Abstract
K562 cells and peripheral blood mononuclear cells were treated with hydrogen peroxide (H2O2) to determine the expression of Krüppel-like factor (KLF) 4. A full-length complementary DNA or an anti-sense oligonucleotide of KLF4 was transfected into cells, and expressions of B-cell lymphoma/leukemia-2 (bcl-2) and bcl-2-associated X (bax) proteins were analyzed. The results showed that H2O2 treatment of cells resulted in an increase in KLF4 levels; KLF4 induced apoptosis and slowed cell growth, potentially resulting from up-regulation of bax and down-regulation of bcl-2. Transcriptional activities on bcl-2 and bax were promoted following KLF4 overexpression potentially through KLF4 binding sites on corresponding promoters. All results indicate that KLF4 induces apoptosis in leukemia cells involving the bcl-2/bax pathway during H2O2 stimulation, suggesting a potential mechanism for research on drug-induced apoptosis.
Keywords: Krüppel-like factor 4, Leukemia, Gene expression, Apoptosis, bcl-2, bax
Introduction
Oxidative stress is caused by an imbalance between the production of reactive oxygen and the ability of a biological system to detoxify reactive intermediates or to repair the resulting damage. In humans, oxidative stress is involved in many diseases such as atherosclerosis, Parkinson’s disease, heart failure, myocardial infarction, and Alzheimer’s disease. Severe oxidative stress can cause cell death, and even moderate oxidation can trigger apoptosis, while more intense stresses may cause necrosis (Lennon et al. 1991). Reactive oxygen species have been demonstrated to participate in drug-induced apoptosis (Davis et al. 2001).
Leukemia cell reduction through anti-leukemic therapy may be due to inhibition of proliferation or direct induction of cell death. In vitro studies with tumor and leukemia cell lines have shown that cytotoxic drugs used in anti-cancer chemotherapy induce cell death by activating diverse apoptosis signaling pathways. Several studies have correlated constitutive apoptosis gene expression of caspases (Campos et al. 1999), mitochondria-related molecules (Hogarth and Hall 1999), and death receptors (Iijima et al. 1997) to treatment outcomes in order to identify apoptosis-signaling molecules relevant to leukemia therapy.
Krüppel-like factors (KLFs) are zinc finger transcription factors that exhibit homology to Krüppel from Drosophila (Ghaleb et al. 2005). Previous studies have demonstrated that KLF proteins typically regulate critical aspects of cellular differentiation and tissue development. Among KLF family members, KLF6 and KLF10 have been shown to be involved in apoptosis in human lung cancer cells (Ito et al. 2004) and pancreatic epithelial cells (Kim et al. 1998), respectively. Moreover, apoptosis has also been implicated in bladder cancer cells by KLF4 (Ohnishi et al. 2003). KLF4 (gut KLF) was first identified in the epithelial lining of the gut and skin, and subsequent studies have shown that KLF4 plays a role in the regulation of cellular growth and differentiation in these tissues (Vaporciyan et al. 1993). In previous studies, we demonstrated that KLF4 induces apoptosis in murine RAW264.7 macrophages and C2C12 cells (Liu et al. 2007a, b, 2008a, b).
K562 cells were the first human immortalized myelogenous leukemia line to be established. They are a BCR-ABL-positive erythroleukemia line derived from a 53-year-old female chronic myeloid leukemia (CML) patient on blast crisis (Lozzio and Lozzio 1975). Investigation into the effect of KLF4 on the apoptotic process of K562 cells after induction by drugs related to oxidative stress may lead to valuable discoveries. In this work, we applied hydrogen peroxide (H2O2) to induce apoptosis and postulated that KLF4 plays an important role in the apoptosis of K562 leukemia cells and peripheral blood mononuclear cells (PBMCs) of CML patients. Our results may explain the mechanism of apoptosis induced by oxidative stress during drug application in CML.
Materials and methods
Cell culture
Human K562 leukemia cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences and routinely grown in RPMI-1640 medium (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum at 37°C and 5% CO2. Human PBMCs were isolated from the donor blood of CML patients by Ficoll density gradient centrifugation and cultured in RPMI-1640 medium with 10% heat-inactivated human serum and 2 mM glutamine overnight. Nonadherent cells were subsequently removed, and adherent monocyte-enriched cultures were stimulated for further treatment. Informed consent was obtained from patients or from close relatives of the patients.
