Abstract
In order to verify the effects of heat and exercise acclimation (HA) on resting and exercise-induced expression of plasma and leukocyte heat shock protein 72 (Hsp72) in humans, nine healthy young male volunteers (25.0 ± 0.7 years; 80.5 ± 2.0 kg; 180 ± 2 cm, mean ± SE) exercised for 60 min in a hot, dry environment (40 ± 0°C and 45 ± 0% relative humidity) for 11 days. The protocol consisted of running on a treadmill using a controlled hyperthermia technique in which the work rate was adjusted to elevate the rectal temperature by 1°C in 30 min and maintain it elevated for another 30 min. Before and after the HA, the volunteers performed a heat stress test (HST) at 50% of their individual maximal power output for 90 min in the same environment. Blood was drawn before (REST), immediately after (POST) and 1 h after (1 h POST) HST, and plasma and leukocytes were separated and stored. Subjects showed expected adaptations to HA: reduced exercise rectal and mean skin temperatures and heart rate, and augmented sweat rate and exercise tolerance. In HST1, plasma Hsp72 increased from REST to POST and then returned to resting values 1 h POST (REST: 1.11 ± 0.07, POST: 1.48 ± 0.10, 1 h POST: 1.22 ± 0.11 ng mL−1; p < 0.05). In HST2, there was no change in plasma Hsp72 (REST: 0.94 ± 0.08, POST: 1.20 ± 0.15, 1 h POST: 1.17 ± 0.16 ng mL−1; p > 0.05). HA increased resting levels of intracellular Hsp72 (HST1: 1 ± 0.02 and HST2: 4.2 ± 1.2 density units, p < 0.05). Exercise-induced increased intracellular Hsp72 expression was observed on HST1 (HST1: REST, 1 ± 0.02 vs. POST, 2.9 ± 0.9 density units, mean ± SE, p < 0.05) but was inhibited on HST2 (HST2: REST, 4.2 ± 1.2 vs. POST, 4.4 ± 1.1 density units, p > 0.05). Regression analysis showed that the lower the pre-exercise expression of intracellular Hsp72, the higher the exercise-induced increase (R = −0.85, p < 0.05). In conclusion, HA increased resting leukocyte Hsp72 levels and inhibited exercise-induced expression. This intracellular adaptation probably induces thermotolerance. In addition, the non-increase in plasma Hsp72 after HA may be related to lower stress at the cellular level in the acclimated individuals.
Keywords: Exercise-heat stress, Heat and exercise acclimation, Heat shock protein 72
Introduction
Heat and exercise acclimation (HA) refers to an organism’s ability to perform exercise or work in high environmental temperatures (Robinson et al. 1943). HA is attained by a series of sessions of sustained increased core temperature, usually generated by performing work or exercise during repeated days in a hot environment (Moseley 1997). The most common reported adaptations to HA are lower rest and exercise core temperature, lower exercise heart rate and augmented sweat rate (Nadel et al. 1974). HA, however, is a complex process involving adaptations not only at whole-body but also at the cellular level (Horowitz 2002; Yamada et al. 2007; McClung et al. 2008).
Heat shock proteins (Hsps) are a highly conserved group of proteins that serve as molecular chaperones and accelerate cellular repair from heat stress, ischemia and endotoxic shock (Kregel 2002; Lau et al. 2000). The most thermosensitive and highly inducible Hsp belongs to the 70-kDa family (Mizzen and Welch 1988) and is commonly known as Hsp72. Although the function of intracellular Hsp72 (iHsp72) has been extensively investigated, it has also been suggested that Hsp72 has a functional role when released by a variety of cells (Febbraio et al. 2002a; Broquet et al. 2003; Hunter-Lavin et al. 2004; Lancaster et al. 2004) into the circulation (Pockley et al. 1998). Extracellular Hsp72 (eHsp72) is thought to stimulate innate immunity (Matzinger 1994), act as a danger signal resulting in increased immune responses and facilitate host defense to pathogenic challenges (Fleshner and Johnson 2005). For example, eHsp72 has been suggested to have an immunological function (Moseley 1998; Asea et al. 2000; Campisi and Fleshner 2003; Fleshner et al. 2003) with the attachment of eHsp72 to the surface of monocytes being found to stimulate the production of several cytokines in vitro (Asea et al. 2000).
While many studies have focused on the human Hsp72 response to acute exercise (Febbraio and Koukoulas 2000; Marshall et al. 2007; Morton et al. 2006; Reichsman et al. 1991; Ryan et al. 1991), changes in the human Hsp72 response that occur during HA have been less frequently investigated. Only two studies have examined iHsp72 expression in humans during a period of HA. Yamada et al. (2007) examined peripheral blood mononuclear cell Hsp72 expression during a period of 10 days of HA and found elevated baseline levels from day 6 through day 10. Similarly, McClung et al. (2008) found that a 10-day exercise-HA protocol increased baseline levels of iHsp72 and that the inducibility of Hsp72 to a 43°C ex vivo heat shock was blunted. Studies on the effects of HA on eHsp72 concentration show contrasting results. Extracellular levels of Hsp72 have been shown to decrease after the initial phase (2 days) (Marshall et al. 2006) and after 5 days of HA (Kresfelder et al. 2006), but no difference was found after a 10-day HA protocol (Yamada et al. 2007).
It also should be noticed that all studies that investigated the effects of HA on iHsp72 or eHsp72 have used constant workload protocols. This procedure has the drawback of reducing the adaptation stimulus as the subjects become acclimated. As increased core temperature is crucial for HA, in order to maintain the same stimulus, workload must be continuously adjusted so that the same elevation in core temperature is maintained throughout the period of HA. Thus, in the present study, we used the controlled hyperthermia technique (Fox et al. 1961, 1967; Cotter et al. 1997; Patterson et al. 2004) to induce HA. Therefore, the aim of the present study was to investigate the effects of a period of HA using the controlled hyperthermia technique on intracellular and extracellular levels of Hsp72 both at rest and after exercise. We hypothesized that after HA, the intracellular levels of Hsp72 would be increased at rest and that the induction after exercise would be blunted. We also hypothesized that extracellular resting levels of Hsp72 would be decreased after HA but that the exercise-induced increase would not be modified.
