Scholar]
- Szuchman A, Aviram M, Soliman K, Tamir S, Vaya J. Exogenous N-linoleoyl tyrosine marker as a tool for the characterization of cellular oxidative stress in macrophages. Free Radic Res. 2006;40(1):41–52. doi: 10.1080/10715760500358787. [DOI] [PubMed] [Google Scholar]
- Szuchman A, Aviram M, Ramadan M, Soliman K, Vaya J. Characterization of oxidative stress in blood from diabetic vs. hypercholesterolaemic patients, using a novel synthesized marker. Biomarkers. 2008;13:119–131. doi: 10.1080/13547500701614556. [DOI] [PubMed] [Google Scholar]
- Thompson PN, Stone A, Schultheiss WA. Use of treatment records and lung lesion scoring to estimate the effect of respiratory disease on growth during early and late finishing periods in South African feedlot cattle. J Anim Sci. 2006;84:488–498. doi: 10.2527/jas.2005-774. [DOI] [PubMed] [Google Scholar]
- Weber PSD, Madsen SA, Smith GW, Ireland JJ, Burton JL. Pre-translational regulation of neutrophila l-selectin in glucocorticoid-challenged cattle. Vet Immunol Immunopathol. 2001;83:213–240. doi: 10.1016/S0165-2427(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Weber PSD, Toelboell T, Chang L-C, Tirrell JD, Saama PM, Smith GW, Burton JL. Mechanisms of glucocorticoid-induced down-regulation of neutrophil l-selectin in cattle: evidence for effects at the gene-expression level and primarily on blood neutrophils. J Leukoc Biol. 2004;75:815–827. doi: 10.1189/jlb.1003505. [DOI] [PubMed] [Google Scholar]
- Weber PSD, Madsen-Bouterse SA, Rosa GJM, Sipkovsky S, Ren X, Almeida PE, Kruska R, Halgren RG, Barrick JL, Burton JL. Analysis of the bovine neutrophil transcriptome during glucocorticoid treatment. Physiol Genom. 2006;28(1):97–112. doi: 10.1152/physiolgenomics.00094.2006. [DOI] [PubMed] [Google Scholar]
- Welch WJ. The mammalian stress response: cell physiology and biochemistry of stress proteins. In: Morimoto RI, Tissieres A, Georgopolous C, editors. Stress proteins in biology and medicine. New York: Cold Spring Harbor; 1990. pp. 223–278. [Google Scholar]
- Williams JHH, Ireland E. Sensing danger—Hsp72 and HMGB1 as candidate signals. J Leukoc Biol. 2008;83:489–492. doi: 10.1189/jlb.0607356. [DOI] [PubMed] [Google Scholar]
- Wittum TE, Woolen NE, Perino LJ, Littledike ET. Relationship among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. JAVMA. 1996;209:814–818. [PubMed] [Google Scholar]
- Yagi Y, Shiono H, Chikayama Y, Ohnuma A, Nakamura I, Yayou K-I. Transportation stress increases somatic cell counts in milk, and enhances the migration capacity of peripheral blood neutrophils of dairy cows. Clin Pathol. 2004;66(4):381–387. doi: 10.1292/jvms.66.381. [DOI] [PubMed] [Google Scholar]
- Yates WDG. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral–bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982;46:225–263. [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bao E, Zhao R, Hartung J. Expression of heat shock protein 60 in the tissues of transported piglets. Cell Stress Chaperones. 2009;14:61–69. doi: 10.1007/s12192-008-0055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]