Abstract
Celastrol, a novel HSP90 inhibitor, has recently attracted much attention due to its potential in multiple applications, such as anti-inflammation use, degenerative neuron disease relief, and tumor management. At present, the studies in celastrol's effects on HSP90's clients have focused on the kinase sub-population, while another key sub-population, nuclear transcription factors (TFs), is not being well-explored. In this study, we observe the effects of celastrol on 18 TFs (belonging to HSP90 clients) in three human cell lines: MCF-7 (breast cancer), HepG2 (hepatoma), and THP-1 (monocytic leukemia). The results show that at least half of the detectable TFs were affected by celastrol, though the effect patterns varied with cell type and dosage. Bi-directional regulations of some TFs were identified, a phenomenon not yet seen with other HSP90 inhibitors. Celastrol's capability to affect multiple TFs was consistent with its altering HSP90/TFs interactions and disrupting HSP90/Hop interaction, in addition to the reported damaging HSP90/Cdc37 interaction. This work confirms, for the first time, that celastrol has broad effects on TFs belonging to HSP90's clients, casts new light on understanding these reported actions, and suggests new possible applications for celastrol, such as diabetes management.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-010-0202-1) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein 90, Nuclear transcription factor, Co-chaperone, Inhibitor, Celastrol
Introduction
Celastrol, also known as tripterine, is a triterpenoid compound extracted from the Chinese herb Tripterygium wilfordii Hook F. In China, this herb has been used in anti-rheumatic treatment for thousands of years. In recent years, celastrol has attracted attention due to its potential for use in anti-inflammation (Jung et al. 2007; Kim et al. 2009a, b; Pinna et al. 2004), anti-tumor (Dai et al. 2009; Ge et al. 2010; He et al 2009a), and neuron degenerative disease amelioration applications (Allison et al. 2001; Faust et al. 2009; Kiaei et al. 2005). Celastrol can inhibit NF–κB activation (He et al. 2009a; Jung et al. 2007; Lee et al. 2006), arrest cell cycle(Ge et al. 2010), and induce heat shock and anti-oxidant response (Faust et al. 2009, Westerheide et al. 2004). The most recent reports attribute these effects to an attack on the heat shock protein (HSP90) complex by celastrol (Zhang et al. 2008, 2009). Yet, the effects of celastrol on one key sub-population of HSP90's clients, some transcription factors (TFs) are far from clear, an issue that should be addressed if celastrol is to be effectively applied in treatment.
Heat shock response induction via activating HSF1 provided the first evidence that celastrol inhibits HSP90 (Morimoto 1998; Westerheide et al. 2004). Analysis of gene expression patterns provided further support to this line of thought (Hieronymus et al. 2006), and corroborating data rapidly accumulated (Sreeramulu et al. 2009; Zhang et al. 2008, 2009). Since HSP90 inhibition clearly explained nearly all of celastrol's reported actions [including inhibition of NF–κB (He et al. 2009a; Zhang et al. 2006), anti-tumor effects in various cell lines (Nagase et al. 2003; Sethi et al. 2007; Yang et al. 2006), and neuron degenerative disease amelioration (Chow and Brown 2007; Faust et al. 2009, Kiaei et al. 2005)], the molecular basis for celastrol's HSP90 inhibition was intensively explored (Sreeramulu et al. 2009; Trott et al. 2008; Zhang et al. 2008, 2009). Celastrol's main target(s) in the HSP90 complex, however, remain debatable. Some propose that celastrol might preferentially affect the interaction between HSP90 and its co-chaperone Cdc37 (Li et al. 2009), while others believe that its affection might be not so specific (Chadli et al. 2010).
