Abstract
As interleukin-6 (IL-6), its soluble receptor (sIL-6R), and the IL-6/sIL-6R complex is transiently elevated in response to prolonged moderate-intensity exercise, this study investigated how these levels would be modulated by an acute bout of high-intensity intermittent (HIIT) exercise in comparison to continuous moderate-intensity exercise (MOD). This study also investigated the expression of the differentially spliced sIL-6R (DS-sIL-6R) in response to exercise. Eleven healthy males completed two exercise trials matched for external work done (582 ± 82 kJ). During MOD, participants cycled at 61.8 (2.6)% VO2peak for 58.7 (1.9) min, while HIIT consisted of ten 4-min intervals cycling at 87.5 (3.4)% 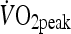 separated by 2-min rest. Blood samples were collected pre-exercise, post-exercise, and 1.5, 6, and 23 h post-exercise. Plasma IL-6, sIL-6R, IL-6/sIL-6R complex, and DS-sIL-6R levels were measured by enzyme-linked immunosorbent assay. HIIT caused a significantly greater increase in IL-6 than MOD (P = 0.018). Both MOD and HIIT resulted in an increase in sIL-6R and IL-6/sIL-6R complex (P < 0.001), however, this was not significantly different between trials. Soluble IL-6R peaked at 6 h post-exercise in both trials. DS-sIL-6R increased significantly with exercise (P = 0.02), representing 0.49% of the total sIL-6R increase. This investigation has demonstrated that the IL-6 response is greater after intermittent high-intensity exercise than comparable moderate-intensity exercise; however, increased IL-6/sIL-6R complex nor sIL-6R was different between HIIT and MOD. The current study has shown for the first time that elevated sIL-6R after HIIT exercise is derived from both proteolytic cleavage and differential splicing.
separated by 2-min rest. Blood samples were collected pre-exercise, post-exercise, and 1.5, 6, and 23 h post-exercise. Plasma IL-6, sIL-6R, IL-6/sIL-6R complex, and DS-sIL-6R levels were measured by enzyme-linked immunosorbent assay. HIIT caused a significantly greater increase in IL-6 than MOD (P = 0.018). Both MOD and HIIT resulted in an increase in sIL-6R and IL-6/sIL-6R complex (P < 0.001), however, this was not significantly different between trials. Soluble IL-6R peaked at 6 h post-exercise in both trials. DS-sIL-6R increased significantly with exercise (P = 0.02), representing 0.49% of the total sIL-6R increase. This investigation has demonstrated that the IL-6 response is greater after intermittent high-intensity exercise than comparable moderate-intensity exercise; however, increased IL-6/sIL-6R complex nor sIL-6R was different between HIIT and MOD. The current study has shown for the first time that elevated sIL-6R after HIIT exercise is derived from both proteolytic cleavage and differential splicing.
Keywords: Intermittent exercise, IL-6R differential splicing
Introduction
A comprehensive review of the myokine, interleukin-6 (IL-6), has provided evidence to support the concept that acute elevations of IL-6 can have beneficial consequences on health through metabolic and anti-inflammatory mechanisms (Pedersen 2009). IL-6 has been classified as having both pro- and anti-inflammatory properties (Jones et al. 2001). Unlike other pro-inflammatory cytokines, IL-6 appears to be the primary inducer of acute-phase proteins, many of which have anti-inflammatory properties, as well as inhibiting TNF-α and IL-1 expression (reviewed in Pedersen et al. 2001). Prolonged moderate-intensity exercise is capable of elevating levels of circulating IL-6 (Keller et al. 2001; Penkowa et al. 2003) and its receptor interleukin-6R (IL-6R) to form a binary complex (IL-6/IL-6R; Taga et al. 1989). The soluble form of the receptor, sIL-6R, allows IL-6 signaling to occur in tissues lacking membrane-bound IL-6R, a process termed trans-signaling (Rose-John and Heinrich 1994). sIL-6R can be generated by two processes: differential mRNA splicing (DS-sIL-6R; Horiuchi et al. 1994) or proteolytic cleavage (PC-sIL-6R) of the membrane-bound receptor (Mullberg et al. 1994). Although the DS-sIL-6R represents <1% of total sIL-6R at rest (Dimitrov et al. 2006), the source of the elevated sIL-6R in response to exercise stress is unknown.
While there is considerable knowledge regarding moderate-intensity exercise and the response of the IL-6 system, there is now increasing evidence that high-intensity exercise may have a greater cardioprotective effect (Babraj et al. 2009; Swain and Franklin 2006). This is in parallel to evidence indicating that IL-6 release is intensity-dependent (Helge et al. 2003; Ostrowski et al. 2000), and that over 50% of the variation can be contributed to the duration of exercise (Fischer 2006). Intermittent exercise offers a solution to both these criteria in that it combines periods of high-intensity exercise intervened with rest periods, allowing the duration to be extended beyond that of continuous high-intensity exercise. Although intermittent exercise is normally the domain of athletes, there is evidence that this form of exercise can be accomplished by recreationally active women (Talanian et al. 2007). If IL-6 is considered a mediating factor for health, then it is important to investigate the response of the IL-6 system to this type of exercise.
