Abstract
We have previously identified the CCT subunit eta as specifically reduced in healing fetal skin wounds by differential display, and observed that this reduction is not seen with any other CCT subunit. We now report the cloning and characterization of the cDNAs for rabbit CCT-eta and its closest evolutionary homolog, CCT-beta. Quantitative examination of CCT-eta and –beta message expression in healing fetal and adult wounds at 12 h post-injury confirms that CCT-eta mRNA is decreased in fetal wound tissues, but actually elevated in adult wound tissues. CCT-beta mRNA, in contrast, remains unchanged in both fetal and adult wound tissues. CCT-eta mRNA remains persistently elevated in healing adult wounds for 28 days following injury, whereas CCT-beta mRNA remains invariant throughout. CCT-eta protein is similarly increased, whereas CCT-beta protein remains unchanged.  -smooth muscle actin (
-smooth muscle actin ( -SMA), a recognized substrate of CCT known to be important in integumentary wound healing, was also measured over the course of wound healing, and both mRNA and protein levels were elevated throughout the 28 days.
-SMA), a recognized substrate of CCT known to be important in integumentary wound healing, was also measured over the course of wound healing, and both mRNA and protein levels were elevated throughout the 28 days.
Keywords: CCT, CCT-eta, Chaperonin, Wound healing, Alpha smooth muscle actin
Introduction
In contrast to adult (postnatal) wound healing, fetal skin wounds heal rapidly with minimal inflammation and no scar formation (Burrington 1971; Krummel et al. 1987; Siebert et al. 1990). Multiple studies have pointed out the biochemical, physiological and mechanical differences (reviews by Yannas 2005; Wilgus 2007; Hantash et al. 2008; Buchanan et al. 2009) observed between fetal and adult skin wound healing, but as yet the critical factors determining the disparity in healing outcome have yet to be identified. Studies from our laboratory have suggested that the eta subunit of the chief cytosolic chaperone, chaperonin containing T-complex polypeptide (CCT), may play a crucial role in differentiating scarless fetal healing from the scirrhous healing of adult skin wounds (Darden et al. 2000; Satish et al. 2008).
CCT is a 16-subunit complex composed of two stacked rings, each composed of eight polypeptide subunits (alpha, beta, gamma, delta, epsilon, eta, theta, and zeta) which are encoded by eight different genes (Rommelaere et al. 1993; Kubota et al. 1994, 1995). The primary substrates for CCT appear to be the cytoskeletal proteins (eg. tubulin and actin) (Sternlicht et al. 1993; Willison and Kubota 1994; Kubota et al. 1995), but the CCT complex is estimated to interact with up to 15% of all cellular proteins and has been implicated in a variety of processes including embryogenesis, ciliary biogenesis, cell viability, and cell proliferation. Alterations in CCT components, therefore, have the potential to cause pleiotropic effects on cellular physiology.
We have previously identified the CCT subunit eta to be reduced in our rabbit model of fetal skin wound healing by differential display and semi-quantitative RT-PCR (Darden et al. 2000). We have observed that no other chaperonin subunit shares this fetal-specific pattern of decrease at the tissue level (Satish et al. 2008), and have further noted that fibroblasts from fetal skin tissues express substantially less CCT-eta mRNA than do fibroblasts from adult skin (Satish et al. manuscript in preparation). We were therefore interested to more fully and precisely characterize the expression pattern of CCT-eta in healing wounds.
Since our model system is rabbit, and because full genomic sequence data was unavailable, we first cloned and sequenced the cDNAs for rabbit CCT-eta and, by way of comparison, CCT-beta, the CCT subunit most closely evolutionarily related to –eta. We then designed and validated primer and probe sequences that would allow quantitation of the CCT subunit messages. These quantitative assays were then used to determine the mRNA expression patterns of both CCT subunits in adult and fetal skin wound and control tissues. We also examined CCT-eta and CCT-beta expression at the protein level. Finally, because actin is a known substrate of the CCT hexadecameric enzyme, and because alpha-smooth muscle actin ( -SMA) is specifically important to the wound healing and scarring process, we also examined the time course of
-SMA) is specifically important to the wound healing and scarring process, we also examined the time course of  -SMA message and protein expression in healing adult wounds.
-SMA message and protein expression in healing adult wounds.