Generation of constructs
Oligonucleotide primers were designed to amplify the coding sequence of mouse KLF4 complementary DNA (cDNA), yielding a 1.5-kb product. The oligonucleotide primers are as follows: 5′-AGT TGG ACC CAG TAT ACA TTC CGC CAC AGC AGC CT-3′ (forward) and 5′-TTA AAA GTG CCT CTT CAT GTG TAA GGC AAG GTG GT-3′ (reverse). The polymerase chain reaction (PCR) product was electrophoresed onto 0.9% agarose, and a 1.5-kb fragment was purified with Gel Extraction Kit (Qiagen, Hilden, Germany). The fragment was then inserted into the pcDNA3.1 vector (Strategene, Cedar Creek, TX, USA) and sequenced commercially (Invitrogen, Carlsbad, CA, USA). Meanwhile, full-length mouse KLF2 cDNA was also generated by PCR and inserted into the pcDNA3.1 vector for plasmid construction, as described previously (Anderson et al. 1995; Conkright et al. 2001).
Lipofectamine-mediated gene transfection
Transfection of cells was carried out in accordance with the manufacturer’s instructions (Lipofectamine 2000™; Invitrogen) (Liu et al. 2007a, b). The cells were transfected separately with 10 μg of pcDNA3.1-KLF4/20 μl of Lipofectamine (experimental group) and 10 μg of pcDNA3.1/20 μl of Lipofectamine (vector control), followed by incubation with serum-free and antibiotics-free RPMI-1640 medium at 37°C in a CO2 incubator for 6 h. The medium was then replaced with fresh RPMI-1640 culture medium containing 10% fetal bovine serum. After the cells had been cultured for 18 h, they were treated in accordance with the experimental protocol.
Loss-of-function assay using morpholino oligos
A KLF4 morpholino anti-sense oligonucleotide (Liu et al. 2003a, b) was designed to target the initiation site for KLF4 translation (AS; agactcgccaggtggctgcctcatt) and was synthesized commercially (Invitrogen). Morpholinos were transfected into K562 cells with Lipofectamine in accordance with the manufacturer’s instructions (Lipofectamine 2000™; Invitrogen) 24 h after plating. The specificity of the anti-sense oligo was validated by employing an invalid oligo (Inv; ttactccgtcggtggaccgctcaga) and a group only treated with Lipofectamine (Ctrl). Cell samples were collected 48 h after transfection with either Trizol® reagent (Invitrogen) for real-time PCR or lysis buffer [62.5 mM Tris–HCl (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerin, and 50 mM dithiothreitol] for Western blot analysis.
Cell Counting Kit-8 for cell proliferation assay
Cells were inoculated in a 96-well plate containing known numbers of viable cells (1 × 104 cells/well), followed by incubation at 37°C in a CO2 incubator for 24, 48, and 72 h. Adding 10 μl of the prepared Cell Counting Kit-8 (CCK-8; Wako, Osaka, Japan) solution into each well of the plate, we incubated the cells for another 4 h. Absorbance was measured at 450 nm by a microplate reader (Perkin-Elmer, Waltham, MA, USA).
Flow cytometry
All groups of cells were cultured into confluence, collected for washing twice in phosphate-buffered saline, and then fixed in 70% ethanol overnight at 4°C. Cell samples were stained with propidium iodide (50 mg/ml; Sigma, St. Louis, MO, USA). Sub-G1 peak was measured by FACScan Flow Cytometry (Becton Dickinson, Franklin Lakes, NJ, USA) and analyzed by Cell Quest software.
Caspase activity assay
Caspase fluorescent assay kits specific for caspase-3 (Biovision, Mountain View, CA, USA) were used to detect caspase activation by measuring the cleavage of a synthetic fluorescent substrate. In brief, cells were cultured in 60-mm dishes and treated with 0.5 mM H2O2 for the indicated periods. Cell lysates were prepared with the lysis buffer provided by the assay kit and centrifuged at 10,000×g for 1 min, and the supernatants were collected. With bovine serum albumin as standard for protein content, equal amounts of protein were reacted with the synthetic fluorescent substrates at 37°C for 1.5 h, and absorbance at 405 nm was read on a microplate reader. The fold increase in caspase-3 activity versus control was determined.