Materials and methods
Subjects
After responding to a medical questionnaire and being shown to be healthy, nine male subjects (25.0 ± 0.7 years; 80.5 ± 1.9 kg; 179.6 ± 1.7 cm, mean ± SE), inhabitants of a tropical region (Belo Horizonte, Minas Gerais, Brazil, latitude 19.5° S and longitude 43° W), volunteered to participate in this study. The subjects were nonsmokers and did not take any medication or drink alcohol during the period of the study. The study was conducted from June to September (end of fall and winter in the Southern Hemisphere). All procedures were in accordance with the Helsinki Declaration of 1975 and the local ethics committee of research approved the present protocol. An informed consent was obtained from all the subjects before the beginning of the study.
Preliminary testing
Subjects performed two aerobic capacity tests in a temperate environment (23 ± 0°C and 59.2 ± 0.4% relative humidity) 2–3 days before the beginning and the end of the HA protocol. The protocol consisted of exercising on a treadmill starting from 3 MET intensity followed by increases in speed and grade that accounted for increases in 3 MET (1 MET = 3.5 mL O2 kg−1 min−1) every 3 min until fatigue. Expired gases were collected and analyzed (BIOPAC System MP35, Goleta, CA/USA) for oxygen and carbon dioxide content breath-by-breath. Peak oxygen consumption (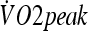 ) was considered the highest oxygen uptake value obtained during the test.
) was considered the highest oxygen uptake value obtained during the test.
HA protocol
All subjects underwent an 11-day HA protocol. HA was induced by a controlled hyperthermia protocol (Fox et al. 1961, 1967; Cotter et al. 1997; Patterson et al. 2004), which consisted of elevating rectal temperature (Tre) by 1°C in 30 min of treadmill exercise (1% grade and 2.20 ± 0.11 m s−1) and then maintaining it elevated for another 30 min (1% grade and 1.69 ± 0.11 m s−1) in an environmental chamber (Russels Technical Products, WMD 1150-5, Holland, MI, USA; dry bulb 40 ± 0°C, relative humidity 45.1 ± 0.2% rh and wind speed 1.4 ± 0.1 m s−1). During the days of HA, work rate was increased to maintain the target increase in Tre. Mean exercise running speed was 1.9 ± 0.1 m s−1 on the first day of HA and 2.1 ± 0.1 m s−1 on the last day of HA (p < 0.05). The HA protocol was completed on average within 13 days (range 12–15 days).
Heat stress test
Subjects performed a heat stress test 2 days before (HST1) and 2 days after (HST2) the HA period. Subjects reported to the laboratory 2 h before the test and ingested a standardized meal (1,720 kJ, 66 g of carbohydrate, 12 g of protein, 11 g of fat) in order to guarantee the same carbohydrate ingestion and avoid changes in the heat shock response as showed by Febbraio et al. (2004). They were instructed to collect a urine sample, void their bladders and insert a Tre probe (Yellow Spring Instruments, Series 400) 12 cm beyond the anal sphincter. Skin temperature probes (Yellow Spring Instruments, Yellow Springs, OH/USA, Series 400A) were attached to three skin sites: chest, arm, and thigh and mean skin temperature (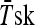 ) was calculated as follows (Roberts et al. 1977): 0.43 Tchest + 0.25 Tarm + 0.32 Tthigh. Mean body temperature (
) was calculated as follows (Roberts et al. 1977): 0.43 Tchest + 0.25 Tarm + 0.32 Tthigh. Mean body temperature (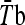 ) was calculated as 0.8 Tre + 0.2
) was calculated as 0.8 Tre + 0.2  . After that, 5 mL of venous blood was drawn in EDTA tubes for hematocrit and hemoglobin concentration, which were used to calculate the plasma volume variation according to Dill and Costill (1974). Subjects were then moved to a climatic chamber with the same environmental condition described above in the HA protocol, where they rested for 5 min in a seated position while the baseline measurements were recorded. After that, subjects ran on a treadmill (Quinton Medtrack ST65, Bothell, WA/USA) at 50% of their individual
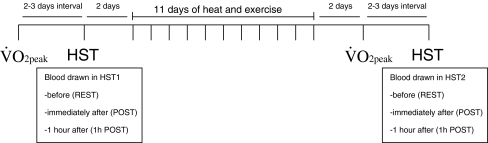
. After that, 5 mL of venous blood was drawn in EDTA tubes for hematocrit and hemoglobin concentration, which were used to calculate the plasma volume variation according to Dill and Costill (1974). Subjects were then moved to a climatic chamber with the same environmental condition described above in the HA protocol, where they rested for 5 min in a seated position while the baseline measurements were recorded. After that, subjects ran on a treadmill (Quinton Medtrack ST65, Bothell, WA/USA) at 50% of their individual 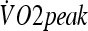 for 90 min. Rectal and skin temperatures were recorded at 6-min intervals. Heart rate was measured continuously (Polar Vantage NV, Kempele, Finland). Individual inability to sustain the predetermined workload or the attainment of a Tre of 39.5°C was taken as parameters to reduce the speed of the treadmill so that the subject could complete the 90 min of exercise. On six occasions in HST1 (three due to individual incapacity and three due to attainment of the limiting Tre) and on three occasions in HST2 (the same three subjects as in HST1, all due to individual incapacity), the running speed had to be reduced. Whole-body sweat rate was calculated by the difference in pre- and post-exercise body weights (Filizola® MF-100 scale, precision of 0.02 kg, São Paulo, Brazil), normalized by body surface area (AD; DuBois and DuBois 1916), divided by time, corrected for water ingested and uncorrected for metabolic or respiratory variations. Pilot studies showed that respiratory and metabolic variations accounted for less than 3% of total weight loss. In all experiments, subjects wore shorts, socks and running shoes. Tests were performed always between 15:30 and 17:00. Figure 1 outlines the procedures during the protocol.