HSP90 inhibitors exert their cellular effects mainly via affecting HSP90's clients. There are two important sub-populations of HSP90's clients, kinases and nuclear TFs. The effects of celastrol on the kinase sub-population, such as Cdks and ERK are firmly confirmed in several cellular models (He et al. 2009b; Kim et al. 2009c, Salminen et al. 2010), but celastrol's effects on TFs sub-population HSP90's client are not well-established, despite of the work on several members in Class I nuclear receptor family (Chadli et al. 2010). At present, whether celastrol has broad effects on TFs sub-population clients remains contrary. For example, androgen receptor (AR) is reported being affected by celastrol in LNCaP cell (Hieronymus et al. 2006) while not in Hela cells (Chadli et al. 2010). Interactions of the HSP90 with the kinase sub-population are believed to involve Cdc37, while the TF's interactions are thought not to involve Cdc37 (Li et al. 2009). The argument over HSP90/Cdc37 interaction as essential target adds further difficulty in predicting the influence of celastrol over the TFs sub-population. If HSP90/Cdc37 interaction is preferentially or even solely affected, then celastrol should have little effect on the TFs sub-population. In contrast, if celastrol's effects are not limited to affect HSP90/Cdc37 interaction, the TFs sub-population might be as affected by treatment as the kinases ones. Therefore, the observation on actual effects of celastrol toward the TFs sub-population clients is urgently needed.
Here, we observed protein level changes in 18 nuclear TFs in three human cell lines (breast cancer cell MCF-7, hepatic cancer cell HepG2, and monocytic leukemia cell THP-1) when treated with different doses of celastrol. The TFs observed here are based on the HSP90 client list at http://www.picard.ch, including four members of class I nuclear receptor, four of class II nuclear receptor, five members of the PER/ARNT/SIM (PAS) family, and five miscellaneous TFs (detailed in Online Resource 1). The results show that celastrol affects many nuclear TFs in a cell type- and dose-dependent way. Consistently, celastrol affects HSP90/TFs interactions and disrupts HSP90/Hop, in addition to reported affecting HSP90/Cdc37 interaction.
Materials and methods
Drugs and reagents
RPMI 1640 medium, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and streptomycin/penicillin for cell culture use were obtained from PAA Laboratories (Linz, Austria). DMEM with no phenol red was obtained from Gibco (Invitrogen Co, Carlsbad, CA). Dimethyl sulfoxide (DMSO), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO). Protein A/G plus agarose beads were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies for estrogen receptor α (ERα), ERβ, glucocorticoid receptor (GR), AR, peroxisome proliferator–activated receptor αPPARα, constitutive androstane receptor (CAR), P53, and tonicity-responsive enhancer binding protein (TonEBP) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against heat shock transcription factor-1 (HSF-1) were obtained from Cell Signaling Technology, Inc (Danvers, MA). Anti-pregnane X receptor (PXR), hypoxia-inducible factor-1α (HIF-1α), HIF-2α, HIF-3α, signal transducer and activator of transcription 3 (STAT3) antibodies were purchased from Affinity BioReagents (Golden, CO). Anti-interferon regulatory factor 3 (IRF3) antibody was obtained from Zymed (Invitrogen Co, Carlsbad, CA). Anti-PPARβ antibody was obtained from LifeSpan, Inc. (Seattle, WA). Antibody for single-minded 2 (Sim2) was obtained from Abnova Co. (Taiwan, China). Anti-HSP90 (H9010) for immunoprecipitation was purchased from Alexis Biochemicals (San Diego, CA), while those for Western blot and flow cytometric analysis were obtained from Sressgen Bioreagents (Ann Arbor, MI). Anti-actin antibody and horseradish peroxidase (HRP)-labeled secondary antibodies, BCA protein assay kit, and Beyo ECL Plus were purchased from Beyotime Biotechnology (Jiangsu, China). All reagents were stored as recommended by the manufactures.
Celastrol was extracted as previously reported by us (Zhang et al. 2006). Celastrol was prepared to 50 mM with DMSO and stored at −20°C, this mixture usable up to 3 months after preparation. The stored solution was diluted further to a proper lower concentration with culture medium just prior to culture introduction.
