Abstract
Xenohormesis is a biological principle that explains how environmentally stressed plants produce bioactive compounds that can confer stress resistance and survival benefits to animals that consume them. Animals can piggyback off products of plants' sophisticated stress response which has evolved as a result of their stationary lifestyle. Factors eliciting the plant stress response can judiciously be employed to maximize yield of health-promoting plant compounds. The xenohormetic plant compounds can, when ingested, improve longevity and fitness by activating the animal's cellular stress response and can be applied in drug discovery, drug production, and nutritional enhancement of diet.
Keywords: Xenohormesis, Hormesis, Mutualism, Heat shock proteins, Ethnopharmacology, Food production
In the last half century, two powerful yet simple concepts have gained increasing prominence in scientific thought. The first is the concept of the adaptive stress response. Research in the science of stress has established that the ability of organisms to respond to physical, chemical, and social stressors is a fundamental process of life (Feder and Hofmann 1999; Selye 1978; Tytell and Hooper 2001; Welch 1993). A particularly key realization in this area is the idea that low levels of stress can improve an organism's health, well-being, adaptability, and fitness through stimulation of the cellular stress response, a phenomenon known as hormesis (Calabrese and Baldwin 2003; Gerber et al. 1999; Rattan 2008). The second concept is the notion of the basic interconnectedness of living organisms on this planet. The field of ecology in particular has established the remarkable degree to which the success or failure of one species depends not only on its inert physical environment but also on the success or failure of the other species sharing that environment (Boucher 1985; Bronstein et al. 2006; Jones et al. 1994; Odling-Smee et al. 2003). The concept of xenohormesis—the process by which one organism benefits from the stress response of another (Howitz et al. 2003; Howitz and Sinclair 2008)—lies at the confluence of these two streams of scientific thought.
The phenomenon of xenohormesis was first named by Howitz and Sinclair (2008; Lamming et al. 2004). As the prefix xeno comes from the Greek word meaning stranger or foreigner, xenohormesis describes the phenomenon of a “foreign” organism's stress response producing chemicals that yield benefits to another organism. The word most often refers to the ability of stressed plants to confer stress tolerance to animals that consume them.
In this paper, we review and synthesize recent research bearing on the phenomenon of xenohormesis and point to a number of practical implications of the concept for medicine and agronomy. The following sections introduce the stress response of plants, outline the types of xenohormetic relationships that can exist between plants and herbivores, and discuss the variety of xenohormetic substances that can positively impact animal survival and longevity. The paper aims to build on the existing published literature on xenohormesis by aggregating the growing number of examples of stressed plants producing added nutrient value for the animal that consumes them. Additionally, while several mechanisms for xenohormetic action have been proposed (Howitz and Sinclair 2008), we hypothesize a novel pathway by which products of the plant stress response confer stress tolerance and extend longevity in animals. Finally, we discuss the implications of the principle of xenohormesis for maximizing the nutritional and medicinal properties of plants and for addressing the challenges of adaptation and survival in an ever-changing environment.
Plant stress response
While the cellular stress response and stress chaperones are thought to be at least two and a half billion years old (Feder and Hofmann 1999), plants and their stress response have been evolving for almost one billion years. Because the sessile plant cannot physically move away from stressors, environmental extremes of temperature variation, water or nutrient availability, or predation must be endured in place. As a result, the complexity of the plant stress response humbles that of animals (Kotak et al. 2007). Yet, when an animal eats a plant that has activated its stress response, the animal may readily benefit from the plant's travail.
It is not intuitive that stressed plants may offer more beneficial nutrient products than plants grown under seemingly ideal conditions that maximize the crop yield in terms of mass alone. A prime example of such an unexpected outcome is observed in the production of wine, where the best grapes in terms of taste and health benefit often result from relatively dry, sun-exposed, infertile soil (de Andrés-de Prado et al. 2007; Gambuti et al. 2007). Indeed, the synthesis of resveratrol—a polyphenol that activates the mammalian stress response system and extends longevity (Baur and Sinclair 2006; Putics et al. 2008)—is stimulated by UV light, ozone, or pathogen stress (Brehm et al. 1999; Preisig-Müller et al. 1999; Versari et al. 2001; Wang et al. 2008). Resveratrol protects the plant itself by reducing UV damage and eliminating pathogenic molds (Adrian et al. 1997; Tang et al. 2010). Similarly, drought-stressed strawberries have better taste, antioxidant capacity, and phenol content (Terry et al. 2007), an observation supported by the rich flavor of strawberries found in the wild in contrast to those that are cultivated under more controlled conditions.
Table 1 provides examples of plants that produce higher concentration of health-promoting substances when enriched by stressful conditions. The table includes hormetic “elicitors” that have been used to increase production of pharmacologically active substances—a practice that could, and perhaps should, be widely applied to future ethno-pharmaceutical production and food agronomy. Indeed, commonly consumed food products like lettuce and fruits can be nutritionally enhanced by cold stress, light stress, water deficit, or nutrient deficit stress (Atkinson et al. 2005; Oh et al. 2009). Heavy metals, salicylic acid, and other compounds can function as hormetic elicitors without compromising crop yield (Kuzel et al. 2009; Zhang et al. 2006). Finally, viral, fungal, and bacterial infection can, in some cases, paradoxically improve the plant's nutritional content via hormesis (Banchio et al. 2010; De Vos et al. 2005; Métraux et al. 1990).
Table 1.