for 90 min. Rectal and skin temperatures were recorded at 6-min intervals. Heart rate was measured continuously (Polar Vantage NV, Kempele, Finland). Individual inability to sustain the predetermined workload or the attainment of a Tre of 39.5°C was taken as parameters to reduce the speed of the treadmill so that the subject could complete the 90 min of exercise. On six occasions in HST1 (three due to individual incapacity and three due to attainment of the limiting Tre) and on three occasions in HST2 (the same three subjects as in HST1, all due to individual incapacity), the running speed had to be reduced. Whole-body sweat rate was calculated by the difference in pre- and post-exercise body weights (Filizola® MF-100 scale, precision of 0.02 kg, São Paulo, Brazil), normalized by body surface area (AD; DuBois and DuBois 1916), divided by time, corrected for water ingested and uncorrected for metabolic or respiratory variations. Pilot studies showed that respiratory and metabolic variations accounted for less than 3% of total weight loss. In all experiments, subjects wore shorts, socks and running shoes. Tests were performed always between 15:30 and 17:00. Figure 1 outlines the procedures during the protocol.
Fig. 1.
Procedures during the heat and exercise acclimation protocol. 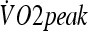 , peak oxygen consumption; HST, heat stress test
, peak oxygen consumption; HST, heat stress test
Hydration status and food intake
Subjects were instructed to drink 500 mL of tap water 2 h before each experimental day. Hydration status was assessed by urine-specific gravity (Ug) both before and after each experimental day using a portable refractometer (JSCP-Uridens, São Paulo, SP, Brazil). The subjects were considered euhydrated in all experimental days (Ug < 1.030; Armstrong 2000). During HST and HA periods, water intake was provided ad libitum (water temperature was 11.1 ± 0.1°C and 10.8 ± 0.2°C for HST1 and HST2, respectively; p > 0.05) during the entire test. Subjects ingested similar amounts of water in HST1 and HST2 (838 ± 117 and 782 ± 66 mL, respectively; p > 0.05). During the entire study, subjects were asked to maintain their habitual diet in order to diminish the effect of food intake variation on the variables measured. The diet content was analyzed using the DietPro 3.0 software (São Paulo, SP/Brazil), and it did not vary among the days of the protocol (11,800 ± 300 kJ/day, 55 ± 0.9% carbohydrates, 16 ± 0.5% proteins, 29 ± 0.9% fat, 1,980 ± 97 mg of sodium).
Isolation of leukocytes
For leukocyte isolation (mononuclear cells and granulocytes), 10 mL of blood was drawn in heparinized tubes three times: before HSTs (REST) and twice after HSTs, one immediately after HSTs (POST) and another after 1 h rest at room temperature (1 h POST). Blood was layered onto Histopaque-1119 (catalog no. 1119-1, Sigma-Aldrich, St. Louis, MO, USA) and centrifuged according to the manufacturer's instructions. Leukocytes were then collected and washed three times using cold PBS. After that, cells were counted on a hemacytometer under a microscope using 0.4% trypan blue {trypan blue solution [0.4% (w/v)] product number T8154, Sigma-Aldrich, St. Louis, MO, USA}; viability was expressed as a percentage (always higher than 95%). The pellet was stored at −80°C until analyses. The cells were then lysed in lyses buffer [20 mM Hepes (pH 7.4), 250 mM NaCl, 2 nM EDTA, 2 mM EGTA, 1 mM NaF, 1 M DTT, 100 mM PMSF and 1% NP-40], centrifuged and the supernatant was used in SDS-PAGE. Total protein concentration of the supernatant was measured using the Bradford assay.
iHsp72 analyses
Thirty micrograms of total protein was separated on an 8% polyacrylamide gel by electrophoresis. Five microliters of a protein standard (recombinant human hsp70 protein, catalog no. Nsp-555, Stressgen Bioreagents, Ann Habor, MI, USA) were used as positive control. Protein was transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA), and then nonspecific binding sites were blocked with 5% nonfat dry milk solution (10 mM Tris–HCl, pH 7.6, 150 mM NaCl and 0.1% Tween 20) for 2 h. To detect Hsp72, the primary antibody (1:1,000, mouse anti-HSP70 SPA810, Stressgen Bioreagents) was applied overnight, and the appropriate secondary antibody was applied for 1 h (1:3,000, horseradish peroxidase rabbit antimouse IgG1, Invitrogen, Carlsbad, CA/USA). Binding of the primary antibody was detected with the use of peroxidase-conjugated secondary antibody. Enhanced chemiluminescence reagents (Amersham Biosciences) were used to visualize the autoradiogram, which was later exposed to photographic film. The film was developed and the bands were analyzed using Scion Image software (Scion Corporation based on NIH image). Ratios were used to express differences in bands, with the first band (pre-exercise, day 1 control) being the control, or 1. The intensities for subsequent bands were divided by the intensity of the first sample and expressed as a ratio.
eHsp72 measurement
A commercially available enzyme-linked immunosorbent assay (EKS-700, Stressgen, lot # 506413) was used to determine Hsp72 concentration in plasma samples. One hundred microliters of prepared Hsp72 standard (0.78–50 ng/mL) and undiluted heparinized plasma samples were added to the microassay plate in duplicate. Wells were covered and incubated at room temperature for 2 h, with gentle rocking. After incubation, liquid was aspirated from all wells, and the wells were washed with wash buffer six times. One hundred microliters of anti-Hsp70: biotin antibody (diluted 1/500) was added to all wells and incubated for 1 h. After incubation, wells were washed a further six times before the addition of 100 μL avidin–HRP conjugate (diluted 1/10000). After 1 h of incubation, wells were washed as described previously and TMB substrate was added and incubated for 10 min at room temperature. One hundred microliters of acid stop solution were added to all wells, and absorbance was measured at 450 nm (EIA Reader, Model 2550, Bio-Rad, Hercules, CA/USA). The absorbance of the assay blank (0 ng/mL Hsp72) was subtracted from all other standards and samples to account for the background absorbance of reagents. The Hsp72 concentrations of each sample were then interpolated from the constructed standard curve plotted on a log–log scale. The concentration of Hsp72 in plasma samples was expressed as the weight of the protein per milliliter of plasma (ng/mL). All plasma samples were run with the same standard curve. Plasma Hsp72 concentrations were corrected for changes in plasma volume.