Cell cultures and treatments
The cell lines from human tumors used here were as follows: MCF-7 (breast cancer), HepG2 (hepatic cancer), and THP-1 (monocytic leukemia), all of which were obtained from Shanghai CellBank of the National Science Academy of China. The cells were maintained in DMEM (for adherent cells) or RPMI 1640 medium (for non-adherent cells). The maintained cells were supplemented with 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin in a humidified 5% CO2 incubator at 37oC. Exponentially growing cells were used for experiments. For depletion of endogenous estrogen, plated cells were changed to phenol red-free DMEM supplemented with 5% charcoal absorbed FBS at least 24 h before treatment with celastrol. Each experiment was repeated at least three times. Culture medium with appropriate amounts of DMSO (vehicle) served as controls. Final DMSO concentration never exceeded 0.1%.
Determination of cell viability
Cell viability was determined by MTT assay. In 96-well culture plates, 4 × 104 cells were plated and treated with indicated concentrations of celastrol for 6 or 24 h. At the end of each time point, the growth medium was removed, and 100 μl of 0.5 mg/ml MTT was added to each well for 1 h at 37°C. The MTT solution was then removed, and 100 μl of DMSO was added. Absorbance at 492 nm was read by Multiskan MK3 Microplate Reader (Thermo Electron Instrument).
Western blot
Cells were treated with indicated amounts of celastrol for 6 h, washed twice with ice-cold phosphate buffer solution (PBS), and then harvested in cell lysis buffer (Beyotime, Jiangsu, China) for Western blot, which contained 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, and protease inhibitors. The lysate was sonicated and then clarified by centrifuging at 13,000×g for 10 min. Protein concentration was determined by the BCA protein assay kit. Equal amounts of proteins were subjected to 6–12% SDS-PAGE and then transferred to polyvinylidene difluoride membranes. Blots were probed with the appropriate antibodies overnight. Detection was accomplished using correspondent HRP-conjugated secondary antibodies and developed on film with Beyo ECL Plus and autoradiography. Protein bands were analyzed by Quantity one 4.4.0 software (Bio-Rad).
Co-immunoprecipitation
Cells were washed twice with ice-cold PBS and harvested in a immunoprecipitation (IP) lysis buffer containing 20 mM Tris·HCl (pH 7.4), 25 mM NaCl, 0.1% Nonidet P40, 2 mM DTT, 20 mM Na2MoO4, and protease inhibitor cocktail. Lysates were sonicated and further incubated for 2 h at 4°C and then centrifuged at 13,000×g for 10 min. One milligram protein was incubated with 2 μg of anti-HSP90 (H9010) antibody overnight at 4°C. Thirty microliters Protein A/G plus agarose beads were then added to each sample and incubated for 3 h at 4°C, rotating. Beads were then washed three times with PBS. The immunoprecipitates were then electrophoresed on 8–10% SDS-PAGE. Blots were probed with anti-HSP90 and the indicated antibodies.
Flow cytometric assay
Following PBS wash, cells were fixed in 100% methanol for 10 min at 4°C, incubated with anti-HIF-1α antibodies for 45 min at 4°C, followed by wash and incubation with the corresponding secondary antibodies in conjunction with FITC for 30 min at 4°C. Appropriate isotypes were used as control. Samples were analyzed by flow cytometer (FACSCalibur, BD). Analyses were performed with CellQuest software (BD Biosciences).
Statistics
Data are presented as mean ± SD. Matched t test analysis was used for statistical evaluation of significant differences among the groups using SPSS 11.5 for Windows software. A value of P < 0.05 was considered to be statistical significance. Experiments were repeated at least three times.
Results
Dose-dependent effects of celastrol on cell viability
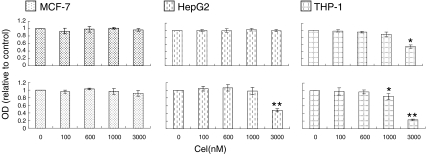
To determine suitable doses of celastrol for each cell line, we observed the dose-effects of celastrol on cells' viability with 6 and 24 h incubation. After 6 h incubation, the maximum doses tested that did not reduce the number of living cells in THP-1, HepG2, and MCF-7 were 1,000; 3,000; and 3,000 nM, respectively (Fig. 1); these amounts were used as the highest dosage for each respective cell type. Low and medium doses for all three cell types were 100 and 600 nM. Unless otherwise stated, incubation time with celastrol was 6 h.