Examples of stressed plants producing higher yields of potentially therapeutic bioactive compounds
| Plant | Stressor | Bioactive product | Benefit |
|---|---|---|---|
| American Mayapple (Podophyllum peltatum) | Light stress (Cushman et al. 2004) | Podophyllom | Cancer, warts, arthritis, psoriasis |
| Artemisia annua | Light and cold stress (Zeng et al. 2008) | Artemisinin | Anti-malarial (Graham et al. 2010) |
| Black chokeberry (Aronia melanocarpa) | Catabolites of polyamine biosynthesis, ornithine decarboxylase inhibitor (Hudec et al. 2006) | Phenolic compounds | Phenolic effects: antioxidant, anti-inflammatory, cancer, aging, diabetes, neurodegenerative diseases, renal disease (Bengmark et al. 2009; Kidd 2009; Knutson and Leeuwenburgh 2008; Leonarduzzi et al. 2009) |
| Black Current (Ribes nigrum) | Ornithine decarboxylase inhibitor, O-phosphoethanolamine, carboxymethyl chitin (Hudec et al. 2009) | Phenolic compounds | Phenolic effects |
| Cistus clusii | Water deficit stress (Hernandez et al. 2004) | Ascorbic acid, phenolic compounds | Common cold (Heimer et al. 2009), phenolic effects |
| Cucumber (Cucumis sativus) | Fungal and viral infection (Métraux et al. 1990) | Salicylate | Arthritis, fever, cardiovascular disease (Rainsford 2007) |
| Curcuma (Curcuma longa) | Nutrient deprivation, heat stress (Li and Zhang 1999; Xia et al. 2005) | Curcumin | Cancer, liver cirrhosis, chronic renal disease, chronic obstructive lung disease, diabetes and Alzheimer's disease, intensive care patients, Crohn's disease (Bengmark et al. 2009) |
| Dandelion (Taxaxacum officinale) | Ornithine decarboxylase inhibitor, O-phosphoethanolamine, carboxymethyl chitin (Hudec et al. 2007) | Phenolic compounds | Phenolic effects |
| Grape (Vitis vinifera) | Heat (Wang et al. 2008), fungal infection (Roldán et al. 2003) | Phenolic compounds | Phenolic effects |
| Gray poplar (Populus x canescens) | Osmotic stress high salinity (Luo et al. 2009) | Abscisic acid | Diabetes (Guri et al. 2007) |
| Green algae (Haematococcus pluvialis) | Light and nutrient stress (Vidhyavathi et al. 2008) | Carotenoids (astoxanthin) | Alzheimers disease, skin and eye diseases (Shudo et al. 2009), cardiovascular disease (Fassett and Coombes 2009) |
| Hypericum brasiliense | Heat and water deficit stress (Nacif de Abreu and Mazzafera 2005) | Phenolic compounds | Phenolic effects |
| Lavender (Lavandula vera) | Vanadyl sulfate (Georgiev et al. 2006) | Rosmarinic acid | Atopic dermatitis (Lee et al. 2008), antiviral (Swarup et al. 2007), sun screen, cancer (Sánchez-Campillo et al. 2009) |
| Lettuce (Lactuca sativa) | Heat, cold, light stress (Oh et al. 2009) | Ascorbic acid, alpha-tocopherol, phenolic compounds | Common cold (Heimer et al. 2009), leg cramps (Oh et al. 2009), phenolic effects |
| Mustard (Arabidopsis) | Bacterial and fungal infection, aphid attack (De Vos et al. 2005) | Jasmonate | Cancer (Cohen and Flescher 2009) |
| Oregano (Origanum x majoricum) | Bacterial infection (Banchio et al. 2010) | Carvacrol | Antibiotic, antiparasitic, diabetes, analgesic, cancer, anti-inflammatory (Baser 2008) |
| Prickly pear cactus (Opuntia ficus indica) | Heat stress and pH stress (Chaidee and Pfeiffer 2006) | Betalains | Diabetes, dyslipidemia, gastritis, prostate hypertrophy (Ennouri et al. 2006) |
| Purple coneflower (Echinacea purpurea) | Acetylsalicylic acid, salicylic acid, methylsalicylate, titanium (IV) ascorbate (Kuzel et al. 2009), ornithine decarboxylase inhibitor, O-phosphoethanolamine, carboxymethyl chitin (Hudec et al. 2007) | Caffeic acid and other phenolic compounds | Cardiovascular disease (Kumaran and Prince 2010), phenolic effects |
| Soy beans (Glycine max) | Salicylic acid, methyl salicylate, ethyl acetate (Zhang et al. 2006) | Phenolic compounds | Phenolic effects |
| St. John's wort Hypericum perforatum | Water deficit stress (Gray et al. 2003) | Hyperforin | Antidepressant (Linde 2009) |
| Strawberry (Fragaria chiloensis) | Water deficit stress (Terry et al. 2007) | Phenolic compounds | Phenolic effects, improved taste |
| Taxus yunnanensis cell suspension cultures | Fungal elicitors, yeast elicitors, methyl jasmonate, chitosan, heavy metals, and heat (Zhang and Fevereiro 2007) | Paclitaxel | Cancer |
| Wheat-rye hybrid (triticale) | Water deficit stress (Hura et al. 2009a, b) | Ferulic acid, phenolic compounds | Neurodegenerative disorders, cardiovascular disease, diabetes, cancer (Barone et al. 2009), phenolic effects |
| Willow seedlings (Salix sericea) | Beetle herbivory (Fields and Orians 2006) | Salicylate | Improved stress response, longevity, antipyretic, anti-inflammatory (Batulan et al. 2005; Strong et al. 2008) |
The class of compounds classified as phenols and polyphenols deserve special comment, as they are important categories of secondary metabolites in higher plants that have diverse therapeutic applications. They are divided into flavonoids—which include rutin, quercetin, epigallocatechin gallate (EGCG), isoflavones, and anthrocyanidins—and non-flavonoids—including hydroxybenzoic acid and stilbene derivatives such as resveratrol, cinnamic acid, caffeic acid, curcumin, rosmarinic acid, and ferulic acid. Key enzymes for synthesis of phenolic compounds are phenylalanine ammonia-lyase and stilbene synthase, both of which respond to plant stressors as part of the plant defense against infection and environmental challenge (Benoît et al. 2000; Brehm et al. 1999). Phenolic compounds activate the mammalian stress response when ingested, have antioxidant and anti-inflammatory effects, and have therapeutic effects against aging, cancer, type 2 diabetes, neurodegenerative diseases, and renal disease (Bengmark et al. 2009; Kidd 2009; Knutson and Leeuwenburgh 2008; Leonarduzzi et al. 2009). Finally, dietary polyphenol intake in both humans and wild primates (about 14 mg/kg/day) is within the range of having real biological effects (Milton 2003).