Materials and methods
Animal wounding and RNA isolation
The Institutional Animal Care and Use Committee (IACUC) reviewed and approved all animal protocols, and guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals were followed. The wounding protocol for our New Zealand white rabbits, and RNA isolation/storage from fetal control (FC), fetal wound (FW), adult control (AC), and adult wound (AW) tissues was performed as previously described (Darden et al. 2000; Kathju et al. 2006; Satish et al. 2008). In brief, pregnant New Zealand white rabbits at 20–21 days gestation were operated via midline laparotomy under general anesthesia. The uterine horns were delivered and limited hysterotomies were performed so as to allow 1 cm linear full-thickness dorsal integumentary incisonal wounds to be placed on selected fetuses; no more than 2–3 fetuses were operated per animal. The amniotic volume was then replaced with pre-warmed saline or Plasmalyte solution, the hysterotomies sutured closed, and the laparotomy incision closed. The shaved dorsums of the adult rabbits were exposed and 2 cm full thickness incisional wounds were placed bilaterally, again taking care to not violate the subcutaneous tissue. These adult incisional wounds were covered by Opsite dressings to allow for undisturbed wound healing. After 12 h, operated rabbits were reanesthetized and a 0.5–1 mm zone of tissue around the wound site was harvested (FW), as well as unwounded fetal skin (FC) from control littermates. Wounded and control adult skin tissue was also harvested and stored immediately at RNAlater® (Ambion, Austin, TX).
To monitor adult wound healing over a longer time course, non-pregnant rabbits carrying adult wounds only were followed to 28 days post-injury, with periodic sacrifice and harvesting of wound and control tissues at indicated intervals. The quality and quantity of total RNA extracted from fetal and adult wounded and control tissues were determined by measuring the OD 260/OD 280 ratio using an ND-1000 spectrophotometer (Nanodrop Technologies Inc., Wilmington, DE) and by capillary electrophoresis with the Agilent 2100 BioAnalyzer (Agilent Technologies Inc., Palo Alto, CA).
Full length cloning and sequencing of rabbit CCT-eta and CCT-beta cDNA
Our working model is a rabbit system, and we first cloned the rabbit CCT-eta and CCT-beta cDNAs; this then allowed us to generate rabbit-appropriate molecular agents for further investigations. We first used the available mouse and human CCT gene sequences to identify regions that are exactly conserved between the two, reasoning that these would likely be highly similar in rabbit as well. Primer sets to CCT-eta and –beta sequences were designed using Primer Express software, and used in RT-PCR from rabbit fetal control RNA to generate amplimers of the predicted molecular weights. These amplimers were then subcloned and sequenced, providing a large segment of rabbit cDNA sequence for both molecules. Further primers were then designed from this obtained sequence for use in 5′- and 3′-RLM-RACE (RNA-ligase mediated rapid amplification of cloned ends) reactions as per the manufacturer’s specifications (GeneRacer, Invitrogen Corp.) The resultant 5′ and 3′ RACE amplimers were themselves subsequently subcloned and sequenced, and a composite draft sequence of the entire cDNA was assembled and then verified experimentally using end-sequence primers spanning the entire cDNA length.
Quantitative real time RT-PCR
Total RNA extracted (RNeasy Mini Kit, Qiagen Inc., Valencia, CA) from FC, FW, AC and AW tissues were subjected to quantitative comparative RT-PCR assays to determine the relative mRNA expression levels of CCT-eta and -beta as well as α-SMA. Primers and probes for these assays were designed by Primer Express software (Applied Biosystems, Foster City, CA) and are listed in Table 2. The primers were obtained from Integrated DNA Technologies and the Taqman probes were purchased from Applied Biosystems. The experimental conditions for RT-PCR were carried out exactly as described previously (Satish et al. 2008). All primer sets yielded amplimers of the expected molecular weight, and the sequence of these amplimers was verified. Using the comparative critical cycle (Ct) method and using GAPDH as the endogenous control, the expression levels of the target gene products were normalized and relative abundance was calculated. Data were analyzed using the 7900 HT SDS software version 2.1 provided by Applied Biosystems.
Table 2.