Fig. 1.
Dose-dependent effects of celastrol on cellular viability in MCF-7, HepG2, and THP-1. Cells were seeded overnight in 96-well plates at a density of 2 × 105/ml in phenol red-free DMEM supplemented with 5% charcoal absorbed FBS, and then indicated celastrol doses were added for 6 or 24 h. The cell's viability was determined by MTT method. Upper panel exposure to celastrol for 6 h. Lower panel exposure to celastrol for 24 h. Each value represents mean ± SD from three independent tests
Effects of celastrol on TFs protein levels in the three cells
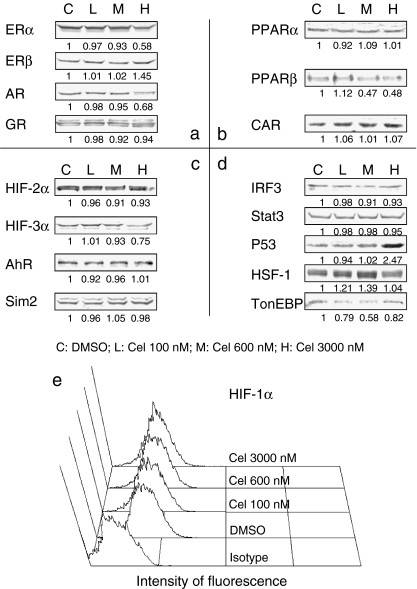
Eighteen TFs belonging to HSP90's clients were examined in MCF-7, HepG2, and THP-1, treated with indicated doses of celastrol for 6 h. Celastrol's vehicle DMSO was used for control. All TFs were successfully detected by Western blot, except for HIF-1α, which was detectable by flow cytometry.
For MCF-7 cells, only PXR tested negative. In the 17 detectable TFs, eight were affected by celastrol (Fig. 2). ERα, AR, HIF-3α, and TonEBP were decreased, with the minimum dose of celastrol needed to cause such changes being different for each TF. In contrast, ERβ, P53, and HSF-1 were elevated. More strikingly, PPARβ showed bi-directional alteration in that its level was elevated by 100 nM celastrol but decreased from 600 nM. Among these noted changes, AR reduction and P53 and HSF-1 elevation have been found in other cellular models (Hieronymus et al. 2006; Sung et al. 2010; Westerheide et al. 2004).
Fig. 2.
MCF-7 TFs alteration detection. MCF-7 cells were treated with different doses of celastrol for 6 h. For Western blot (WB) assay, treated cells were incubated in lysis buffer, and cell lysates were obtained by centrifugation. Equal amounts of whole cell proteins were subjected to 6–12% SDS-PAGE, as detailed in Materials and methods. For flow cytometry (FCM) assay, treated cells were washed with PBS and fixed in 100% methanol, followed by incubation in anti-HIF-1α antibodies and the corresponding secondary antibodies in conjunction with FITC, as detailed in Materials and methods. a Class I nuclear TFs detected by WB. b Class II nuclear TFs detected by WB. c Factors belonging to the PAS family except for HIF-1α detected by WB. d Other factors detected by WB. e Histogram of HIF-1α expression by FCM. Negative WB assay results are not shown. Cel celastrol. The experiment was repeated three times and the value under each band represents the mean density of the three detections
In HepG2 cells, 15 TFs tested positively, of which eight were affected by celastrol (Table 1 and Fig. S1 in Online Resource 1). PPARβ, HIF-1α, and HIF-2α were decreased in celastrol's presence while P53 was elevated. PXR, AhR, HSF-1, and TonEBP were seen with bi-directional alterations.
Table 1.