Statistical analyses
Based on previous studies (Yamada et al. 2007; McClung et al. 2008), we calculated that eight subjects would be required to detect a difference in iHsp72 expression and seven to detect a difference in eHsp72 concentration with a 95% probability and a power of 80%. A two-way repeated-measures analysis of variance was used to compare Hsp72 values, body temperatures and heart rate (HST vs. time), and a Student's t test was used to compare calculated whole-body sweat rate values. When a significant F value was found, we performed a Student–Newman–Keuls as a post hoc analysis. Regression analyses curves were constructed and the r value calculated. Pearson’s correlation was used to evaluate the significance. The α level was set at .05. Data are shown as mean ± SE, unless otherwise stated.
Results
Heat and exercise acclimation
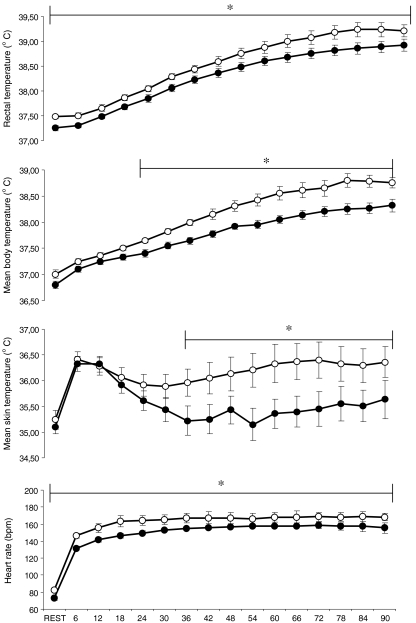
HA was confirmed, as the subjects exercised during HST2 with lower exercise Tre,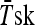 ,
,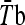 ody heart rate (Fig. 2) and showed higher
ody heart rate (Fig. 2) and showed higher 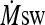 (HST1: 8.6 ± 1.0 and HST2: 10.0 ± 1.2 g m−2 min−1, p < 0.05). Subjects also exercised at a higher intensity in HST2 in comparison to HST1 (1.90 ± 0.03 and 2.04 ± 0.05 m s−1, respectively; p < 0.0001).
(HST1: 8.6 ± 1.0 and HST2: 10.0 ± 1.2 g m−2 min−1, p < 0.05). Subjects also exercised at a higher intensity in HST2 in comparison to HST1 (1.90 ± 0.03 and 2.04 ± 0.05 m s−1, respectively; p < 0.0001).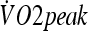 was not different before and after the adaptation period (50.5 ± 1.0 and 52.3 ± 1.3 mL kg−1 min−1, respectively; p = 0.11). Hematocrit (HST1: 44.8 ± 1.1 and HST2: 44.0 ± 1.1%, p < 0.05) and hemoglobin concentration (HST1: 16.2 ± 0.4 and HST2: 15.3 ± 0.4 g dL−1, p < 0.05) were diminished after the HA period, reflecting plasma volume expansion. In fact, calculated plasma volume expansion was 5.2 ± 1.3% (p < 0.001).
was not different before and after the adaptation period (50.5 ± 1.0 and 52.3 ± 1.3 mL kg−1 min−1, respectively; p = 0.11). Hematocrit (HST1: 44.8 ± 1.1 and HST2: 44.0 ± 1.1%, p < 0.05) and hemoglobin concentration (HST1: 16.2 ± 0.4 and HST2: 15.3 ± 0.4 g dL−1, p < 0.05) were diminished after the HA period, reflecting plasma volume expansion. In fact, calculated plasma volume expansion was 5.2 ± 1.3% (p < 0.001).
Fig. 2.
Rectal temperature (a, n = 9), mean skin temperature (b, n = 8), mean body temperature (c, n = 8) and heart rate (d, n = 9) during heat stress tests before (HST1, white circles) and after (HST2, black circles) the heat and exercise acclimation period. Values are mean with SEs. *p < 0.05, difference between HST
Before the HA, plasma volume decreased 8 ± 3% (range −13% to −4%) in the POST situation and 7 ± 3% (range −11% to −4%) in the 1 h POST situation. After the HA, plasma volume decreased 5 ± 4% (range −12% to −2%) in the POST situation and 1 ± 3% (range −6% to +2%) in the 1 h POST situation.
Intracellular expression of Hsp72
iHsp72 expression before and after the HA period is shown in Fig. 3. There were significant day × time interactions (p < 0.0001). Following HA, a baseline increase in iHsp72 expression was observed (p < 0.01). Furthermore, there was also an increase in iHsp72 expression in response to the exercise bout in HST1 (p < 0.01), and this increase was maintained after 1 h of rest following the exercise bout. However, this response was blunted in HST2 (REST vs. POST, p = 0.22; REST vs. 1 h POST, p = 0.45).
Fig. 3.