RNA extraction and real-time PCR
Total RNA was isolated using Trizol® reagent (Invitrogen) in accordance with the manufacturer’s protocol. After extraction, 5 µg of total RNA was then used as template to synthesize cDNA using a First Strand Synthesis Kit (Invitrogen). The cDNA from this synthesis was then used in quantitative real-time PCR analysis with the TaqMan system (ABI-Prism 7700 Sequence Detection System; Applied Biosystems, Foster City, CA, USA) using SYBR Green dye. The following primer pairs of human origin were used (Cheok et al. 2003; Lin et al. 2006; Ai et al. 2007): KLF4, 5′-CAA GTC CCG CCG CTC CAT TAC CAA-3′ (forward) and 5′-CCA CAG CCG TCC CAG TCA CAG TGG-3′ (reverse); bcl-2, 5′-AGT ACC TGA ACC GGC ATC TG-3′ (forward) and 5′-GCT GAG CAG GGT CTT CAG AG-3′ (reverse); bax protein, 5′-TGG AGC TGC AGA GGA TGA TTG-3′ (forward) and 5′-GAA GTT GCC GTC AGA AAA CAT G-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GAC ATC AAG AAG GTG GTG AAG C-3′ (forward) and 5′-GTC CAC CAC CCT GTT GCT GTA G-3′ (reverse).
Western blot analysis
After various treatments, proteins in whole-cell lysate were resolved on 10% SDS-PAGE and then transferred onto PVDF membranes (Schleicher and Schuell, Dassel, Germany). Membranes were blocked overnight in phosphate-buffered saline containing 10% nonfat dry milk and 0.5% Tween-20, and incubated with primary antibodies for 2 h. Horseradish-peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as secondary antibody. Immunoreactive bands were visualized using DAB (Boster Biological Technology, Wuhan, Hubei, China). GAPDH antibody was used to normalize for equal amounts of proteins and to calculate the relative induction ratio. The following antibodies were used: mouse bcl-2 monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse bax monoclonal antibody (1:1,000; Santa Cruz Biotechnology); rabbit KLF4 polyclonal antibody (1:1,000; Santa Cruz Biotechnology); rabbit cleaved caspase-3 monoclonal antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA); rabbit cleaved poly ADP-ribose polymerase (PARP) monoclonal antibody (1:1,000; Cell Signaling Technology); mouse GAPDH monoclonal antibody (1:1,000; Sigma); and horseradish-peroxidase-conjugated anti-mouse and anti-rabbit IgG (1:1,000; Boster Biological Technology).
Luciferase reporter gene assay
The assay was performed in accordance with the instructions of the Dual Luciferase Reporter System (Promega, Madison, WI, USA). Generation of the homo bcl-2 promoter construct (−500 to +10) was performed by PCR using human genomic DNA as template and cloned into pGL3-Basic, and authenticity was verified by sequencing (data not shown) and by the homo bax promoter. Moreover, the mutant promoter construct with point mutations (T to G at positions −173 and −169 for pGL3-mutbcl-2; A to C at positions −27 and −19 for pGL3-mutbax) was also determined using the pGL3-bcl-2 construct or the pGL3-bax construct as template for overlap extension PCR. For the luciferase reporter assay, exponentially growing cells were seeded on 24-well culture dishes. Transfections were performed as described previously. All transfections from at least three independent experiments were performed in triplicate. Each transfection experiment contained 500 ng of the pGL3-bcl-2 promoter reporter construct or the pGL3-mutbcl-2 promoter construct, with 500 ng of the pcDNA3.1-KLF4 vector or 500 ng of the pcDNA3.1-KLF2 vector as specificity control, and with 20 ng of pRL-null vector (Promega) as internal transfection control, while a similar transfection of the pGL3-bax promoter construct or the pGL3-mutbax construct was also performed.
Statistical analysis
Each experiment was performed three times, and the data were expressed as mean±SEM, or representative data were shown. Statistical analysis was performed using two-tailed Student’s t test. P < 0.05 was considered significant.