Plant–animal xenohormetic relationships
There are multiple potential forms of xenohormetic relationships between plants and animals, including our own species. In some cases, xenohormesis is embedded within an active mutualistic relationship between plant and animal species, and the substances produced by the stressed plant are specifically targeted to produce benefits for the helper animal species. For example, trees like Cecropia, Acacia, and Macaranda have evolved indirect defense to herbivores by attracting and nourishing other organisms that reduce plant herbivore damage (Heil 2008). Over a century ago, Francis Darwin described a “standing army of ants” that consume Acacia food bodies and protect the plant from the ravages of herbivores (Darwin 1877). The damage from herbivore feeding elicits an octadecanoid cascade, which leads to jasmonic acid synthesis and the production of extrafloral nectar and food bodies. These food bodies provide carbohydrates, lipids, amino acids, and micronutrients that attract ants and parasitic wasps that protect against the offending herbivores (Heil 2008; Linsenmair et al. 2001). Plant–animal mutualisms also occur to facilitate the dispersal of seeds and pollination of flowers by animals (Bronstein et al. 2006); consumption of xenohormetic substances in stressed plants may enable animals to more successfully distribute the plant's genes when conditions are difficult.
While mutualism between plants and animals can support a co-evolutionary impetus for xenohormesis, xenohormesis can also occur with no apparent benefit to the plant. In these cases, the animal is able to piggyback on the self-directed adaptive response of the plant. In fact, most of the bioactive compounds listed in Table 1 are simply products of the plant’s stress response that protect it from further environmental damage.
Xenohormetic plant compounds can yield benefits to the animal directly, or by activating the animal's own stress defense pathways. The xenohormetic benefit of the Cecropia–ant relationship, for example, comes mainly in the form of calories (Heil 2008). Ascorbic acid, flavonoids, and alpha-tocopherol produced by lettuce and soy act primarily as antioxidants (Leonarduzzi et al. 2009; Oh et al. 2009; Zhang et al. 2006). Other hormetic compounds like podophyllum and paclitaxel have a low dose therapeutic effect, but are quite toxic at higher doses (Biganzoli et al. 2009; Kao et al. 1992). Polyphenols such as resveratrol, curcumin, and carvacrol, on the other hand, are able to stimulate the animal's stress response with minimal systemic toxicity (Allard et al. 2009; Baur and Sinclair 2006; Kato et al. 1998; Scapagnini et al. 2001).
There are several possible explanations for why the animal's stress response should be activated by products of a plant's stress response (Howitz and Sinclair 2008). First, it may occur as a fortunate coincidence, especially in the case of marginally toxic products that produce hormetic benefits via the animal's generalized stress response. Second, it may be due to shared evolutionary history and physiology. Animals and plants, for instance, share a high degree of sequence homology between their stress response signaling pathways, particularly for highly conserved kinases and heat shock proteins (Jiménez et al. 2007). Finally, selection may have favored animal physiology that responds to products of the plant's stress response in order to gauge changing environmental conditions, a hypothesis that has been particularly emphasized in the work of Howitz and colleagues (Howitz et al. 2003; Howitz and Sinclair 2008; Lamming et al. 2004).
Xenohormetic compounds and longevity pathways
Many bioactive plant compounds that are associated with improved animal longevity confer their benefits by activating or priming cellular survival pathways. The caloric restriction model of inducing robustness and longevity has been used to explain much of hormetic action (Rattan 2008). In fact, the term “caloric restriction mimetics” is often used to describe bioactive compounds with hormetic effects (Ingram et al. 2006; Redman et al. 2008). Recent studies associate caloric deprivation signaling with both activation of the stress response and restoration of energy supply (Canto et al. 2009; Saunders and Verdin 2009).
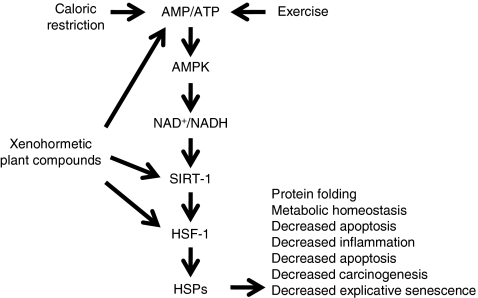
The key alarm that initiates the response to caloric restriction is lack of available energy in the form of ATP deficit and/or oxidative stress. Figure 1 illustrates a direct pathway that responds to a low energy state and results in a robust stress response and energy recovery. A high AMP/ATP ratio, as occurs with caloric restriction and exercise, activates AMPK (AMP-activated protein kinase), which then, via the NAD+/NADH redox state, stimulates sirtuin 1 (SIRT-1) deacetylation of key transcription factors including heat shock factor-1 (HSF-1; Canto et al. 2009). HSF-1 activates most heat shock proteins (Hsps) and is associated with stress survival and longevity extension across diverse species (Saunders and Verdin 2009).
Fig. 1.