Statistics of TFs in HepG2 and THP-1 caused by celastrol
| Cell line | HepG2 | THP-1 | ||||
|---|---|---|---|---|---|---|
| Cel (nM) | 100 | 600 | 3,000 | 100 | 600 | 1,000 |
| ERα | 1.05 ± 0.07 | 0.97 ± 0.05 | 0.94 ± 0.08 | 1.11 ± 0.06* | 1.03 ± 0.05 | 0.3 ± 0.05** |
| ERβ | 0.95 ± 0.06 | 0.98 ± 0.03 | 0.95 ± 0.05 | ND | ND | ND |
| AR | 1.03 ± 0.08 | 1.02 ± 0.05 | 1.05 ± 0.06 | 1.05 ± 0.07 | 0.99 ± 0.05 | 0.84 ± 0.03** |
| GR | 0.96 ± 0.05 | 0.94 ± 0.07 | 1.01±0.04 | 1.08 ± 0.06 | 2.04 ± 0.06** | 2.17 ± 0.03** |
| PPARα | ND | ND | ND | ND | ND | ND |
| PPARβ | 0.66 ± 0.07** | 1.09 ± 0.08 | 1.04 ± 0.06 | ND | ND | ND |
| CAR | ND | ND | ND | 1.44 ± 0.03** | 1.8 ± 0.04** | 2.11 ± 0.02** |
| PXR | 1.85 ± 0.05** | 0.97 ± 0.05 | 0.31 ± 0.03** | ND | ND | ND |
| HIF-1α | 0.64 ± 0.03** | 0.76 ± 0.02** | 0.6 ± 0.02** | 0.81 ± 0.02** | 1.02 ± 0.04 | 0.84 ± 0.03** |
| HIF-2α | 1.04 ± 0.08 | 0.65 ± 0.05** | 0.51 ± 0.02** | 0.93 ± 0.08 | 0.15 ± 0.06** | 0.01 ± 0.01** |
| HIF-3α | 1.01 ± 0.06 | 0.97 ± 0.05 | 0.94 ± 0.08 | 1.02 ± 0.05 | 0.93 ± 0.08 | 0.82 ± 0.06** |
| AhR | 1.31 ± 0.02** | 0.97 ± 0.05 | 0.68 ± 0.01** | 0.77 ± 0.04** | 0.27 ± 0.03** | 0.02 ± 0.01** |
| Sim2 | 0.99 ± 0.05 | 1.00 ± 0.03 | 0.94 ± 0.07 | 0.65 ± 0.05** | 0.63 ± 0.06** | 0.29 ± 0.03** |
| IRF3 | ND | ND | ND | ND | ND | ND |
| Stat3 | 1.01 ± 0.04 | 1.06 ± 0.08 | 1.02 ± 0.05 | 1.03 ± 0.09 | 1.01 ± 0.05 | 1.07 ± 0.08 |
| P53 | 1.32 ± 0.06** | 1.13 ± 0.04** | 1.59 ± 0.03** | 0.96 ± 0.07 | 0.98 ± 0.05 | 0.37 ± 0.03** |
| HSF-1 | 1.25 ± 0.04** | 0.77 ± 0.02** | 0.81 ± 0.03** | 1.18 ± 0.05** | 0.7 ± 0.03** | 0.02 ± 0.01** |
| TonEBP | 1.21 ± 0.03** | 1.13 ± 0.05** | 0.51 ± 0.05** | 0.89 ± 0.02** | 0.75 ± 0.03** | 0.18 ± 0.02** |
All values are represented as mean ± SD; experiments were independently repeated at least three times and tested relative to DMSO. ND not detectable
*P < 0.05; **P < 0.01
While only 13 of THP-1's TFs (the lowest of the three cell lines) tested positively, 12 of them were affected by celastrol (Table 1 and Fig. S2 in Online Resource 1): AR, HIF-1α, HIF-2α, HIF-3α, AhR, Sim2, P53, and TonEBP were decreased; GR and CAR were increased, and ERα and HSF-1 showed bi-directional changes.