Primer and probe sequences used for quantitative real time RT-PCR
| Gene | Sequences |
|---|---|
| Rabbit CCT-eta Forward | 5′-GTGGTGCTGAGCAGTTTATGGA-3′ |
| Rabbit CCT-eta Reverse | 5′-TTCTTAATGGCCCTCCTGACA-3′ |
| Rabbit CCT-eta Probe | 5′-6 FAM- AGACGGAGCGGTCCCTGCATGA-TAMRA-3′ |
| Rabbit CCT- beta Forward | 5′-CATCAAAGCAGCACCAAGGA-3′ |
| Rabbit CCT- beta Reverse | 5′- TGGTCCAGAGATCAAGATGCAT-3′ |
| Rabbit CCT- beta Probe | 5′- 6 FAM -TGATCACCATCCTTGTTAAGCATACCCATATAMRA-3′ |
| Rabbit GAPDH Forward | 5′- CGCCTGGAGAAAGCTGCTAA-3′ |
| Rabbit GAPDH Reverse | 5′- CTCGGTGTAGCCCAGGAT-3′ |
| Rabbit GAPDH Probe | 5′-6 FAM-AAGCAGGCATCCGAGGGCCC-TAMRA-3′ |
| Rabbit α-SMA Forward | 5′-GACAGCTACGTGGGTGATGAAGC-3′ |
| Rabbit α-SMA Reverse | 5′-TCGTCCCAGTTGGTGATGATG-3′ |
| Rabbit α-SMA Probe | 5′-6 FAM-ACCTTGAAGTACCCGATCGAACATGGC-TAMRA-3′ |
The primers and probes were designed using PRIMER EXPRESS® software v2.0 provided by Applied Biosystems.
Immunoblotting
Proteins were extracted from unwounded control and wounded adult skin using Tissue Protein Extraction Reagent (T-PER) obtained from Thermo Fisher Scientific (Rockford, IL). Protein concentration was measured using the Bradford assay. Equal quantities of protein extract were resolved by SDS-PAGE and transferred to a Whatman™ Protran pure nitrocellulose immobilization membrane (GE Health Care, Piscataway, NJ). The membranes were probed with antibodies specific for CCT-eta (AbD SeroTec, Raleigh, NC) CCT-beta (AbD SeroTec), and α-SMA (Abcam, Cambridge, MA), conjugated with HRP-labelled secondary antibody, and the signals detected using Amersham™ ECL™- Western Blotting Detection System obtained from GE Health Care. To confirm equal loading of proteins, immunoblots were probed against GAPDH (Abcam). The band intensity was measured using the AlphaImager from Alpha Innotech Corporation (San Leandro, CA).
Results
Cloning and sequencing of CCT-eta and CCT-beta subunits
Our initial differential display experiments demonstrated that the chaperonin containing T-complex polypeptide (CCT) subunit eta is reduced in a healing fetal wound but not in an adult wound (Darden et al. 2000). Further, semi-quantitative RT-PCR assays showed that no other chaperonin subunit shares this fetal wound-specific decrease (Satish et al. 2008). In order to better develop molecular tools for the study of CCT-eta in our model system, we first cloned the rabbit CCT-eta cDNA (GenBank accession number FJ349099). The rabbit cDNA clone was highly homologous as expected to both mouse and human cDNA sequences, with a total length of 1,875 bp, a 5′ UTR of 78 bp, a 3′ UTR of 165 bp, with a consensus polyadenylation signal at nucleotide 1,829 bp.
CCT-beta has been described as the most closely evolutionarily related subunit to CCT-eta (Archibald et al. 2000; Fares and Wolfe 2003) and showed no apparent differential expression in either healing fetal wound or adult wound tissues; these characteristics recommended it as the most appropriate CCT subunit for comparison with CCT-eta. We therefore cloned the rabbit CCT-beta cDNA sequence as well (GenBank accession number FJ349100); unsurprisingly, it too was highly homologous to mouse and human sequence. The CCT-beta cDNA spans 1,931 bp, with 82 bp of 5′ UTR, 240 bp of 3′ UTR, and a consensus polyA sequence at nucleotide 1,892 bp.
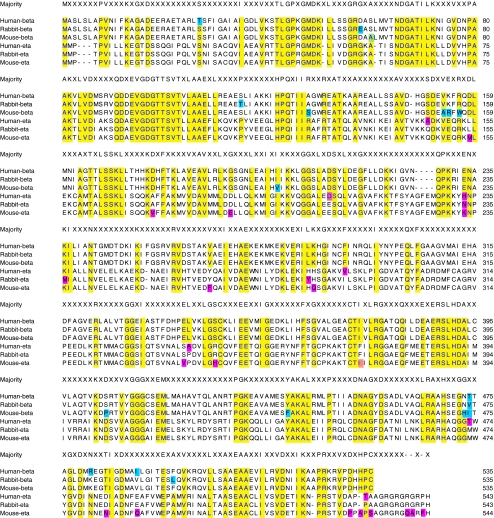
Figure 1 show the amino acid alignments of human, rabbit, and mouse CCT-eta and –beta protein sequences. A substantial degree of homology across subunits and species is readily apparent. Table 1 shows the percent identity values of each sequence pair among CCT-eta and beta subunits from human, rabbit and mouse.