Results
H2O2-induced expression of KLF4 in apoptotic K562 cells and PBMCs
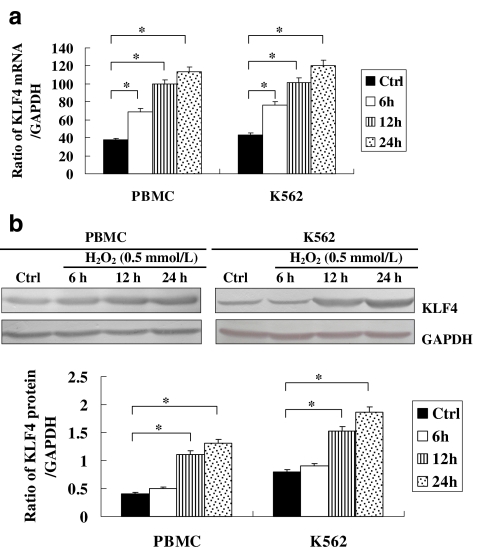
In this report, we first determined KLF4 expression in K562 cells and PBMCs treated with H2O2 (0.5 mmol/l) for various periods of time. The dose of H2O2 (0.5 mmol/l) was the demonstrated apoptotic dose previously used on K562 cells in our laboratory (Liu et al. 2003a, b). As shown in Fig. 1, H2O2 treatment on K562 cells and PBMCs led to a sustainable increase in messenger RNA (mRNA) levels of KLF4 from 6 to 24 h (Fig. 1a) and in protein levels from 12 to 24 h (Fig. 1b); KLF4 levels increased significantly in response to H2O2 stimulation. The increased levels of KLF4 suggested a potential role in the apoptosis of cells induced by H2O2.
Fig. 1.
a Expression of KLF4 mRNA in apoptotic K562 cells and PBMCs induced by H2O2. K562 cells and PBMCs were stimulated with H2O2 at indicated doses for varying periods of time. mRNA levels of KLF4 at varying time periods were determined by real-time PCR. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. b Expression of KLF4 protein in apoptotic K562 cells and PBMCs induced by H2O2. K562 cells and PBMCs were stimulated with H2O2 at indicated doses for varying periods of time. Protein levels of KLF4 at varying time periods were determined by Western blot analysis. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05
Apoptosis of K562 cells and PBMCs overexpressing KLF4
We overexpressed KLF4 in K562 cells and PBMCs by transfecting with the pcDNA3.1-KLF4 construct, with the null pcDNA3.1 as control. The overexpression of KLF4 is shown in Fig. 2a. Cell proliferation assay results showed that the overexpression of KLF4 delayed the growth of K562 cells and PBMCs (Fig. 2b), while the apoptosis rates of KLF4-overexpressed K562 cells and PBMCs were increased versus those of the null control group cells (Fig. 2c), in the presence or in the absence of H2O2 treatment. Furthermore, KLF4 overexpression resulted in activation of caspase-3 in H2O2-induced K562 cells and PBMCs, as evidenced by increased fluorescence in the caspase assay (Fig. 2d) and increased levels of cleaved caspase-3 and PARP upon cleavage of caspase-3 (Fig. 2e). Cells in S phase were decreased in the group of KLF4 overexpression, while cells in G1 phase were relatively increased versus controls (data not shown). These results indicated that overexpression of KLF4 resulted in the apoptosis and delayed growth of K562 cells.
Fig. 2.