Intracellular Hsp72 expression before (REST), immediately after (POST) and after 1 h rest at room temperature (1 h POST) heat stress tests before (HST1, white bars) and after (HST2, black bars) the heat and exercise acclimation period. Values are means with SEs. *p < 0.05, different from HST1 at REST
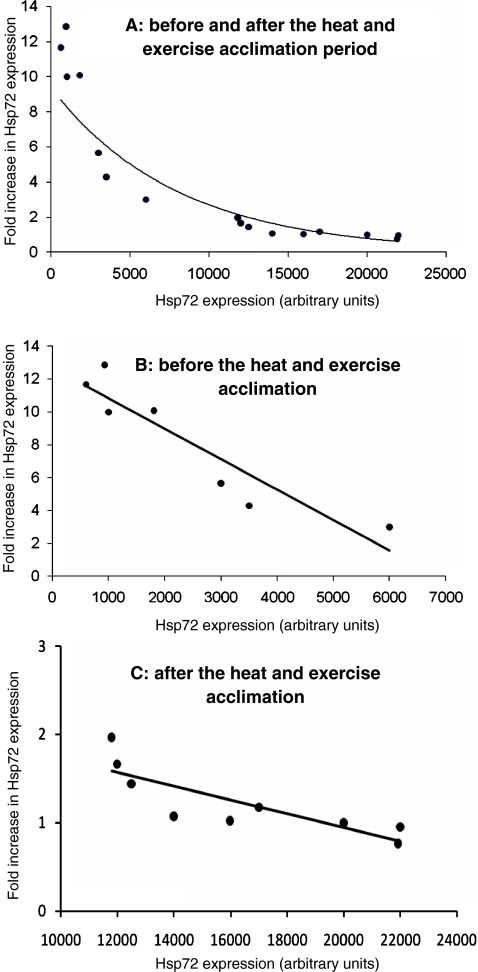
In order to investigate the relationship between pre-exercise and exercise-induced expression of iHsp72, we performed Pearson correlation analysis (Fig. 4). We observed a highly significant negative hyperbolic correlation between the pre-exercise expression and the increase in iHsp72 induced by exercise (Fig. 4a). A linear negative correlation is still found when data from before (Fig. 4b) and after (Fig. 4c) the acclimation period are analyzed separately.
Fig. 4.
Relationship between pre-exercise intracellular expression of Hsp72 and Hsp72 inducibility (fold induction from pre-exercise values). Pearson’s correlation showed a negative significant correlation between variables when data were analyzed together (a, before and after the acclimation period, R = −0.94, R2 = 0.91, p < 0.0001), or separately (b, before the acclimation period, R = −0.69, R2 = 0.83, p < 0.0001; c, after the acclimation period, R = −0.93, R2 = 0.86, p < 0.0001)
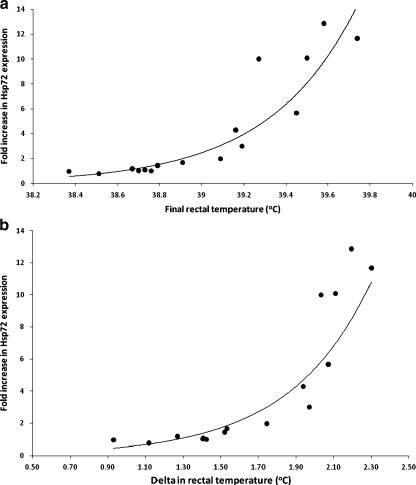
The induction of iHsp72 expression may occur when there is a certain increase in internal temperature irrespective of its initial value. To address this possibility, we analyzed the relationship between final rectal temperature and iHsp72 expression (Fig. 5a) and delta rectal temperature and iHsp72 expression in HSTs (Fig. 5b). We observed a highly significant positive correlation between iHsp72 induction and final rectal temperature (Fig. 5a) and delta rectal temperature (Fig. 5b). These results suggest that both final rectal temperature and/or delta rectal temperature influence iHsp72 expression during exercise and heat acclimation.
Fig. 5.
Relationship between iHsp72 expression and final rectal temperature and delta rectal temperature. Pearson’s correlation showed a positive significant correlation between iHsp72 expression and final rectal temperature (a, R = 0.95, R2 = 0.90) and rectal temperature variation (b, R = 0.93, R2 = 0.87)
We also performed a differential leukocyte count in all situations. Neither the total (HST1: 5,944 ± 856 cells vs. HST2: 5,828 ± 452 cells) nor the differential count (neutrophils, HST1: 65 ± 2.6% vs. HST2: 62 ± 2.3% and mononucleated cells, HST1: 35 ± 2.6% vs. HST2: 38 ± 2.3%) was different on REST before and after HA (p > 0.05 to all). On POST, there was an increase in the total leukocyte count both before and after HA compared to REST (p < 0.05). However, this increase was not different between HST1 and HST2 (total leukocyte count, HST1: 8,113 ± 656 cells vs. HST2: 8,714 ± 687 cells, p > 0.05). Despite this increase in the total leukocyte count, the differential count showed no difference between REST and POST. The differential count on POST was as follows: neutrophils, HST1: 63 ± 3.5% vs. HST2: 62 ± 2.1% and mononucleated cells, HST1: 37 ± 2.7 vs. HST2: 38 ± 2.1 cells. 1 h POST, the increase in the total leukocyte count persisted both before and after the HA (HST1: 9,612 ± 1,019 cells vs. HST2: 9,871 ± 743 cells); however, the differential count showed that the percentage of neutrophils increased both before and after HA (neutrophils, HST1: 75 ± 2.1% vs. HST2: 70 ± 2.2% and mononucleated cells, HST1: 25 ± 2.1% vs. HST2: 30 ± 2.2%, p < 0.05).
Extracellular concentration of Hsp72
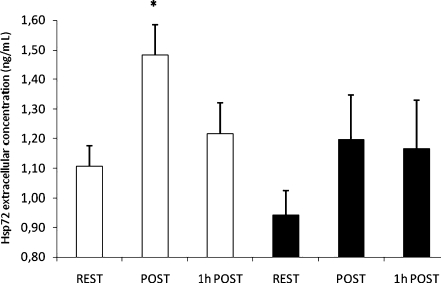
eHsp72 concentration is shown in Fig. 6. A significant main effect for eHsp72 [F(3,25), p = 0.04] was found. Post hoc test showed that before the HA period, an increase in eHsp72 was observed in response to the exercise bout (REST vs. POST, p < 0.05) and a return to pre-exercise levels was observed after 1 h rest following exercise (REST vs. 1 h POST, p = 0.53). No change in eHsp72 concentration was observed at baseline before and after HA. Moreover, after HA, no exercise-induced increase in eHsp72 was observed (REST vs. POST, p = 0.15; REST vs. 1 h POST, p = 0.22).
Fig. 6.