Longevity pathway demonstrating how caloric restriction, exercise, and stress-derived plant products activate the mammalian stress response
HSF-1 specifically orchestrates cellular viability by limiting replicative senescence, apoptosis, inflammation, and stress vulnerability (Logan et al. 2009; Meldrum et al. 2003; Pandita et al. 2004; Saunders and Verdin 2009). AMPK activation also limits protein and fat synthesis while stimulating fatty acid oxidation and glycolysis in order to restore the availability of ATP. The net effect on the organism is to meet demands for immediate energy availability and cellular organelle protection. We suggest that an activated stress response plays a fundamental role in conferring fitness and survival benefits to animals consuming stressed plants. In support of this notion, overexpression of HSF-1 extends longevity, while underexpression reduces it in transgenic animal models (Calderwood et al. 2009; Hsu et al. 2003; Morley and Morimoto 2004). Importantly, plant caloric restriction mimetics can have effects at several steps of the pathway illustrated in Fig. 1. In some cases, it is difficult to identify the predominate level that any particular xenohormetic compound activates; for instance, resveratrol activates both AMPK (Fullerton and Steinberg 2010) and SIRT1 (Westerheide et al. 2009), but it is not yet definitively clear which activation dominates its effect.
To date, the primary bioactive plant compounds known to raise Hsps and to be associated with extension of animal life span are resveratrol (Putics et al. 2008; Westerheide et al. 2009), curcumin (Chen et al. 2001; Kitani et al. 2007; Scapagnini et al. 2001), salicylate (Batulan et al. 2005; Strong et al. 2008), and EGCG (Abbas and Wink 2009; Zhang et al. 2009). Plant compounds that raise Hsps but currently have no proven longevity effects are rosmarinic acid (Rattan et al. 2009), ferulic acid (Calabrese et al. 2008), jasmonic acid (Oh et al. 2005), and carvacrol (Wieten et al. 2010). As a class, these compounds are associated with pleiotropic medicinal benefits that include improved stress tolerance, anti-carcinogenesis, improved glycemic control in diabetes, and reduced cardiovascular disease (see Table 1). The therapeutic promise of these Hsp-augmenting substances and their derivatives are substantial. We should note, however, that because most studies to date have been carried out in cell culture and at super-physiological concentrations, there remain high returns to research focusing on the magnitude of these benefits under more natural conditions.
Another pathway associated with longevity extension that is activated by xenohormetic compounds involves transcription factor nuclear factor-E2-related factor 2 (Nrf2; Godman et al. 2009; Onken and Driscoll 2010). Nrf2 binds antioxidant response elements upstream of cytoprotective stress response proteins such as heme oxygenase and Hsp 22/40/90. The xenohormetic compound curcumin, for example, activates heme oxygenase by regulating Nrf2 (Balogun et al. 2003), while other plant compounds that are not necessarily xenohormetic like sulforaphane (in broccoli) and allicin (in garlic) also activate Nrf2 (Chen et al. 2004; Dinkova-Kostova et al. 2002).
Remarkably, antioxidants like ascorbate, alpha-tocopherol, and some flavonoids have not been associated with improved longevity (Muller et al. 2007). The lack of long-term therapeutic benefit of antioxidants may be partially explained by their suppression of the endogenous stress response. Oxidation products are potent stimulants of HSF-1 activity, and decreasing oxidation in the cell can reduce the HSF-1 stress response (Hooper and Hooper 2004).
Implications of xenohormesis
How can humans leverage the lessons of xenohormesis? As mentioned above, elicitors can improve production of ethno-pharmaceutical products and the nutrient quality of commonly consumed agricultural products. For instance, the global spread of drug-resistant malaria has created a major demand for the anti-malarial artemisinin, a sesquiterpenoid compound that is synthesized in glandular trichomes of Artemisia annua (Graham et al. 2010). The fact that A. annua's glandular trichomes are similar to the food bodies described by Darwin, in that they increase in density and size in response to stress, is probably not a random coincidence; however, unlike the food bodies, they release oils that directly protect the plant from herbivore attack and/or attract pollinators (Schilmiller et al. 2008). Indeed, the hormetic elicitors of cold and light stress increase artemisinin yield in A. annua (Zeng et al. 2008) and may be employed to address pharmaceutical production shortages.
While the original concept of xenohormesis focused on micronutrients (Howitz et al. 2003), the concept can also be extended at the macronutrient level. Vígh and colleagues observed that plants exposed to cold shock increase synthesis of unsaturated fatty acids, increasing membrane fluidity and stabilizing the stressed membrane (Quinn et al. 1989; Vígh et al. 1985). Pertinently, animal consumption of these less saturated fats lowers the animal's threshold for triggering the stress response and is associated with a hearty, less disease-prone state. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response (Balogh et al. 2005; Nagy et al. 2007). In fact, evidence supporting the positive impact of “healthy” plant and animal oils (e.g., olive oil and omega-3 fatty acids) on our well-being is rapidly expanding (Sofi 2009; Zevenbergen et al. 2009). This xenohormetic process also provides a natural model for the action of the Hsp co-inducing hydroxylamines: like unsaturated plant lipids, these membrane-intercalating compounds are capable of simultaneously reducing the molecular order of specific membrane domains and correcting dysregulated expression of Hsps (Török et al. 2003).
Continued focused research on plants' adaptive stress response will likely lead to the discovery of new health-promoting compounds with significant pharmaceutical promise. Close examination of bioactive products found in plants that survive and thrive in environmentally harsh conditions may reveal potent therapeutic substances. For example, extracts from the prickly pear cactus (Opuntia ficus indica) can be effective in reducing the symptoms of excessive alcohol consumption. When ingested, the cactus extract increases Hsp levels and is promoted for use by endurance athletes (Wiesse et al. 2004). The active ingredient may be a betalain, which is associated with potentially broad medicinal benefits, including treatment of diabetes, dyslipidemia, gastritis, and prostate hypertrophy (Ennouri et al. 2006).