Our hypothesis is that high-intensity intermittent exercise will elevate all components of the IL-6 system above that of continuous moderate-intensity exercise. A secondary hypothesis is that exercise will increase the DS-sIL-6R.
Materials and methods
Participant characteristics
Eleven healthy male volunteers participated in this study (mean (SD); age 22.3 (4.0) years, body mass 73.5 (5.4) kg, height 1.79 (0.1) m, body mass index 23.0 (1.76) kg·m−2). None of the participants were specifically trained to a particular sport, however, all were physically active and reported participating in exercise equating to three or four 30 min exercise sessions per week. Participants who reported taking any anti-inflammatory medication were excluded from this study. The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki (2008). All participants were informed of the possible risks and discomforts associated with taking part in the study before giving informed written consent.
Preliminary measurements
During visit 1 to the laboratory, peak oxygen uptake 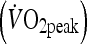 was determined using a continuous incremental exercise test on an electromagnetically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) performed to volitional exhaustion. Expired air was measured continuously using an online breath-by-breath gas analysis system (Ultima CPX, MedGraphics, MN, USA) as well as continuous monitoring of heart rate (RS200, Polar Electro, Kempele, Finland). The starting workload for the incremental exercise test was 100 W and increased by 35 W every 3 min with participants cycling at a pedal cadence of 70 rpm.
was determined using a continuous incremental exercise test on an electromagnetically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) performed to volitional exhaustion. Expired air was measured continuously using an online breath-by-breath gas analysis system (Ultima CPX, MedGraphics, MN, USA) as well as continuous monitoring of heart rate (RS200, Polar Electro, Kempele, Finland). The starting workload for the incremental exercise test was 100 W and increased by 35 W every 3 min with participants cycling at a pedal cadence of 70 rpm. 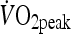 was identified as the oxygen uptake
was identified as the oxygen uptake 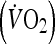 averaged over the highest 30-s period during the test. One week later, participants attended the laboratory to complete a familiarization trial. During this visit, participants completed five 4 min intervals separated by a 2 min rest at a load corresponding to that which would elicit 85–90%
averaged over the highest 30-s period during the test. One week later, participants attended the laboratory to complete a familiarization trial. During this visit, participants completed five 4 min intervals separated by a 2 min rest at a load corresponding to that which would elicit 85–90% 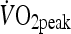 . This session allowed the external work to be calculated for the HIIT trial. This value was then used to determine the duration required during the MOD trial (undertaken at 60%
. This session allowed the external work to be calculated for the HIIT trial. This value was then used to determine the duration required during the MOD trial (undertaken at 60% 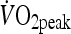 ) in order that the same amount of external work was undertaken in both trials.
) in order that the same amount of external work was undertaken in both trials.
Experimental protocol
Participants attended the laboratory on two other occasions separated by 1 week to complete a HIIT and a MOD trial in a randomized counterbalanced order. On both occasions, participants arrived at the laboratory at 8 a.m. following an overnight fast, having abstained from caffeine, alcohol and strenuous exercise during the 24 h period prior to testing. On arrival, a cannula was inserted into the antecubital vein, with the participant seated in a semi-supine position for blood sampling. This cannula provided the blood samples for pre- and post-exercise and 1.5 h post-exercise. The cannula was kept patent throughout the trial via regular flushing with 0.9% saline solution. Once a pre-exercise blood sample was taken, the participant moved to the cycle ergometer and completed ten 4-min intervals at a power output to elicit 85–90% of 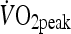 or cycled continuously at an intensity of approximately 60%
or cycled continuously at an intensity of approximately 60% 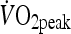 . Participants completed the same external work in both experimental trials. Immediately upon the cessation of exercise, a post-exercise blood sample was taken. Participants then rested in a seated position for 1.5 h, at which point another blood sample was taken. Following this sample, the cannula was removed and the participant was permitted to leave the laboratory, however was requested to reduce physical activity to a minimum. The participant returned to the laboratory at 6 h post-exercise and 24 h from the start of the exercise where further blood samples were obtained.
. Participants completed the same external work in both experimental trials. Immediately upon the cessation of exercise, a post-exercise blood sample was taken. Participants then rested in a seated position for 1.5 h, at which point another blood sample was taken. Following this sample, the cannula was removed and the participant was permitted to leave the laboratory, however was requested to reduce physical activity to a minimum. The participant returned to the laboratory at 6 h post-exercise and 24 h from the start of the exercise where further blood samples were obtained.
Blood sampling
Arterialized blood was collected in two 10 mL vacutainers at each time point which had been pretreated with K+EDTA (BD Biosciences, San Diego, USA). Whole blood was used to determine hematocrit using the microcapillary technique and hemoglobin concentration using a commercially available kit (Randox, Co Antrim, UK). The remaining sample was immediately centrifuged at 4,000×g for 10 min at 4°C, and the resulting plasma was aliquoted and stored at −80°C for subsequent analysis. Plasma volume changes were calculated according to Dill and Costill (1974).