Extracellular hsp72 before (REST), immediately after (POST) and after 1 h rest at room temperature (1 h POST) heat stress tests before (HST1, white bars) and after (HST2, black bars) the heat and exercise acclimation period. Values are means with SEs. *p < 0.05, different from HST1 at REST
Discussion
The present study investigated the effects of HA using the controlled hyperthermia technique on intracellular and extracellular levels of Hsp72 both at baseline and after a bout of exercise. The major findings were (1) an increased baseline expression of iHsp72, (2) the exercise-heat-induced increase in intracellular and extracellular Hsp72 levels was inhibited and (3) the lower the iHsp72 expression at baseline, the higher its induction by exercise.
Heat and exercise acclimation
In this study we employed 11 days of controlled hyperthermia technique to induce HA in our subjects. The classic adaptations to HA were all observed in the present study, i.e., reduced resting and exercising internal temperature and heart rate, reduced exercising skin temperature, increased sweat rate and exercise tolerance. These data show that the protocol used in the present study was effective in inducing HA in our subjects.
Intracellular heat shock protein 72
An acute exercise-induced increase in iHsp72 expression before HA was observed in the present study. Interestingly, the previous studies of Yamada et al. (2007) and Marshall et al. (2007) did not observe such increase. The different internal temperatures induced in the two studies might explain this discrepancy. Of note, most of our subjects reached over 39°C core temperature; in fact, maximal Tre induced in the present study was 39.2°C on average. Furthermore, our subjects reached on average 39°C at 60 min of exercise, and maintained this internal temperature until the end of the protocol, while the subjects of previous studies did not reach 39°C on average. This 30 min with internal temperature over 39°C might have been sufficiently stressful to induce an increase in iHsp72.
The cell population isolated in the present study contained only leukocytes. In contrast, the previous studies of Hsp72 response in HA isolated peripheral blood mononuclear cells (Marshall et al. 2006; Yamada et al. 2007; McClung et al. 2008). The responses observed in the present study could be related to changes in circulating numbers of individual leukocyte populations. However, it is unlikely that the differences observed between the present study and other studies are due to the cell population analyzed, since the differential leukocyte count before and after the HA was not different under any of the conditions analyzed. Additionally, Fehrenbach et al. (2000) reported that the responses of monocytes and granulocytes expression of Hsp72 in people who ran a half marathon were similar. Therefore, the changes observed were due to changes in iHsp72 expression and not to changes in the differential leukocyte count.
Furthermore, baseline iHsp72 expression was increased following the HA protocol and the exercise-induced increase in Hsp72 was inhibited following HA. These responses are in agreement with our hypothesis and with recent studies on iHsp72 and HA in humans and animals. Maloyan et al. (1999) showed that passive HA increases basal expression of Hsp72 in rat cardiac muscle and reduces in vitro heat shock-induced increase in Hsp72. In 2007, Marshall et al. showed no change in iHsp72 expression after 2 days of exercise in the heat. Although some physiological adaptations were noticed, 2 days might not have been long enough for a complete acclimation, since this response may take 5–10 days (Lind 1963). Studying Hsp72 adaptation following 7 days of HA at the muscular level, Watkins et al. (2007) also did not observe any alteration in Hsp72 expression in the vastus lateralis, although the subjects of that study exercised for only 30 min daily, which might not have been sufficient to induce adaptation at the cellular level. More recently, Yamada et al. (2007) and McClung et al. (2008) showed that after 10 days of HA the baseline levels of iHsp72 increased. In their study, McClung et al. also observed that when cells from acclimated subjects were submitted to an in vitro heat shock, there was a blunted Hsp72 increase. Our results agree with those of Yamada et al. and McClung et al. showing that HA increases Hsp72 expression in humans. We expand beyond the in vitro findings to show in an in vivo model that when baseline levels of Hsp72 are elevated, the stress-induced increase in Hsp72 is blunted.
We show that in HST1 rectal and mean body temperatures were greater than in HST2, which could have affected the iHsp72 expression. However, rectal temperature variation in HST1 (1.02 ± 0.24°C) was not different in comparison to the HST2 (1.08 ± 0.21°C, p = 0.26), neither was mean body temperature variation (HST1: 1.04 ± 0.22°C and HST2: 1.02 ± 0.18°C, p = 0.39) or heat storage (HST1: 44.2 ± 4.7 W/m2 and HST2: 39.0 ± 3.3 W/m2, p = 0.09). Therefore, we believe that the thermal stimulus between the HSTs was not different and might not have influenced the iHsp72 response to exercise.
To better address the issue above, we analyzed the relationship between iHsp72 expression and final rectal temperature, and between iHsp72 expression and delta rectal temperature (Fig. 5). Although there was a clear relationship between internal temperature and iHsp72 expression (Fig. 5a), the delta rectal temperature also showed a relationship with iHsp72 induction (Fig. 5b). Although there may exist an internal temperature threshold for iHsp72 induction, it is also possible that this response occurs once a certain variation of internal temperature is reached.
The mechanism by which increased baseline Hsp72 may inhibit its own expression is related to Hsp72 binding to the heat shock factor 1 (HSF-1). In the unstressed cell, the HSF-1 is inactive and is present as a homodimer bound to Hsp72. Under stress, Hsp72 binds to denatured proteins and frees HSF-1 that trimerizes, migrates to the nucleus and binds to the heat shock element. This increases the intracellular levels of Hsp72, which can bind to HSF-1 and stop its own transcription (Morimoto 1998). Possibly, after HA, as the expression of Hsp72 is increased, the cellular stress to induce HSF-1 activation would have to be higher (McClung et al. 2008) and our results support this hypothesis. Although speculative, we propose that the increase in iHsp72 due to repeated increases in core temperature is an interesting adaptation at the cellular level because, under future stress, its transcriptional and translational machineries do not have to be directed to the synthesis of heat shock proteins. In that situation, once stress is inflicted upon the cell, its Hsp72 content is already enough to deal with that particular challenge and, therefore, normal cell function and cell homeostasis are maintained. The physiological significance of the increase in iHsp72 following HA is not known. The increase in iHsp72 has been associated with enhanced thermotolerance in vitro (Kregel 2002) and in vivo (Maloyan et al. 1999), and HA increases heat endurance in various species including humans (Patterson et al. 2004). Therefore, augmented iHsp72 may play a role in HA at the cellular level, enhancing cell tolerance to subsequent heat insults.