Fig. 1.
Amino acid alignment of the CCT beta and eta subunits from human, rabbit and mouse. Yellow: residues that align to a consensus created from all six sequences; blue: residues that differ amongst the highly similar beta subunits; pink: residues that differ amongst the highly similar eta subunits
Table 1.
The percent identity values of each sequence pair amongst CCT-eta and beta subunits from human, mouse, and rabbit
| Human-beta | Rabbit-beta | Mouse-beta | Human-eta | Rabbit-eta | Mouse-eta | |
|---|---|---|---|---|---|---|
| Human-beta | 100 | 98.7 | 97.6 | 31.4 | 30.8 | 31.4 |
| Rabbit-beta | 100 | 97.6 | 31.4 | 31 | 31.6 | |
| Mouse-beta | 100 | 30.7 | 30.3 | 30.8 | ||
| Human-eta | 100 | 98.3 | 95.4 | |||
| Rabbit-eta | 100 | 96.1 | ||||
| Mouse-eta | 100 |
With full-length rabbit cDNA sequences in hand for our subunits of interest, we then developed (and validated) quantitative real time RT-PCR assays for both CCT-eta and CCT-beta. The primers and probes used for these assays are listed in Table 2.
CCT-eta mRNA is reduced in fetal wounds and actually elevated in adult wounds, whereas CCT-beta mRNA remains unchanged
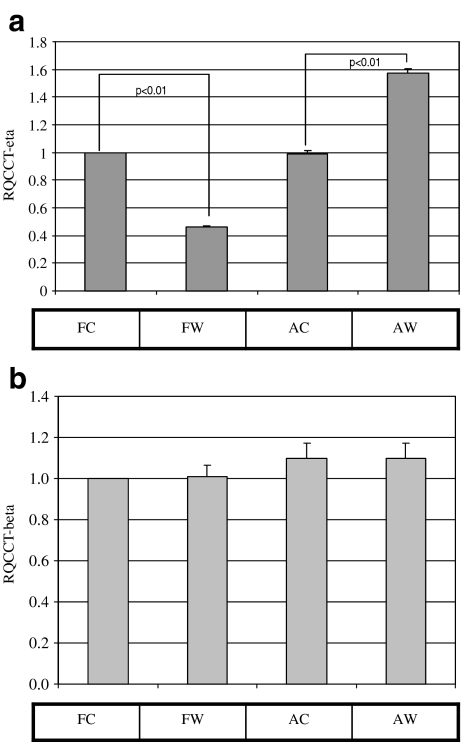
The quantitative RT-PCR assays for CCT-eta and –beta were first employed to verify our previous observations, specifically that CCT-eta mRNA is underexpressed in fetal wounds relative to fetal control, and that CCT-beta mRNA is in contrast unchanged. Direct examination of fetal and adult wound and control tissues showed that CCT-eta message was substantially reduced in healing fetal wounds, and actually elevated in healing adult cutaneous wounds by two-fold at 12 h post-injury. CCT-beta message remained unaltered in both fetal and adult wound tissues relative to control, confirming our previous observations (Satish et al. 2008), (Fig. 2a and b).
Fig. 2.