Another insight from an understanding of xenohormesis is that it may partially explain the conundrum currently surrounding many ethno-pharmaceutical studies that too often cannot stand up to standards of scientific reproducibility. From study to study, researchers may not take into consideration variability in the concentration of bioactive compounds elicited by the conditions under which the medicinal plant was grown. For instance, while a number of studies suggest that cinnamon consumption should improve glycemic control in patients with type 2 diabetes milletus, the result has failed to be confirmed by meta-analysis (Baker et al. 2008). In other words, cinnamon used in one study may not have the same biological effect as cinnamon in another study because it does not have the same concentration of environment-dependent bioactive compounds.
The use of herbs and spices in traditional ethnic cuisines may harness both hormetic and xenohormetic principles. In particular, the use of curcumin, lavender, oregano, mustard (see Table 1), thyme (a source of carvacrol: Baser 2008), and red wine may not only enhance taste but also yield health benefits by priming the consumer's stress response pathways. More generally, while adaptive explanations for the use of spices have focused on their potential effectiveness against pathogens (Billing and Sherman 1998), the theory and data presented here suggest that spices—especially those grown under stressed conditions—may also improve human health via hormetic and xenohormetic pathways (Gerber et al. 1999).
A more global question in this context is whether we are losing important health and nutritional benefits by consuming agricultural products grown in soils and conditions that maximize crop yield but that minimize the stress that would have existed in more natural environments. Has the popularity of controlled mono-cropping in the last few decades affected other species as well? The epidemic of the colony collapse syndrome, for example, has devastated honeybee populations. Is the honeybee no longer ingesting stress-derived and stress-protective bioactive nutrients from a variety of plant species, and therefore becoming more vulnerable itself to life's stresses (Alaux et al. 2010)? Does humanity share a similar risk? As our climate and environment change, it will be more essential than ever for humanity to engage in mutualistic interplay with species at all trophic levels. Perhaps our species can harness the sophisticated stress response of plants to better survive and thrive in a stressful and ever-changing world.
Acknowledgements
The authors thank Ben Sherwood of the Linnean Society library for references and David Campbell, Jeff Leung, and Konrad Howitz for helpful feedback.
Contributor Information
Philip L. Hooper, Phone: +1-970-5865669, Email: phoopermd@gmail.com
Paul L. Hooper, Email: phooper@unm.edu
Michael Tytell, Email: mtytell@wfubmc.edu.
Lászlo Vígh, Email: vigh@brc.hu.
References
- Abbas S, Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009;75:216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- Adrian M, Jeandet P, Veneau J, Weston LA, Bessis R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J Chem Ecol. 1997;23:1689–1702. doi: 10.1023/B:JOEC.0000006444.79951.75. [DOI] [Google Scholar]
- Alaux C, Ducloz F, Crauser D, Le Conte Y (2010) Diet effects on honeybee immunocompetence. Biol Lett (in press) [DOI] [PMC free article] [PubMed]
- Allard JS, Perez E, Zou S, Cabo R. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009;299:58–63. doi: 10.1016/j.mce.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson CJ, Nestby R, Ford YY, Dodds PA. Enhancing beneficial antioxidants in fruits: a plant physiological perspective. Biofactors. 2005;23:229–234. doi: 10.1002/biof.5520230408. [DOI] [PubMed] [Google Scholar]
- Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diab Care. 2008;31:41–43. doi: 10.2337/dc07-1711. [DOI] [PubMed] [Google Scholar]
- Balogh G, Horvàth I, Nagy E, Hoyk Z, Benkõ S, Bensaude O, Vígh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchio E, Bogino PC, Santoro M, Torres L, Zygadlo J, Giordano W. Systemic induction of monoterpene biosynthesis in Origanumxmajoricum by soil bacteria. J Agric Food Chem. 2010;58:650–654. doi: 10.1021/jf9030629. [DOI] [PubMed] [Google Scholar]
- Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10:97–108. doi: 10.1007/s10522-008-9160-8. [DOI] [PubMed] [Google Scholar]
- Baser KH. Biological and pharmacological activies of carvacrol and carvacrol bearing essential oils. Curr Pharm Des. 2008;14(29):3106–1219. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- Batulan Z, Nalbantoglu J, Durham HD. Nonsteroidal anti-inflammatory drugs differentially affect the heat shock response in cultured spinal cord cells. Cell Stress Chaperones. 2005;10:185–196. doi: 10.1379/CSC-30R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bengmark S, Mesa MD, Gil A. Plant-derived health - the effects of tumeric and curcuminoids. Nutr Hosp. 2009;24:273–281. [PubMed] [Google Scholar]
- Benoît MA, D'Aprano G, Lacroix M. Effect of γ-irradiation on phenylalanine ammonia-lyase activity, total phenolic content, and respiration of mushrooms (Agaricus bisporus) J Agric Food Chem. 2000;48:6312–6316. doi: 10.1021/jf000543s. [DOI] [PubMed] [Google Scholar]
- Biganzoli L, Licitra S, Moretti E, Pestrin M, Zafarana E, Leio A. Taxanes in the elderly: can we gain as much and be less toxic? Crit Rev Oncol Hematol. 2009;70:262–271. doi: 10.1016/j.critrevonc.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Billing J, Sherman PW. Antimicrobial functions of spices: why some like it hot. Q Rev Biol. 1998;73:3–49. doi: 10.1086/420058. [DOI] [PubMed] [Google Scholar]
- Boucher DH, editor. The biology of mutualism: ecology and evolution. New York: Oxford University Press; 1985. [Google Scholar]