a Expression of KLF4 protein in KLF4-overexpressed K562 cells and PBMCs. K562 cells and PBMCs were transfected with pcDNA3.1-KLF4, and the expression level of KLF4 was identified by Western blot analysis. Neo vector control group, KLF4 KLF4 overexpression group. b Effect of KLF4 overexpression on cell proliferation determined by CCK-8. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. c Effect of KLF4 overexpression on the cell apoptosis rate determined by flow cytometry. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. d Effect of KLF4 overexpression on caspase-3 activity assayed using caspase fluorescence assay kits. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. e Effect of KLF4 overexpression on the levels of cleaved caspase-3 and PARP measured by Western blot analysis. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. Cells were treated with H2O2 (0.5 mmol/l) for 48 h. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05
Expressions of bax and bcl-2 in K562 cells or PBMCswith overexpression or inhibition of KLF4
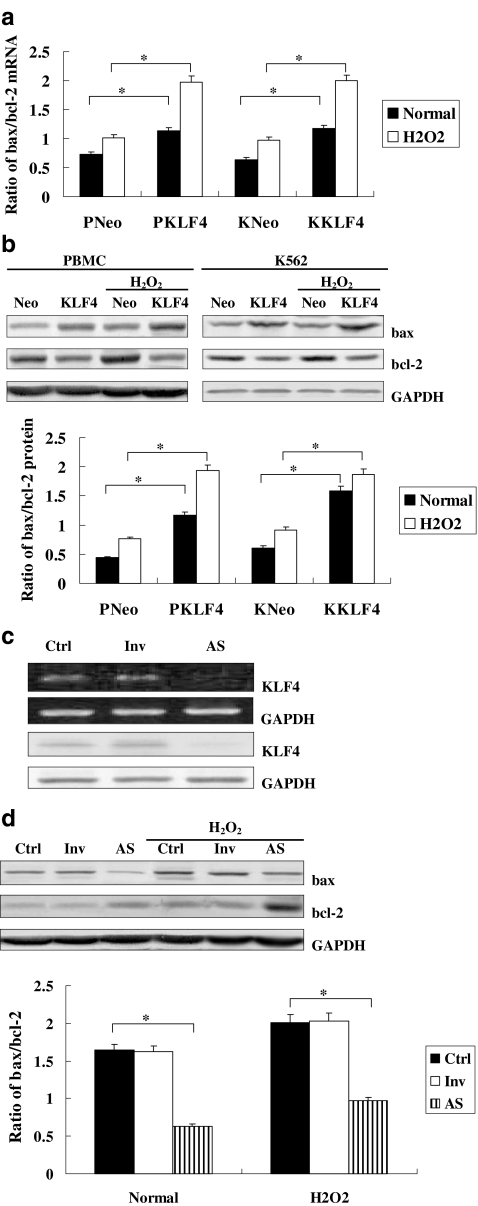
On further study, we investigated the expression changes of the above genes with overexpression or inhibition of KLF4 in cells with or without treatment with H2O2. The expressions of bax and bcl-2 in response to H2O2 stimulation were increased in all cell groups. Moreover, KLF4 overexpression could up-regulate the expression of bax while down-regulating the expression of bcl-2, further intensifying the bax/bcl-2 ratio in the H2O2 group (Fig. 3a, b). The inhibition of KLF4 by morpholino anti-sense oligonucleotide was detected by real-time PCR and Western blot analysis (Fig. 3c), resulting in reduction of the expressions of the above two genes under H2O2 stimulation (Fig. 3d), suggesting a potentially active effect of KLF4 on the apoptosis of K562 cells and PBMCs.
Fig. 3.
a Effect of KLF4 overexpression on the mRNA levels of bcl-2 and bax measured by real-time PCR. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. Cells were treated with H2O2 (0.5 mmol/l) for 12 h. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. b Effect of KLF4 overexpression on the protein levels of bcl-2 and bax measured by Western blot analysis. PNeo vector control group of PBMCs, PKLF4 KLF4 overexpression group of PBMCs, KNeo vector control group of K562 cells, KKLF4 KLF4 overexpression group of K562 cells. Cells were treated with H2O2 (0.5 mmol/l) for 12 h. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. c Expression of KLF4 in KLF4-deficient K562 cells. K562 cells were transiently transfected with KLF4 morpholino anti-sense oligonucleotide. Expression of KLF4 was detected by real-time PCR and Western blot analysis for identification of basal KLF4 inhibition. Ctrl K562 cells were treated only with Lipofectamine, Inv K562 cells were transiently transfected with random oligonucleotides of KLF4, AS K562 cells were transiently transfected with morpholino anti-sense oligonucleotide of KLF4. d Effect of KLF4 inhibition on the protein levels of bcl-2 and bax measured by Western blot analysis. Ctrl K562 cells were treated only with Lipofectamine, Inv K562 cells were transiently transfected with random oligonucleotides of KLF4, AS K562 cells were transiently transfected with morpholino anti-sense oligonucleotide of KLF4. Cells were treated with H2O2 (0.5 mmol/l) for 12 h. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05