Effects of celastrol on components of the HSP90 complex
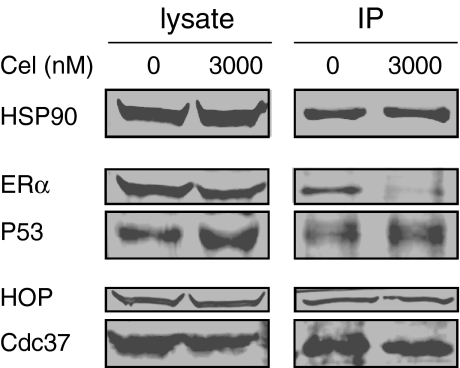
The protein levels of TFs are affected by degradation and synthesis. We found that celastrol caused reduction in ERα persisted in MCF-7 when pre-treated with actinomycin D or cycloheximide, indicating that increasing degradation is involved in reduction of TFs (Fig. S3 in Online Resource 1). HSP inhibition usually leads to protein degradation, and it has been reported that celastrol's reduction of client proteins is related to disruption of HSP90's binding ability (Zhang et al. 2008), we thought that this might hold true in our model. Thus, we observed celastrol's effect on the interaction between HSP90/TFs and HSP90/co-chaperone. Figure 3 shows that the decrease in ERα and elevation of P53 in MCF-7 whole cell lysates were accompanied by similar alterations of these two proteins in the HSP90 complex by co-immunoprecipitation. This result indicated that changes in TFs were related to changes in their interaction with HSP90. The Co-IP experiment also showed reduction of both Hop and Cdc37 in the HSP90 complex. The HSP90–Hop–HSP70 complex is considered to be the minimal HSP90 core complex (Li et al. 2009); celastrol's disruption of HSP90/Hop could explain why this agent affects so many TFs. That both HSP90/Hop and HSP90/Cdc37 were affected suggests that celastrol have more effects than specifically disrupting HSP90/Cdc37 interaction (Zhang et al. 2009). During this manuscript's preparation, it was reported that another co-chaperone, P23, is also affected by celastrol (Chadli et al. 2010).
Fig. 3.
Disrupting effects of celastrol on the interaction between HSP90 and its clients and co-chaperones. MCF-7 cells treated with 3,000 nM celastrol were co-immunoprecipitated by anti-HSP90 antibody and the indicated proteins assayed by WB, as detailed in Materials and methods. The experiments were repeated three times
Discussion
In this study, we observed celastrol's effects on 18 nuclear TFs belonging to HSP90's clients in three cell lines and found that half or more of the detectable TFs in each cell line are affected by celastrol. There is no consistent pattern to the observed effects on TFs among the three cells types. Dose-related effects are also revealed, including bi-directional regulation of some TFs (for example, low doses up-regulating and high doses down-regulating). Effects on the multiple TFs are accompanied by alteration of HSP90 complex TFs and co-chaperone levels. This suggests that celastrol have more affection on HSP90 than disrupting HSP90/Cdc37 interaction.
Our results clearly demonstrate that celastrol has profound effects on TFs. The 18 TFs observed in this study are of four different classes, including four members of class I nuclear receptor family, four of class II nuclear receptor family, five PAS family members, and five miscellaneous TFs. Fifteen of the 18 TFs are affected by celastrol in at least one type of the cell lines used in this study. Though TFs expression patterns are different among the three cell lines, at least half of the positive-testing TFs in each cell are affected by celastrol. Therefore, celastrol's effects on TFs are broad and not limited to one type of TFs or one type of cell lines.
However, cell type influences celastrol's ability to affect TFs. In MCF-7, HepG2, and THP-1, the ratios of TFs affected by celastrol to positive-testing TFs are 8/17, 8/15, and 12/13, respectively, with THP-1 most dramatically affected in spite of expressing the fewest TFs of the three lines. THP-1 also shows highest sensitivity to celastrol-triggered cell death. It is suggested that celastrol can kill leukemia stem cells (Hassane et al. 2008); our findings support that this agent may be effective in anti-leukemia treatment. Further indicating that cell type determines celastrol's effectiveness, AR is reported as affected by celastrol in prostate cancer cells but not in Hela cells (Chadli et al. 2010; Hieronymus et al. 2006). Cell type dependence means that a systematic evaluation of celastrol's effects on different tissues is necessary before clinical trials.