CCT-eta mRNA levels are reduced in fetal wounds; CCT-beta mRNA levels remain unchanged. RNA extracted from fetal and adult wounded and unwounded tissues at 12 h post-injury was subjected to qRT-PCR analysis. CCT-eta mRNA levels are significantly decreased in healing fetal wounds at 12 h post-injury and actually elevated in adult wounds (a). In contrast, CCT-beta message levels are unaffected by wounding in either fetal or adult tissues (b). FC-fetal control, FW-fetal wound, AC-adult control, AW-adult wound. Values are means ± SEM of six independent studies performed in duplicate. Statistical analyses were performed using Student’s t test
CCT-eta mRNA is persistently elevated in healing adult wounds, whereas CCT-beta mRNA remains unchanged
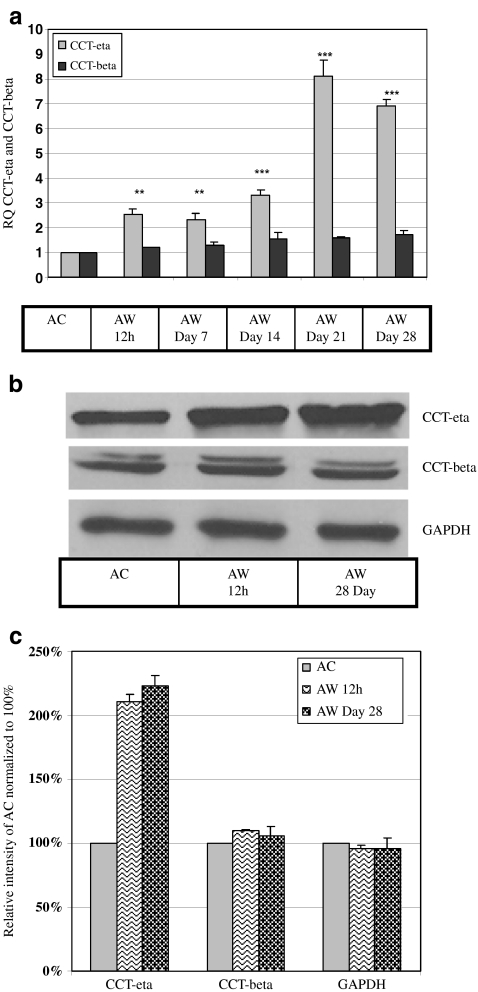
Our finding that CCT-eta (but not CCT-beta) mRNA was increased in adult wounds at 12 h lead us to investigate whether CCT-eta would remain elevated as wounds progressed to healing. We found that, at the mRNA level, CCT-eta expression remained elevated by some two- to three-fold through post-injury day 14, but appeared to increase substantially more at post-injury days 21 and 28. In sharp contrast, we observed no significant change in the overall expression of CCT-beta mRNA in wounded versus control tissues throughout the entire 28 day time course (Fig. 3a). No significant difference was observed in the amount of elevation of CCT-eta mRNA at 12 h post-injury in pregnant versus non-pregnant rabbits.
Fig. 3.
CCT-eta mRNA and protein levels remain elevated in healing adult wounds but CCT-beta levels remain constant. a RNA extracted from 12 h post-injury until 28 day post-injury from adult wounds was subjected to qRT-PCR analysis. CCT-eta mRNA levels gradually increased from 12 h and remain elevated until Day 28. In contrast, there was no significant difference in CCT-beta mRNA levels due to wounding. Values are means ± SEM of three independent studies performed in duplicate. Statistical analyses were performed using Student’s t test **p < 0.01; ***p < 0.001. b Equal amounts of protein loaded from post-injury time point 12 h and Day 28 showed substantial increase in CCT-eta protein levels, but CCT-beta protein remained constant. c Densitometry analysis of protein bands shows an increase in CCT-eta protein accumulation with no corresponding change in CCT-beta protein expression at 12 h and Day 28. GAPDH was used as the internal loading control. Blots shown here are representative of at least three different experiments. AC-adult control; AW-adult wound
CCT-eta protein is elevated in adult wounds, whereas CCT-beta protein remains unchanged
We next sought to determine whether CCT subunit protein levels would mirror the differential expression pattern exhibited by CCT-eta and CCT-beta mRNA. Tissue levels of CCT-eta and –beta protein were directly assayed by Western blot and quantitated; results are shown in Fig. 3b and c. CCT-eta protein was significantly elevated in wounded tissues by 12 h post-injury (211% ± 5.52% compared to adult control), and remained elevated at 28 days (223% ± 12.13% compared to adult control). CCT-beta, again in sharp contrast, displayed no significant variance in the amount of protein present in wounded versus control tissues.