Induction of bcl-2 and bax promoter activities by KLF4 in K562 cells
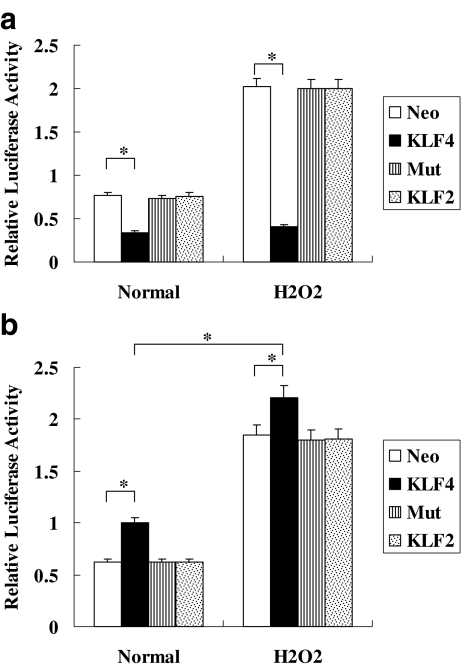
Promoter sequences of bax and bcl-2 were analyzed by a Matinspector Professional program (www.genomatix.de) and a Transcription Element Search System (TESS; www.cbil.upenn.edu) to predict KLF4 binding elements in the promoters. There is one KLF4 binding element at nt −177/−163 in the promoter of bcl-2, and one KLF4 binding element at nt −30/−16 in the promoter of bax. In order to understand how KLF4 can regulate bcl-2 and bax, we detected its effects on their promoter activities. A strong transinactivation effect on the bcl-2 promoter is shown in Fig. 4a, and the strong transactivation effect of KLF4 on the bax promoter is shown in Fig. 4b. The stimulation of H2O2 may further promote the transactivation of the bax promoter while not inducing the activity of the bcl-2 promoter dramatically. Moreover, the specificity of the transcriptional activity of KLF4 for the bcl-2 and bax promoters was further confirmed with KLF2 or the bcl-2/bax mutant as control.
Fig. 4.
a Transcription activity of KLF4 to bax promoter in K562 cells. K562 cells were cotransfected transiently with an expression plasmid of full-length KLF4 (500 ng) and a reporter driven by bax promoter (500 ng) or mutant bax promoter (500 ng), pcDNA3.1-KLF2 (500 ng), or pcDNA3.1 (500 ng) as control. Luciferase activity was detected using the Dual Luciferase Reporter System. Cells were treated with H2O2 (0.5 mmol/l) for 12 h. Neo vector control group, KLF4 KLF4 overexpression group, Mut cell group transfected with pGL3-mutbax, KLF2 KLF2 overexpression group. All transfections were performed at least three times in triplicate. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05. b Transcription activity of KLF4 to bcl-2 promoter in K562 cells. K562 cells were cotransfected transiently with an expression plasmid of full-length KLF4 (500 ng) and a reporter driven by bcl-2 promoter (500 ng) or mutant bcl-2 promoter (500 ng), pcDNA3.1-KLF2 (500 ng), or pcDNA3.1 (500 ng) as control. Luciferase activity was detected using the Dual Luciferase Reporter System. Cells were treated with H2O2 (0.5 mmol/l) for 12 h. Neo vector control group, KLF4 KLF4 overexpression group, Mut cell group transfected with pGL3-mutbcl-2, KLF2 KLF2 overexpression group. All transfections were performed at least three times in triplicate. The relative values of all results were determined and expressed as the mean±SEM of three experiments performed in duplicate. *P < 0.05
Discussion
KLF4 is an epithelial-cell-enriched zinc-finger-containing transcription factor that has been widely investigated in both normal development and carcinogenesis (Ghaleb et al. 2005). Under normal conditions, the expression of KLF4 mRNA is most abundant in the colon and skin in mice, while the expression of KLF4 is decreased in intestinal adenomas of mice with multiple intestinal neoplasia and in colonic adenomas of patients with familial adenomatous polyposis. In this investigation, we first detected the expression of KLF4 in the apoptotic K562 cells and PBMCs isolated from blood samples of CML patients and found that KLF4 levels were increased with apoptosis of the cells, suggesting a potential role for KLF4 in apoptosis. Moreover, apoptosis and delayed growth of cells induced by overexpression of KLF4 further demonstrated the hypothesis. Subsequent investigations may elucidate the mechanism by which KLF4 induces apoptosis of K562 cells.