Extracellular heat shock protein 72
Serum HSP70 has been detected in peripheral circulation of apparent healthy individuals (Pockley et al. 1998) and increases in response to different stressors including exercise (Walsh et al. 2001). Increase in eHsp72 after a single bout of exercise has been previously observed (Walsh et al. 2001; Febbraio et al. 2004; Fehrenbach et al. 2005) and our results corroborate those findings, as increased eHsp72 (∼34%) was observed after the exercise bout before the HA.
Evidence suggests that eHsp can act as an inflammatory molecule and induce cytokine production in immune cells (Multhoff et al. 1999; Asea et al. 2000). Asea et al. (2000) showed that Hsp72 bound with high affinity to the plasma membrane and upregulated the expression of proinflammatory cytokines tumor necrosis factor-α, interleukin-1β and interleukin-6 in human monocytes.
The reason for extracellular increases in Hsp72 after exercise is not currently known, but it may partly contribute to the exercise-related inflammatory reaction which mainly happens after prolonged, intensive exercise (Asea 2003).
Although we observed an increase in eHsp72 before HA, this response was completely inhibited after HA. This finding was unexpected and contradicts our initial hypothesis. Only a few studies so far have investigated the effects of HA on extracellular concentration of Hsp72. Earlier, Kresfelder et al. (2006) also showed that baseline eHsp72 levels decreased after 5 days of HA, but only in subjects who showed signs of HA, while subjects who were not acclimated showed an increase in eHsp72. More recently, Yamada et al. (2007) showed no alterations in eHsp72 after either exercise or HA. The reason for such discrepancies between our results and previous studies is not clear. They may be related to the different HA protocols used, as previous studies used a constant workload protocol to induce HA, whereas in the present study we used a controlled hyperthermia technique. As the controlled hyperthermia technique imposes constant thermal stress upon the organism during HA, we might have induced a greater degree of adaptation in our subjects. Other possible differences between the present and previous studies may be related to different exercise duration or to differences in blood handling [serum vs. heparinized plasma (Whitham and Fortes 2006)].
The source of eHsp72 is not completely known, but it has been shown that the liver (Febbraio et al. 2002a), the brain (Lancaster et al. 2004) and some cells including leukocytes (Hunter-Lavin et al. 2004), but not the skeletal muscle (Febbraio et al. 2002b), can release Hsp72 into the circulation. Johnson and Fleshner (2006) proposed that α-adrenergic stimulation is responsible for Hsp72 release into the circulation. Whitham et al. (2006) showed that exercise with caffeine supplementation led to a higher increase in eHsp72 and that this increase was associated with higher plasma levels of catecholamines. Therefore, it is possible that reduced sympathetic activation might have caused this diminished response after HA. This hypothesis is strengthened by previous data showing that HA does not change resting but reduces exercise plasma levels of epinephrine (Febbraio et al. 1994). On the other hand, heart rate elevation (the difference between final exercise and rest heart rates), an indirect measure of sympathetic activation in HST1, was not different in comparison to HST2 (HST1: 83 ± 5 and HST2: 81 ± 7 bpm, p = 0.18), and, thus, sympathetic activation may have been similar between the HSTs.
During the preparation of this manuscript, Ogura et al. (2008) showed that body temperature elevation during exercise is important for induction of exercise-increased eHsp72. In the present study, core temperature was higher in HST1 in comparison to HST2 and, therefore, temperature elevation might have been an important factor in eHsp72 elevation. However, as stated before, rectal and mean body temperature variation and heat storage were not different in HST1 and HST2. At present, it is not known whether eHsp72 increases in response to a threshold temperature or to a temperature variation in vivo.
Even though the significance of a post-exercise increase in eHsp72 remains unclear, it has been proposed to have immunological functions (Matzinger 1994), to function as a danger signal to the organism and to activate various cytokines and inflammatory pathways (Asea et al. 2000). The release of Hsp72 into the circulation before the HA might indicate that the exercise bout in that situation was sensed by the organism as a stress capable of inducing an immunological response. However, as the subjects of the present study became adapted to heat, the exercise bout after the HA may not have been sensed by the organism as a stress capable of eliciting an immunological response and, therefore, there was no need for Hsp72 release into the circulation. We might even speculate that the higher iHsp72 observed after the HA may have protected the cells from the stress induced by the exercise bout, and, thus, a mechanism involving release of Hsp72 to induce an inflammatory response was inhibited.
Some limitations of the present study should be addressed. We measured iHsp72 expression on total leukocyte population instead of measuring intracellular expression of Hsp72 in monocytes and neutrophils separately. The observed responses were probably related to Hsp72 expression in neutrophils as this population represents the highest percentage of circulating leukocytes (>60%). However, the differential count was not different between PRE and POST neither before nor after the HA. Therefore, it is likely that the responses observed were indeed related to changes in iHsp72 expression and not to changes in the differential leukocyte count.
In conclusion, in the present study, we showed that HA led to (1) increased baseline levels of iHsp72 and (2) inhibition of the exercise-induced increase in intracellular and eHsp72. These findings expand beyond previous animal, human and in vitro studies and suggest that the repeated increase in internal temperature induce iHsp72 synthesis and that this enhanced level inhibits this further iHsp72 increase. Furthermore, eHsp72 does not increase after exercise following HA even with a similar mean body temperature increase.
Acknowledgements
The authors are indebted to Maria Aparecida Vasconcelos Faria, technician at the Exercise Physiology Laboratory, for helping in data collection and to Carlos Roberto Bueno Júnior and Stéphano Freitas Soares Melo from the Laboratory of Biochemistry of Physical Activity for helping in intracellular Hsp72 data analysis. We also thank the volunteers for their willingness to participate in the study. CAPES, FAPESP, FAPEMIG and CNPq supported this study.