- Brehm I, Preisig-Müller R, Kindl H. Grapevine protoplasts as a transient expression system for comparison of stilbene synthase genes containing cGMP-responsive promotor elements. Z Naturforsch. 1999;54:220–229. doi: 10.1515/znc-1999-3-412. [DOI] [PubMed] [Google Scholar]
- Bronstein JL, Alarcón R, Geber M. The evolution of plant-insect mutualisms. New Phytol. 2006;172:412–428. doi: 10.1111/j.1469-8137.2006.01864.x. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421:691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Calafato S, Puleo E, Cornelius C, Sapienza M, Morganti P, Mancuso C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. Clin Dermatol. 2008;26:358–363. doi: 10.1016/j.clindermatol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidee A, Pfeiffer W. Parameters for cellular viability and membrane function in chenopodium cells show a specific response of extracellular pH to heat shock with extreme Q10. Plant Biol. 2006;8:42–51. doi: 10.1055/s-2005-872945. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR. Alternative activation of extracellular signal-regulated protein kinases in curcumin and arsenite-induced HSP70 gene expression in human colorectal carcinoma cells. Eur J Cell Biol. 2001;80:213–221. doi: 10.1078/0171-9335-00158. [DOI] [PubMed] [Google Scholar]
- Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cohen S, Flescher E. Methyl jasmonate: a plant stress hormone as an anti-cancer drug. Phytochemistry. 2009;70:1600–1609. doi: 10.1016/j.phytochem.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Cushman KE, Maqbool M, Lata H, Bedir E, Khan IA, Moraes RM. Podophyllotoxin content and yield of American mayapple leaves in sun and shade. Hort Science. 2004;40(1):60–63. [Google Scholar]
- Darwin F. On the glandular bodies on Acacia sphærocephala and Cecropia peltata serving as food for ants. With an appendix on the nectar-glands of the common brake fern. J Linn Soc Lond Bot. 1877;15:298–409. [Google Scholar]
- Andrés-de Prado R, Yuste-Rojas M, Sort X, Andrés-Lacueva C, Torres M, Lamuela-Raventós RM. Effect of soil type on wines produced from Vitis vinefera L. Cv. grenache in commercial vineyards. J Agric Food Chem. 2007;55:779–786. doi: 10.1021/jf062446q. [DOI] [PubMed] [Google Scholar]
- Vos M, Oosten VR, Poecke RM, Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Loon LC, Dicke M, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microb Interact. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennouri M, Fetoui H, Bourret E, Zeghal N, Guermazi F, Attia H. Evaluation of some biological parameters of Opuntia ficus indica. 2. Influence of seed supplemented diet on rats. Bioresour Technol. 2006;97:2136–2140. doi: 10.1016/j.biortech.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5:333–342. doi: 10.2217/fca.09.19. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fields MJ, Orians CM. Specificity of phenolic glycoside induction in willow seedlings (Salix sericea) in response to herbivory. J Chem Ecol. 2006;32:2647–2656. doi: 10.1007/s10886-006-9188-7. [DOI] [PubMed] [Google Scholar]
- Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010;59:551–553. doi: 10.2337/db09-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambuti A, Strollo D, Erbaggio A, Lecce L, Moio L. Effect of winemaking practices on color indexes and selected bioactive phenolics of Aglianico wine. J Food Sci. 2007;72:S623–S628. doi: 10.1111/j.1750-3841.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Georgiev M, Kuzeva S, Pavlov A, Kovacheva E, Ilieva M. Enhanced rosmarinic acid production by Lavandula vera MM cell suspension culture through elicitation with vanadyl sulfate. Z Naturforsch C. 2006;61:241–244. doi: 10.1515/znc-2006-3-414. [DOI] [PubMed] [Google Scholar]
- Gerber LM, Williams GC, Gray SJ. The nutrient-toxin dosage continuum in human evolution and modern health. Q Rev Biol. 1999;74:273–289. doi: 10.1086/393162. [DOI] [PubMed] [Google Scholar]
- Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin DG, Giardina C (2009) Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones (in press) [DOI] [PMC free article] [PubMed]
- Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, et al. The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science. 2010;327:328–331. doi: 10.1126/science.1182612. [DOI] [PubMed] [Google Scholar]
- Gray DE, Pallardy SG, Garrett HE, Rottinghaus GE. Effect of acute drought stress and time of harvest on phytochemistry and dry weight of St. John's wort leaves and flowers. Planta Med. 2003;69:1024–1030. doi: 10.1055/s-2003-37026. [DOI] [PubMed] [Google Scholar]
- Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–116. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Heil M. Indirect defence via tritophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- Heimer KA, Hart AM, Martin LG, Rubio-Wallace S. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J Am Acad Nurse Pract. 2009;21:295–300. doi: 10.1111/j.1745-7599.2009.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez I, Alegre L, Munne-Bosch S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004;24:1303–1311. doi: 10.1093/treephys/24.11.1303. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Vitamin E and atherosclerosis. Prev Cardiol. 2004;7:144. doi: 10.1111/j.1520-037X.2004.3520.x. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hudec J, Bakos D, Mravec D, Kobida L, Burdova M, Turianica I, Hlusek J. Content of phenolic compounds and free polyamines in black chokeberry (Aronia melanocarpa) after application of polyamine biosynthesis regulators. J Agric Food Chem. 2006;54:3625–3628. doi: 10.1021/jf060299q. [DOI] [PubMed] [Google Scholar]
- Hudec J, Burdova M, Kobida L, Komora L, Macho V, Kogan G, Turianica I, Kochanova R, Lozek O, Haban M, et al. Antioxidant capacity changes and phenolic profile of Echinacea purpurea, nettle (Urtica dioica L.), and dandelion (Taraxacum officinale) after application of polyamine and phenolic biosynthesis regulators. J Agric Food Chem. 2007;55:5689–5696. doi: 10.1021/jf070777c. [DOI] [PubMed] [Google Scholar]