Cancer is one of the world’s leading causes of death and occurs when the homeostatic balance between cell growth and cell death is disturbed. Research in cancer biology has discovered that a variety of aberrations in the gene expression of anti-apoptotic, pro-apoptotic, and BH3-only proteins can contribute to the many forms of the disease. Apoptosis plays a very important role in regulating a variety of diseases that have enormous social impact. Bcl-2 is essential to the process of apoptosis because it suppresses the initiation of the cell death process; moreover, bax protein or BAX gene was the first identified pro-apoptotic member of the bcl-2 protein family. Since KLF4 influenced the expression of bcl-2 and bax genes in other myoblast cells (Liu et al. 2008a, b), we also detected the expression of the above genes in K562 cells and PBMCs associated with the overexpression and inhibition of KLF4. The results suggested the induction effect of KLF4 on bax and the reduction effect of KLF4 on bcl-2. Oxidative stress is involved in drug-induced apoptosis during various pathophysiological processes, especially cancer chemotherapy. The KLF4-regulated mechanism on the expression of bax and bcl-2 in response to H2O2 stimulation could partially elucidate the related apoptotic pathway.
Many target genes of KLF4, including CYP1A1, human keratin 4, intestinal alkaline phosphatase, ornithine decarboxylase, histidine decarboxylase, and cyclin D1, have been identified as transcriptional factors (Okano et al. 2000; Shie et al. 2000). KLF4 regulates target genes by binding to the potential KLF4 binding elements in the promoters. Our laboratory also demonstrated that KLF4 activated the IL-10 promoter and the HMGB1 promoter by binding to the corresponding KLF DNA binding sites, indicating the potential effect of KLF4 on inflammatory mediator genes (Liu et al. 2007a, b, 2008a, b). By using Matinspector Professional program (www.genomatix.de) and TESS (www.cbil.upenn.edu), we found one KLF4 binding element at nt −177/−163 in the promoter of bcl-2, and another KLF4 binding element at nt −30/−16 in the promoter of bax. We further found that KLF4 could transactivate the promoter activity of bax and transinactivate the promoter activity of bcl-2. The regulation effect of KLF4 on bcl-2 and bax genes explores a novel pathway for the transcriptional mechanism of these two genes. bcl-2 family members are regulated at the transcriptional level: several pro-survival genes are induced transcriptionally by certain cytokines (Dijkers et al. 2000; Cory et al. 2003), while the expression of bax is up-regulated by the tumor-suppressor protein p53, and bax has been shown to be involved in p53-mediated apoptosis (Chipuk et al. 2004). Furthermore, it has been reported that p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway (Thomas et al. 2000). It has also been demonstrated that KLF4 suppresses the expression of p53 by directly acting on its promoter, which considers KLF4 as a regulator of p53 that oncogenically transforms cells as a function of p21CIP1 status (Rowland et al. 2005). We could assume that the serial expression changes of bcl-2 and bax might result from the interaction of KLF4, p53, bcl-2, and bax genes. However, this hypothesis needs to be demonstrated by further studies.
In summary, our study demonstrated the increasing expression of KLF4 in apoptotic K562 cells and PBMCs, and identified that KLF4 induced the expression of bax and reduced the expression of bcl-2, potentially resulting in the apoptosis of K562 cells and PBMCs induced by H2O2. Further research will provide us with a more complete picture of how these proteins interact with each other to promote and inhibit apoptosis. An understanding of the mechanisms involved will help us find potential treatments (for instance inhibitors to target overexpressed proteins such as KLF4) that may disable cancer, neurodegenerative diseases, and autoimmune diseases.
Acknowledgments
This work was supported by funding from the Science and Technology Program of Hunan Province (2009FJ3169), the National Natural Science Foundation of China (30900623), and the Doctoral Fund of the Ministry of Education of China (Fund for New Teacher, 20090162120020).
Footnotes
Zhongdong Li and Jie Zhao contributed equally to this work.