The patterns of affected TFs also change based on celastrol dosage; this is demonstrated by two facts. First, the number of the affected TFs is dose-dependent (for example, MCF-7 had only three affected TFs when treated with 100 or 600 nM celastrol, but had seven affected TFs when 3,000 nM was used). Second, celastrol can cause bi-directional protein level changes (from elevation to decrease) in several TFs. This bi-directional effect, to our knowledge, has not been reported as caused by other HSP90 inhibitors, and is thus unique for celastrol and deserving of further investigation.
Our work casts new light on understanding the mechanism underling celastrol's effect on the HSP90 complex. Since Cdc37 disruption is believed to have little effect on TFs levels, the disruption of multiple TFs in our study suggests that celastrol's effects be not limited to disrupting HSP90/Cdc37 interaction. To support this notion, we show that, in addition to disrupting HSP90/Cdc37, celastrol can alter HSP90/TFs and HSP90/Hop interaction. In line with our thinking, HSP90/P23 interaction is also reported as disrupted by celastrol (Chadli et al. 2010). Since the combination of HSP90 and Cdc37 is reported to inhibit ATPase activity, direct celastrol inhibition of ATPase in yeast Hsp82 (Zhang et al. 2009) and in HSP90 complex as pulled down by anti-HSP90 in our model (data not shown) puts doubt upon the suggestion that the basis for celastrol's effects is solely or preferentially HSP90/Cdc37 inhibition. We thought that celastrol might attack an import region in HSP90 thus exert broad effects. To support this, celastrol has been reported to interact directly with the C-terminal of HSP90 (Zhang et al. 2009), and we have also got preliminary evidence that celastrol can directly attack the thiols on HSP90's C-terminal (data not shown), in addition to the already reported Cdc37 thiols (Sreeramulu et al. 2009).
Our results offer new evidences for the reported applications while also suggesting novel potential applications of cleastrol. For instance, celastrol's elevation of GR levels supports its useful anti-inflammation effects (Huang et al. 1998), while celastrol's down-regulation effects on AR and the elevation effect on P53 back up celastrol as a useful prostate cancer inhibitor in vitro and in vivo (Pang et al. 2010; Yang et al. 2006). Even the effect of ER elevation adds rationale to celastrol's role in treating neuron degenerative disease (Faust et al. 2009; Kiaei et al. 2005). CAR induction suggests a new application for celastrol, in diabetes, based on recent findings that CAR can increase cellular sensitivity to insulin (Gao et al. 2009). THP-1's high sensitivity also suggests celastrol's usefulness in anti-leukemia application, as mentioned above.
In conclusion, we show for the first time that celastrol can affect multiple TFs belonging to HSP90's clients in a cell type- and dose-dependent way and suggest the necessity of systematic elevation of celastrol's actions upon different tissues. The bi-directional effects of celastrol, especially low-dosage TFs elevations, are unique and worthy of investigation. Our work not only supports other reports on celastrol's potential applications, but also suggests the compound could have novel action upon diabetes, an increasingly common worldwide health problem. Lastly, celastrol's apparent ability to affect many TFs sub-population clients of HSP90 suggests that the important region in HSP90 is celastrol's primary target, thus exerting broad effects on HSP90 complex, including but not limited to the reported damaging HSP90/Cdc37 interaction.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
(DOC 176 kb)
Acknowledgements
This work is supported by Pujiang Overseas Return Talent Project of Shanghai Government (07pj14075).