α-SMA mRNA and protein levels are persistently elevated in healing adult wounds
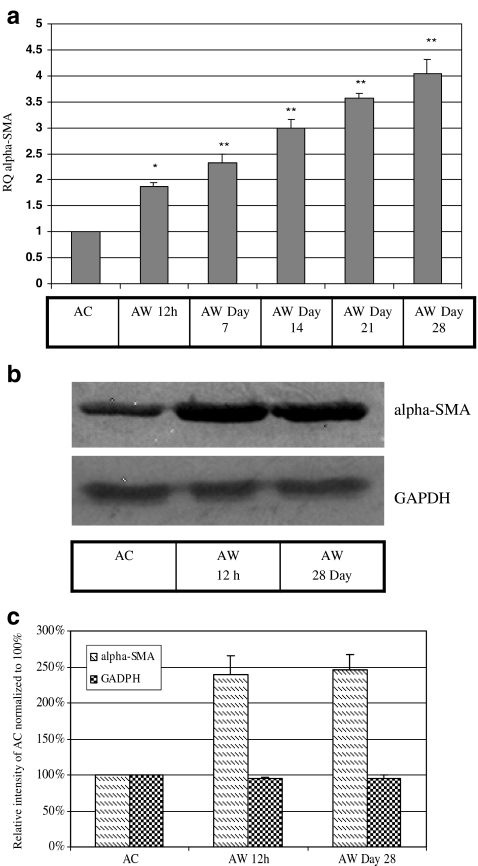
Because actin is a substrate of CCT, and because the α-SMA isoform is specifically known to be a characteristic feature of the transformation of fibroblasts to myofibroblasts during scirrhous wound healing, we also examined the expression of α-SMA in our model. We found that α-SMA mRNA was significantly increased by 12 h post-injury, and appeared to increase further (to four-fold versus unwounded control) at 28 days. α-SMA protein was similarly elevated by 12 h post-injury (240% ± 25% compared to adult control), and similarly remained elevated at 28 days (246% ± 22% compared to adult control) (Fig. 4a, b and c). This increased expression is consistent with the time course of accumulation of myofibroblast cells observed in other animal model systems, eg. minipig, where myofibroblasts appear shortly after wounding and persist for up to 8 weeks until wound closure (Rudolph et al. 1977).
Fig. 4.
α-SMA mRNA and protein levels are significantly and persistently increased in adult wounds. a Total RNA extracted from 12 h post-injury until day 28 post-injury from adult wounds and subjected to qRT-PCR analysis showed that α- SMA mRNA levels increased significantly from 12 h and remained elevated until Day 28. Values are means ± SEM of three independent studies performed in duplicate. Statistical analyses were performed using Student’s t test. **p < 0.01; *p < 0.05. b Western blots using α- SMA antibody (1:100) showed that adult wounds express significantly greater α- SMA protein from 12 h to Day 28. c Densitometry analysis of α- SMA protein bands showed persistently elevated expression at 12 h and Day 28. GAPDH was used as loading control. Shown here is a representative immunoblot of up to three similar such blots. AC-adult control; AW-adult wound
Discussion
Although a substantial amount of work has been carried out to mechanistically examine the function of the hexadecameric CCT molecule, comparatively less attention has been paid to the potential for discrete individualized functions for the CCT component subunits without the CCT holoenzyme. Our intriguing observation that only the CCT-eta subunit was identified as reduced in scarlessly healing fetal wound tissues by differential display led us to further examine whether CCT-eta may also conversely have a unique (and potentially countervailing) role in scirrhous adult wound healing. To further these investigations in our rabbit model, we herein report the cloning and characterization of the rabbit CCT-eta and CCT-beta cDNAs. Sequence analysis confirms that these subunit isoforms in rabbit have a significant degree of homology to each other (and to cognate human and mouse sequence), reinforcing the notion that they have evolved from a common ancestor gene.
Direct quantitation of CCT-eta mRNA confirmed our earlier observation that CCT-eta message is decreased in the fetal wound milieu. Interestingly, quantitative examination of adult wounds revealed an actual two-fold increase in CCT-eta message at 12 h post-injury, although our initial limiting dilution RT-PCR had suggested no change. Since the limiting dilution RT-PCR technique is admittedly semi-quantitative, we conclude that the increase reported herein is a more accurate representation of wound tissue biology. Direct quantitation of CCT-beta mRNA confirmed the invariant pattern of expression previously noted with limiting dilution RT-PCR.
More interestingly, CCT-eta message and protein were found to be persistently elevated as adult wounds progressed to healing, whereas CCT-beta message and protein expression remained unchanged. This finding is highly suggestive of a unique role for CCT-eta in wound healing biology, possibly outside its role as a CCT holoenzyme constituent. Since the CCT hexadecamer requires a fixed stoichiometry of subunits for its function, it may be argued that a specific increase in any single subunit implies a function separate from the subunit’s contribution to CCT chaperonin activity.