References
- Ai W, Zheng H, Yang X, Liu Y, Wang TC. Tip60 functions as a potential corepressor of KLF4 in regulation of HDC promoter activity. Nucleic Acids Res. 2007;35(18):6137–6149. doi: 10.1093/nar/gkm656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol Cell Biol. 1995;15(11):5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos L, Sabido O, Viallet A, Vasselon C, Guyotat D. Expression of apoptosis-controlling proteins in acute leukemia cells. Leuk Lymphoma. 1999;33(5–6):499–509. doi: 10.3109/10428199909058454. [DOI] [PubMed] [Google Scholar]
- Cheok MH, Yang W, Pui CH, et al. Treatment-specific changes in gene expression discriminate in vivo drug response in human leukemia cells. Nat Genet. 2003;34(1):85–90. doi: 10.1038/ng1151. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Wani MA, Lingrel JB. Lung Kruppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem. 2001;276(31):29299–29306. doi: 10.1074/jbc.M103670200. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296(1):1–6. [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10(19):1201–1204. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15(2):92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999;93(8):2671–2678. [PubMed] [Google Scholar]
- Iijima N, Miyamura K, Itou T, Tanimoto M, Sobue R, Saito H. Functional expression of Fas (CD95) in acute myeloid leukemia cells in the context of CD34 and CD38 expression: possible correlation with sensitivity to chemotherapy. Blood. 1997;90(12):4901–4909. [PubMed] [Google Scholar]
- Ito G, Uchiyama M, Kondo M, et al. Kruppel-like factor 6 is frequently down-regulated and induces apoptosis in non-small cell lung cancer cells. Cancer Res. 2004;64(11):3838–3843. doi: 10.1158/0008-5472.CAN-04-0185. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ratziu V, Choi SG, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273(50):33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- Lennon SV, Martin SJ, Cotter TG. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Lin SS, Bassik MC, Suh H, et al. PP2A regulates BCL-2 phosphorylation and proteasome-mediated degradation at the endoplasmic reticulum. J Biol Chem. 2006;281(32):23003–23012. doi: 10.1074/jbc.M602648200. [DOI] [PubMed] [Google Scholar]
- Liu MD, Xiao WM, Jiang BM, Shi YZ, Wang KK, Xiao XZ. Effect of hydrogen peroxide on the apoptosis of K562 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2003;28(5):499–501. [Google Scholar]
- Liu Y, Sinha S, Owens G. A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278(48):48004–48011. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang H, Liu Y, Wang K, Feng Y, Liu M, Xiao X. KLF4 regulates the expression of interleukin-10 in RAW264.7 macrophages. Biochem Biophys Res Commun. 2007;362(3):575–581. doi: 10.1016/j.bbrc.2007.07.157. [DOI] [PubMed] [Google Scholar]
- Liu MD, Liu Y, Liu JW, Zhang HL, Xiao XZ. Effect of Kruppel-like factor 4 overexpression on heat stress-induced apoptosis of Raw264.7 macrophages. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32(6):1002–1006. [PubMed] [Google Scholar]
- Liu J, Liu Y, Zhang H, Chen G, Wang K, Xiao X. KLF4 promotes the expression, translocation, and release of HMGB1 in RAW264.7 macrophages in response to LPS. Shock. 2008;30(3):260–266. doi: 10.1097/shk.0b013e318162bef7. [DOI] [PubMed] [Google Scholar]
- Liu MD, Liu Y, Liu JW, Zhang HL, Xiao XZ. Effect of Kruppel-like factor 4 overexpression on heat stress-induced apoptosis of C2C12 cells. Chin J Arterioscler. 2008;16(4):265–267. [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- Ohnishi S, Ohnami S, Laub F, et al. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308(2):251–256. doi: 10.1016/S0006-291X(03)01356-1. [DOI] [PubMed] [Google Scholar]
- Okano J, Opitz OG, Nakagawa H, Jenkins TD, Friedman SL, Rustgi AK. The Kruppel-like transcriptional factors Zf9 and GKLF coactivate the human keratin 4 promoter and physically interact. FEBS Lett. 2000;473(1):95–100. doi: 10.1016/S0014-5793(00)01468-X. [DOI] [PubMed] [Google Scholar]
- Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7(11):1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Shie JL, Chen ZY, Fu M, Pestell RG, Tseng CC. Gut-enriched Kruppel-like factor represses cyclin D1 promoter activity through Sp1 motif. Nucleic Acids Res. 2000;28(15):2969–2976. doi: 10.1093/nar/28.15.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Giesler T, White E. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene. 2000;19(46):5259–5269. doi: 10.1038/sj.onc.1203895. [DOI] [PubMed] [Google Scholar]
- Vaporciyan AA, Jones ML, Ward PA. Rapid analysis of leukocyte–endothelial adhesion. J Immunol Methods. 1993;159(1–2):93–100. doi: 10.1016/0022-1759(93)90145-W. [DOI] [PubMed] [Google Scholar]