Abbreviations
- HA
heat and exercise acclimation
- iHsp72
intracellular heat shock protein 72
- eHsp72
extracellular heat shock protein 72
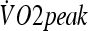
peak oxygen consumption
- HST
heat stress test
- AD
body surface area
- Ug
urine-specific gravity
- Tre
rectal temperature
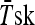
mean skin temperature
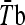
mean body temperature
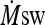
whole-body sweat rate
Footnotes
Study concept and design: Magalhães and Rodrigues; acquisition of data: Magalhães, Passos, Fonseca, Moreira, Lima, Bohen, Ferreira Jr., Martini; analysis and interpretation of data: Magalhaes, Rodrigues, Menezes, Trigueiro, Lima, Soares; drafting of the manuscript: Magalhaes, Rodrigues, Trigueiro; critical revision of the manuscript: Menezes; study supervision: Rodrigues.
References
- Armstrong LE. Performing in extreme environments. Champaign, Illinois, USA: Human Kinetics; 2000. [Google Scholar]
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;13:21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Campisi J, Fleshner M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol. 2003;94:43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- Cotter JD, Patterson MJ, Taylor NA. Sweat distribution before and after repeated heat exposure. Eur J Appl Physiol Occup Physiol. 1997;76:181–186. doi: 10.1007/s004210050232. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intl Med. 1916;17:836–837. [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol. 1994;76:589–597. doi: 10.1152/jappl.1994.76.2.589. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol. 2002;538:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones. 2004;9:390–396. doi: 10.1379/CSC-24R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C, Dickhuth HH, Northoff H (2000) HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc 32:592–600 [DOI] [PubMed]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Johnsonl JD. Endogenous extra-cellular heat shock protein 72: releasing signail(s) and function. Int J Hyperthermia. 2005;21:457–471. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Johnson JD. Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc Immunol Rev. 2003;9:6–24. [PubMed] [Google Scholar]
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization of the sweating mechanism in man. Proc Physiol Soc. 1961;14:56P–57P. [Google Scholar]
- Fox RH, Goldsmith R, Hampton IFG. Heat acclimatization by controlled hyperthermia in hot-dry and hot-wet climates. J Appl Physiol. 1967;22:39–46. doi: 10.1152/jappl.1967.22.1.39. [DOI] [PubMed] [Google Scholar]
- Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:475–483. doi: 10.1016/S1095-6433(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kresfelder TL, Claassen N, Cronjé MJ. Hsp70 induction and hsp70 gene polymorphisms as indicators of acclimatization under hyperthermic conditions. J Therm Biol. 2006;31:406–415. doi: 10.1016/j.jtherbio.2006.02.001. [DOI] [Google Scholar]
- Lancaster GI, Møller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1439–H1445. doi: 10.1152/ajpheart.2000.278.5.H1439. [DOI] [PubMed] [Google Scholar]
- Lind AR. Physiological effects of continuous or intermittent work in the heat. J Appl Physiol. 1963;18:57–60. doi: 10.1152/jappl.1963.18.1.57. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol. 1999;276:R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Marshall HC, Ferguson RA, Nimmo MA. Human resting extracellular heat shock protein 72 concentration decreases during the initial adaptation to exercise in a hot, humid environment. Cell Stress Chaperones. 2006;11:129–134. doi: 10.1379/CSC-158R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HC, Campbell SA, Roberts CW, Nimmo MA. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol. 2007;32:341–348. doi: 10.1016/j.jtherbio.2007.04.003. [DOI] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988;106:1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol. 2006;101:176–182. doi: 10.1152/japplphysiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and the inflammatory response. Ann N Y Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, Kampinga HH, Laumbacher B, Johnson J (1999) Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol 27:1627–1636 [DOI] [PubMed]
- Nadel ER, Pandolf KB, Roberts MF, Stolwijk JAJ. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Naito H, Akin S, Ichinoseki-Sekine N, Kurosaka M, Kakigi R, Sugiura T, Powers SK, Katamoto S, Demirel HA. Elevation of body temperature is an essential factor for exercise-increased extracellular heat shock protein 72 level in rat plasma. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1600–R1607. doi: 10.1152/ajpregu.00581.2007. [DOI] [PubMed] [Google Scholar]
- Patterson MJ, Stocks JM, Taylor NA. Humid heat acclimation does not elicit a preferential sweat redistribution toward the limbs. Am J Physiol Regul Integr Comp Physiol. 2004;286:R512–R518. doi: 10.1152/ajpregu.00359.2003. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Reichsman F, Scordilis SP, Clarkson PM, Evans WJ. Muscle protein changes following eccentric exercise in humans. Eur J Appl Physiol Occup Physiol. 1991;62:245–250. doi: 10.1007/BF00571547. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Wenger CB, Stolwijk JAJ. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol. 1977;43:133–137. doi: 10.1152/jappl.1977.43.1.133. [DOI] [PubMed] [Google Scholar]
- Robinson S, Turrell ES, Belding HS. Rapid acclimatization to work in hot climates. Am J Physiol. 1943;140:168–176. [Google Scholar]
- Ryan AJ, Gisolfi CV, Moseley PL. Synthesis of 70 K stress protein by human leukocytes: effect of exercise in the heat. J Appl Physiol. 1991;70:466–471. doi: 10.1152/jappl.1991.70.1.466. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AM, Cheek DJ, Harvey AE, Goodwin JD, Blair KE, Mitchell JB. Heat shock protein (HSP-72) levels in skeletal muscle following work in heat. Aviat Space Environ Med. 2007;78:901–905. [PubMed] [Google Scholar]
- Whitham M, Fortes MB. Effect of blood handling on extracellular Hsp72 concentration after high-intensity exercise in humans. Cell Stress Chaperones. 2006;11:304–308. doi: 10.1379/CSC-212.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, Walker GJ, Bishop NC. Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol. 2006;101:1222–1227. doi: 10.1152/japplphysiol.00409.2006. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103:1196–1204. doi: 10.1152/japplphysiol.00242.2007. [DOI] [PubMed] [Google Scholar]