- Hudec J, Kochanova R, Burdova M, Kobida L, Kogan G, Turianica I, Chlebo P, Hanackova E, Slamka P (2009) Regulation of the phenolic profile of berries can increase their antioxidant activity. J Agric Food Chem (in press) [DOI] [PubMed]
- Hura T, Hura K, Grzesiak S. Leaf dehydration induces different content of phenolics and ferulic acid in drought-resistant and -sensitive genotypes of spring triticale. Z Naturforsch C. 2009;64:85–95. doi: 10.1515/znc-2009-1-215. [DOI] [PubMed] [Google Scholar]
- Hura T, Hura K, Grzesiak S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J Plant Physiol. 2009;166:1720–1733. doi: 10.1016/j.jplph.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetrics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Jiménez C, Cossío BR, Rivard CJ, Berl T, Capasso JM. Cell division in the unicellular microalga Dunaliella viridis depends on phosphorylation of extracellular signal-regulated kinases (ERKs) J Exp Bot. 2007;58:1001–1011. doi: 10.1093/jxb/erl260. [DOI] [PubMed] [Google Scholar]
- Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. doi: 10.2307/3545850. [DOI] [Google Scholar]
- Kao WF, Hung DZ, Tsai WJ, Lin KP, Deng JF. Podophyllotoxin intoxication: toxic effect of bajiaolian in herbal therapeutics. Hum Exp Toxicol. 1992;11:480–487. doi: 10.1177/096032719201100607. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones. 1998;3:152–160. doi: 10.1379/1466-1268(1998)003<0152:SOTSIE>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226–246. [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Kotak S, Larkindale J, Lee U, Koskull-Döring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Kumaran KS, Prince PS (2010) Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones (in press) [DOI] [PMC free article] [PubMed]
- Kuzel S, Vydra J, Triska J, Vrchotova N, Hruby M, Cigler P. Elicitation of pharmacologically active substances in an intact medical plant. J Agric Food Chem. 2009;57:7907–7911. doi: 10.1021/jf9011246. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Jung E, Koh J, Kim YS, Park D. Effect of rosmarinic acid on atopic dermatitis. J Dermatol. 2008;35:768–771. doi: 10.1111/j.1346-8138.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Testa G, Sottero B, Gamba P, Poli G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr Med Chem. 2010;17(1):74–95. doi: 10.2174/092986710789957760. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang Y. Effects of cultivating measures on rhizome yield and some main active constituents of Curcuma longa L. Zhongguo Zhong Yao Za Zhi. 1999;24(531–533):574. [PubMed] [Google Scholar]
- Linde K. St. John's wort—an overview. Forsch Komplement Med. 2009;16:146–155. doi: 10.1159/000209290. [DOI] [PubMed] [Google Scholar]
- Linsenmair KE, Heil M, Kaiser WM, Fiala B, Koch T, Boland W. Adaptations to biotic and abiotic stress: Macaranda-ant plants optimize investment in biotice defence. J Exp Bot. 2001;52:2057–2065. doi: 10.1093/jexbot/52.363.2057. [DOI] [PubMed] [Google Scholar]
- Logan IR, McNeill HV, Cook S, Lu X, Meek DW, Fuller-Pace FV, Lunec J, Robson CN. Heat shock factor-1 modulates p53 activity in the transcriptional response to DNA damage. Nucleic Acids Res. 2009;37:2962–2973. doi: 10.1093/nar/gkp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZB, Janz D, Jiang X, Gobel C, Wildhagen H, Tan Y, Rennenberg H, Feussner I, Polle A. Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 2009;151:1902–1917. doi: 10.1104/pp.109.143735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum KK, Burnett AL, Meng X, Misseri R, Shaw MB, Gearhart JP, Meldrum DR. Liposomal delivery of heat shock protein 72 into renal tubular cells blocks nuclear factor-kappaB activation, tumor necrosis factor-alpha production, and subsequent ischemia-induced apoptosis. Circ Res. 2003;92:293–299. doi: 10.1161/01.RES.0000057754.35180.99. [DOI] [PubMed] [Google Scholar]
- Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Milton K. Micronutrient intakes of wild primates: are humans different? Comp Biochem Physiol A Mol Integr Physiol. 2003;136:47–59. doi: 10.1016/S1095-6433(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Nacif de Abreu I, Mazzafera P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem. 2005;43:241–248. doi: 10.1016/j.plaphy.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Nagy E, Balogi Z, Gombos I, Åkerfelt M, Björkbom A, Balogh G, Török G, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, et al. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. PNAS. 2007;104:7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche construction: the neglected process in evolution. Princeton: Princeton University Press; 2003. [Google Scholar]
- Oh SY, Kim JH, Park MJ, Kim SM, Yoon CS, Joo YM, Park JS, Han SI, Park HG, Kang HS. Induction of heat shock protein 72 in C6 glioma cells by methyl jasmonate through ROS-dependent heat shock factor 1 activation. Int J Mol Med. 2005;16:833–839. [PubMed] [Google Scholar]
- Oh MM, Carey EE, Rajashekar CB. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol Biochem. 2009;47:578–583. doi: 10.1016/j.plaphy.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita TK, Higashikubo R, Hunt CR. HSP70 and genomic stability. Cell Cycle. 2004;3:591–592. doi: 10.4161/cc.3.5.863. [DOI] [PubMed] [Google Scholar]
- Preisig-Müller R, Schwekendiek A, Brehm I, Reif HJ, Kindl H. Characterization of a pine multigene family containing elicitor-responsive stilbene synthase genes. Plant Mol Biol. 1999;39:221–229. doi: 10.1023/A:1006163030646. [DOI] [PubMed] [Google Scholar]