References
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/S0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Chadli A, Felts SJ, Wang Q, Sullivan WP, Botuyan MV, Fauq A, Ramirez-Alvarado M, Mer G. Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the Co-chaperone p23. J Biol Chem. 2010;285:4224–4231. doi: 10.1074/jbc.M109.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones. 2007;12:237–244. doi: 10.1379/CSC-269.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, DeSano JT, Meng Y, Ji Q, Ljungman M, Lawrence TS, Xu L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–1225. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson's disease. BMC Neurosci. 2009;10:109. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, Xie W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009;284:25984–25992. doi: 10.1074/jbc.M109.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Ji X, Ding Y, Wang X, Fu S, Meng F, Jin X, Ling F, Luo Y. Celastrol causes apoptosis and cell cycle arrest in rat glioma cells. Neurol Res. 2010;32:94–100. doi: 10.1179/016164109X12518779082273. [DOI] [PubMed] [Google Scholar]
- Hassane DC, Guzman ML, Corbett C, Li X, Abboud R, Young F, Liesveld JL, Carroll M, Jordan CT. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood. 2008;111:5654–5662. doi: 10.1182/blood-2007-11-126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Xu Q, Yan M, Zhang P, Zhou X, Zhang Z, Duan W, Zhong L, Ye D, Chen W. The NF–kappa B inhibitor, celastrol, could enhance the anti-cancer effect of gambogic acid on oral squamous cell carcinoma. BMC Cancer. 2009;9:343. doi: 10.1186/1471-2407-9-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MF, Liu L, Ge W, Shaw PC, Jiang R, Wu LW, But PP. Antiangiogenic activity of Tripterygium wilfordii and its terpenoids. J Ethnopharmacol. 2009;121:61–68. doi: 10.1016/j.jep.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, Maloney KN, Clardy J, Hahn WC, Chiosis G, Golub TR. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Huang FC, Chan WK, Moriarty KJ, Zhang DC, Chang MN, He W, Yu KT, Zilberstein A. Novel cytokine release inhibitors. Part I: triterpenes. Bioorg Med Chem Lett. 1998;8:1883–1886. doi: 10.1016/S0960-894X(98)00331-X. [DOI] [PubMed] [Google Scholar]
- Jung HW, Chung YS, Kim YS, Park YK. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med. 2007;39:715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Invest. 2009;39:819–827. doi: 10.1111/j.1365-2362.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- Kim DY, Park JW, Jeoung D, Ro JY. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612:98–105. doi: 10.1016/j.ejphar.2009.03.078. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim K, Lee H, Han S, Lee YS, Choe J, Kim YM, Hahn JH, Ro JY, Jeoung D. Celastrol binds to ERK and inhibits FcepsilonRI signaling to exert an anti-allergic effect. Eur J Pharmacol. 2009;612:131–142. doi: 10.1016/j.ejphar.2009.03.071. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, Hong YS, Lee JJ. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Schwartz SJ, Sun D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resist Updat. 2009;12:17–27. doi: 10.1016/j.drup.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Nagase M, Oto J, Sugiyama S, Yube K, Takaishi Y, Sakato N. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci Biotechnol Biochem. 2003;67:1883–1887. doi: 10.1271/bbb.67.1883. [DOI] [PubMed] [Google Scholar]
- Pang X, Yi Z, Zhang J, Lu B, Sung B, Qu W, Aggarwal BB, Liu M. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn's disease biopsies. Biochem Biophys Res Commun. 2004;322:778–786. doi: 10.1016/j.bbrc.2004.07.186. [DOI] [PubMed] [Google Scholar]
- Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of Thunder God Vine. Biochem Biophys Res Commun. 2010;394:439–442. doi: 10.1016/j.bbrc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- Sreeramulu S, Gande SL, Gobel M, Schwalbe H. Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37, a kinome chaperone-cochaperone, by triterpene celastrol. Angew Chem Int Ed Engl. 2009;48:5853–5855. doi: 10.1002/anie.200900929. [DOI] [PubMed] [Google Scholar]
- Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285:11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Trott A, West JD, Klaic L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- Zhang DH, Marconi A, Xu LM, Yang CX, Sun GW, Feng XL, Ling CQ, Qin WZ, Uzan G, d'Alessio P. Tripterine inhibits the expression of adhesion molecules in activated endothelial cells. J Leukoc Biol. 2006;80:309–319. doi: 10.1189/jlb.1005611. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hamza A, Cao X, Wang B, Yu S, Zhan CG, Sun D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7:162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li Y, Yu Y, Zou P, Jiang Y, Sun D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J Biol Chem. 2009;284:35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 176 kb)