Multiple lines of evidence suggest that individual CCT subunits do have such secondary individual functions. In several systems an incoordinate pattern of CCT subunit expression has been observed, even as we see here (Satish et al. 2008; Tam et al. 2006; Kitamura et al. 2006). Two CCT subunit promoters (alpha and theta) have been functionally examined in HeLa cells, and found to be subject to the control of different sets of transcription factors, signifying an ability to respond differentially to cellular stimuli at the transcriptional level (Kubota et al. 2000; Yamazaki et al. 2003). Roobol and co-workers (1995) have observed that different CCT subunits localize dissimilarly intracellularly; if their only function in the cell were as members of the stoichiometrically fixed CCT hexadecamer, there would be no advantage to such an arrangement. Similarly, Coghlin et al. (2006) have demonstrated differing subcellular locations for CCT-beta versus CCT-epsilon in colorectal adenocarcinoma.
The most compelling evidence for individual subunit function is direct identification of an independent physiological activity for the subunit. In the case of CCT-eta such an activity has been demonstrated: as an inhibitory co-factor for the soluble guanylyl cyclase (sGC), the chief intracellular mediator of nitric oxide (NO) signaling (Hanafy et al. 2004). Since CCT-eta is elevated in healing adult wounds, it may be supposed that sGC activity is therefore suppressed, suggesting an inhibition of nitric oxide signaling in the wound milieu in toto. Since arginine (and other agents that stimulate nitric oxide signaling pathways) have been shown to have favorable effects on wound healing, we may speculate that the increase in CCT-eta seen here contributes to the scirrhous nature of adult wound healing by inhibiting NO-mediated effects. Conversely, we may speculate that reduced CCT-eta in healing fetal wounds allows for improved regenerative wound healing by allowing increased NO signaling.
Our own laboratory has identified a potentially even more direct connection between CCT-eta and wound healing/ scar formation: in fibroblasts in vitro it appears that CCT-eta but not CCT-beta is necessary for accumulation of α-SMA protein (Satish et al. manuscript in preparation). α-SMA is a marker for the transformation of fibroblasts to myofibroblasts in a healing wound, with myofibroblasts thought to be the principal effector agents behind the contractile forces of scar. Thus, increased CCT-eta may permit increased myofibroblast development and thereby increased scar contracture; our observation herein that α-SMA protein levels more or less track CCT-eta accumulation is congruent with this possibility. Conversely, the reduction of CCT-eta seen in healing fetal wounds may (by this model) inhibit scar development by preventing fibroblastic transformation to myofibroblasts.
More experimental work is required to dissect the mechanistic possibilities noted above (or others). Since the healing wound milieu is a complex mixture of cell types, especially in adults, identification of the specific cell populations expressing relatively increased CCT-eta versus CCT-beta will be informative. The interplay between CCT-eta and nitric oxide signaling vis a vis wound healing also warrants further investigation. Overall, the observations reported here contribute to an emerging body of evidence for a role for CCT-eta in determining wound healing biology, and contribute also to a growing mass of evidence to suggest possible unique physiological importances for CCT subunits not strictly confined to participation in the hexadecameric molecule.
Acknowledgements
This study was supported by the Allegheny Singer Research Institute, Allegheny General Hospital and Pittsburgh Tissue Engineering Institute (PTEI). This work was funded from the grants awarded to S.K. (DE014780; AFIRM), J.C.P. (DC 05659), G.D.E. (DC 04173) and L.S. (3M Fellowship). We extend our thanks to Ms. Mary O’Toole for her assistance in preparing this manuscript and Dr. N. Luisa Hiller for assisting in preparation of Fig. 1 and Table 1.