- Putics A, Végh EM, Csermely P, Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid Redox Signal. 2008;10:65–75. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- Quinn PJ, Joo F, Vígh L. The role of unsaturated lipids on membrane structure and stability. Prog Biophys Mol Biol. 1989;53:71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response. 2009;7:90–103. doi: 10.2203/dose-response.08-014.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008;94:643–648. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán A, Palacios V, Caro I, Pérez L. Resveratrol content of Palomino fino grapes: influence of vintage and fungal infection. J Agric Food Chem. 2003;51:1464–1468. doi: 10.1021/jf020774u. [DOI] [PubMed] [Google Scholar]
- Sánchez-Campillo M, Gabaldon JA, Castillo J, Benavente-García O, Baño MJ, Alcaraz M, Vicente V, Alvarez N, Lozano JA. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386–392. doi: 10.1016/j.fct.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Stress response and aging. Science. 2009;323:1021–1022. doi: 10.1126/science.1170007. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Foresti R, Calabrese V, Guiffrida Stell AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. J Cell Biol. 2001;80:213–221. doi: 10.1078/0171-9335-00158. [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Last RL, Pichersky E. Harnessing plant trichome biochemisty for the production of useful compounds. Plant J. 2008;54:702–711. doi: 10.1111/j.1365-313X.2008.03432.x. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress of life. 2. New York: McGraw-Hill; 1978. [Google Scholar]
- Shudo K, Fukasawa H, Nakagomi M, Yamagata N. Towards retinoid therapy for Alzheimer's disease. Curr Alzheimer Res. 2009;6:302–311. doi: 10.2174/156720509788486581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F. The Medditerranean diet revisited: evidence of its effectiveness grows. Curr Opin Cardiol. 2009;24:442–446. doi: 10.1097/HCO.0b013e32832f056e. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother. 2007;51:3367–3370. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Zhan JC, Yang HR, Huang WD. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J Plant Physiol. 2010;167:95–102. doi: 10.1016/j.jplph.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Terry LA, Chope GA, Bordonaba JG. Effect of water deficit irrigation and inoculation with Botrytis cinerea on strawberry (Fragaria x ananassa) fruit quality. J Agric Food Chem. 2007;55:10812–10819. doi: 10.1021/jf072101n. [DOI] [PubMed] [Google Scholar]
- Török Z, Tsvetkova NM, Balogh G, Horváth I, Nagy E, Pénzes Z, Hargitai J, Bensaude O, Csermely P, Crowe JH, et al. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. PNAS. 2003;100:3131–3136. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Emerg Ther Targets. 2001;5:267–287. doi: 10.1517/14728222.5.2.267. [DOI] [PubMed] [Google Scholar]
- Versari A, Parpinello GP, Tornielli GB, Ferrarini R, Guilivo C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J Agric Food Chem. 2001;49:5531–5536. doi: 10.1021/jf010672o. [DOI] [PubMed] [Google Scholar]
- Vidhyavathi R, Venkatachalam L, Sarada R, Ravishankar GA. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot. 2008;59:1409–1418. doi: 10.1093/jxb/ern048. [DOI] [PubMed] [Google Scholar]
- Vígh L, Horvàth I, Hasselt PR, Kuiper PJC. Effect of frost hardening on lipid and fatty acid composition of chloroplast thylakoid membranes in two wheat varieties of contrasting hardiness. Plant Physiol. 1985;79:756–759. doi: 10.1104/pp.79.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wan SB, Zhang P, Wang HL, Zhan JC, Huang WD. Prokaryotic expression, polyclonal antibody preparation of the stilbene synthase gene from grape berry and its different expression in fruit development and under heat acclimation. Plant Physiol Biochem. 2008;46:1085–1092. doi: 10.1016/j.plaphy.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Welch WJ. How cells respond to stress. Sci Am. 1993;268:56–64. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;232:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesse J, McPherson S, Odden MC, Shlipak MG. Effect of Opuntia ficus indica on symptoms of the alcohol hangover. Arch Intern Med. 2004;164:1334–1340. doi: 10.1001/archinte.164.12.1334. [DOI] [PubMed] [Google Scholar]
- Wieten L, Zee R, Goedemans R, Sijtsma J, Serafini M, Lubsen NH, Eden W, Boroere F. Hsp70 expression and induction as a readout for detection of immune modulatory components in foods. Cell Stress Chaperones. 2010;15:25–37. doi: 10.1007/s12192-009-0119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Zhao KJ, Huang ZG, Zhang P, Dong TT, Li SP, Tsim KW. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J Agric Food Chem. 2005;53:6019–6026. doi: 10.1021/jf0508495. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zhao C, Yin L, Yang R, Zeng X, Huang Y, Feng L, Yang X. Cloning of artemisinin biosynthetic cDNAs and novel ESTs and quantification of low temperature-induced gene overexpression. Sci China C Life Sci. 2008;51:232–244. doi: 10.1007/s11427-008-0032-x. [DOI] [PubMed] [Google Scholar]
- Zevenbergen H, Bree A, Zeelenberg M, Laitinen K, Duijn G, Flöter E. Foods with a high fat quality are essential for healthy diets. Ann Nutr Metab. 2009;59(Suppl 1):15–24. doi: 10.1159/000220823. [DOI] [PubMed] [Google Scholar]
- Zhang C, Fevereiro PS. The effect of heat shock on paclitaxel production in Taxus yunnanensis cell suspension cultures: role of abscisic acid pretreatment. Biotechnol Bioeng. 2007;96:506–514. doi: 10.1002/bit.21122. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hettiarachchy N, Chen P, Horax R, Cornelious B, Zhu D. Influence of the application of three different elicitors on soybean plants on the concentrations of several isoflavones in soybean seeds. J Agric Food Chem. 2006;54:5548–5554. doi: 10.1021/jf0531048. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic Biol Med. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]