References
- Archibald JM, Logsdon JM, Jr, Doolittle WF. Origin and evolution of eukaryotic chaperonins: phylogenetic evidence for ancient duplications in CCT genes. Mol Biol Evol. 2000;17:1456–1466. doi: 10.1093/oxfordjournals.molbev.a026246. [DOI] [PubMed] [Google Scholar]
- Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–161. doi: 10.1016/S0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg. 1971;6:523–528. doi: 10.1016/0022-3468(71)90373-3. [DOI] [PubMed] [Google Scholar]
- Coghlin C, Carpenter B, Dundas SR, Lawrie LC, Telfer C, Murray GI. Characterization and over-expression of chaperonin t-complex proteins in colorectal cancer. J Pathol. 2006;210:351–357. doi: 10.1002/path.2056. [DOI] [PubMed] [Google Scholar]
- Darden DL, Hu FZ, Ehrlich MD, Gorry MC, Dressman D, Li HS, Whitcomb DC, Hebda PA, Dohar JE, Ehrlich GD. RNA differential display of scarless wound healing in fetal rabbit indicates downregulation of a CCT chaperonin subunit and upregulation of a glycophorin-like gene transcript. J Pediatr Surg. 2000;35:406–419. doi: 10.1016/S0022-3468(00)90204-5. [DOI] [PubMed] [Google Scholar]
- Fares MA, Wolfe KH. Positive selection and subfunctionalization of duplicated CCT chaperonin subunits. Mol Biol Evol. 2003;20:1588–1597. doi: 10.1093/molbev/msg160. [DOI] [PubMed] [Google Scholar]
- Hanafy KA, Martin E, Murad F. CCTeta, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem. 2004;279:46946–46953. doi: 10.1074/jbc.M404134200. [DOI] [PubMed] [Google Scholar]
- Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- Kathju S, Satish L, Rabik S, Rupert T, Oswald D, Johnson S, Hu FZ, Post JC, Ehrlich GD. Identification of differentially expressed genes in scarless wound healing utilizing polymerase chain reaction-suppression subtractive hybridization. Wound Repair Regen. 2006;14:413–420. doi: 10.1111/j.1743-6109.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Kitamura A, Kubota H, Pack CG, Matsumoto G, Hirayama S, Takahashi Y, Kimura H, Kinjo M, Morimoto RI, Nagata K. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol. 2006;8:1163–1170. doi: 10.1038/ncb1478. [DOI] [PubMed] [Google Scholar]
- Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, Cohen IK. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/S0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994;4:89–99. doi: 10.1016/S0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison K. The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- Kubota H, Yokata S, Yanagi H, Yura T. Transcriptional regulation of the mouse cytosolic chaperonin subunit gene Cctz/t-complex polypeptide 1 by selenocysteine tRNA gene transcription activating factor family zinc finger proteins. J Biol Chem. 2000;275:28641–28648. doi: 10.1074/jbc.M005009200. [DOI] [PubMed] [Google Scholar]
- Rommelaere H, Troys M, Gao Y, Melki R, Cowan NJ, Vandekerckhove J, Ampe C. Eukaryotic cytosolic chaperonin contains t-complex polypeptide 1 and seven related subunits. Proc Natl Acad Sci USA. 1993;90:11975–11979. doi: 10.1073/pnas.90.24.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A, Holmes FE, Hayes NV, Baines AJ, Carden MJ. Cytoplasmic chaperonin complexes enter neuritis developing in vitro and differ in subunit composition within single cells. J Cell Sci. 1995;108:1477–1488. doi: 10.1242/jcs.108.4.1477. [DOI] [PubMed] [Google Scholar]
- Rudolph R, Guber S, Suzuki M, Woodward M. The life cycle of the myofibroblast. Surg Gynecol Obstet. 1977;145:389–394. [PubMed] [Google Scholar]
- Satish L, Abdulally A, Oswald D, Johnson S, Hu FZ, Post JC, Ehrlich GD, Kathju S. Differential expression of chaperonin containing T-complex polypeptide (CCT) subunits during fetal and adult skin wound healing. Cell Stress Chaperones. 2008;13:527–533. doi: 10.1007/s12192-008-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JW, Burd AR, McCarthy JG, Weinzweig J, Ehrlich HP. Fetal wound healing: a biochemical study of scarless healing. Plast Reconstr Surg. 1990;85:495–502. doi: 10.1097/00006534-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB. The T-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci USA. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S, Geller R, Spiess C, Frydman J. the chaperonin TriC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol. 2006;8:1155–1162. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA. Regenerative healing in fetal skin: A review of the Literature. Ostomy Wound Manage. 2007;53:16–31. [PubMed] [Google Scholar]
- Willison KR, Kubota H. The structure, function, and genetics of the chaperonin containing TCP-1 (CCT) in eukaryotic cytosol. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1994. pp. 299–312. [Google Scholar]
- Yamazaki Y, Kubota H, Nozaki M, Nagata K. Transcritional regulation of the cytosolic chaperonin theta subunit gene, Cctq, by Ets domain transcription factors E1k-1, Sap-1a, and Net in the absence of serum response factor. J Biol Chem. 2003;278:30642–30651. doi: 10.1074/jbc.M212242200. [DOI] [PubMed] [Google Scholar]
- Yannas IV. Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J R Soc Interface. 2005;2:403–417. doi: 10.1098/rsif.2005.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]