Dietary and physical activity control
Participants were asked to standardize their food intake and physical activity for the 24 h prior to both trials and were provided with standardized meals for both experimental trial days. They were provided with lunch, a snack, dinner and drinks for both trials. Energy intake was the same for every participant and totaled 10,962 kJ per trial day. On the experimental days, participants completed physical activity diaries and were asked to replicate activity for both trial days.
Enzyme-linked immunosorbent assays
Plasma IL-6, sIL-6R, IL-6/sIL-6R complex and DS-sIL-6R levels were analyzed via sandwich enzyme-linked immunosorbent assays (ELISAs). All materials and chemical reagents were obtained from Sigma-Aldrich Ltd. (Poole, UK) unless otherwise specified. All incubation periods were at room temperature, and during each incubation stage, the plate was placed on a horizontal orbital plate shaker (Mini Orbital Shaker SOB, Stuart Scientific, UK) at 60 rpm unless otherwise stated. Protein concentrations were determined in relation to a four-parameter standard curve (GraphPad Prism, version 4.00, San Diego California, USA) and were corrected for changes in plasma volume from pre-exercise levels.
Interleukin-6 assay
Plates were coated with anti-human IL-6 monoclonal capture antibody (OptEIA, BD Biosciences, Oxford, UK) diluted 1:250 in 0.1 M sodium carbonate. The next day, the plates were washed then blocked with 5% bovine serum albumin (BSA; Probumin, Millipore, Illinois, USA) in Tris-buffered saline (TBS). The plates were incubated for 1 h at room temperature. After 1 h, plates were washed and samples or standards were added to the wells. Samples were diluted 1:5 in TBS with 10% fetal calf serum (FCS). After 2 h, plates were washed and 100 μL IL-6 detection antibody (OptEIA, BD Biosciences, Oxford, UK) diluted 1:250 in TBS-T with 1% BSA was added per well. Plates were incubated for a further 1 h before being washed. The enzyme streptavidin alkaline phosphatase was diluted 1:2,000 in TBS with 1% BSA and 100 µL was added per well. Plates were then incubated for 45 min. After washing, an ELISA amplification system was used (Invitrogen, Paisley, UK). The reaction was stopped with 50 µL of 10% sulfuric acid (stop solution), and the absorbance of the wells was read at 490 nm with a correction of 690 nm (Varioskan Flash, Thermo Scientific, Vantaa, Finland). Samples were analyzed in duplicate with an inter-assay coefficient of 7.4%. This assay measures total IL-6 content as it does not distinguish between the soluble and receptor-bound IL-6.
Soluble interleukin-6 receptor assay
Plasma sIL-6R concentration was measured as described in detail previously (Gray et al. 2008). Briefly, antibody pairs M5 (capture) and M182 (detection) were used to detect sIL-6R (BD Biosciences, San Diego, USA). Plates were coated with the capture antibody overnight. The next day, plates were washed and blocked with assay diluent (phosphate-buffered saline (PBS) with 10% FCS). Plates were then incubated for 1 h before being washed and adding standards/samples to the wells. Samples were diluted 1:200 in assay diluent. After 2 h, the plates were washed and the detection antibody was added. The plate was incubated for a further hour before washing the plate again. The enzyme streptavidin horse radish peroxidise (HRP) was diluted in assay diluent and added to the wells. After 45 min, the plate was washed and a substrate solution was added for 30 min before adding a stop solution. The final absorbance was then read at 450 nm with a correction at 570 nm. Samples were analyzed in duplicate with an inter-assay coefficient of 2.5% and an intra-assay coefficient of 7.9%.
IL-6/sIL-6R complex assay
IL-6/sIL-6R complex determination as been described in detail previously (Gray et al. 2009). This ELISA measures the active binary form of the IL-6/sIL-6R complex and does not measure the inactive tertiary IL-6/sIL-6R/sgp130 complex. This assay used a similar protocol to the sIL-6R assay; however, wells were coated with 100 µL of anti-human IL-6 monoclonal capture antibody, and M182 sIL-6R antibody was used for detection. Samples were diluted 1:2 in assay diluent and were added to wells in duplicate. There are currently no standards available for this assay; therefore, results are presented as fold change in optical density from pre-exercise, calculated independently for each participant. There was an inter-assay coefficient of 3.3%.
Differentially spliced sIL-6R assay
DS-sIL-6R concentration determination has been described in detail previously (Horiuchi et al. 1998). The 2F3 antibody and the recombinant DS-sIL-6R protein was kindly provided by Dr. Horiuchi (Tokyo). DS-sIL-6R was measured in pre-exercise and 6 h post-exercise plasma samples from the HIIT trial. Briefly, plates were coated overnight with 0.5 µg mL−1 anti-DS-sIL-6R monoclonal antibody (mAb 2F3, which was raised against the unique COOH terminal sequence of DS-sIL-6R GSRRRGSCGL) in PBS. The following morning, the plate was washed and the wells were blocked with 5% BSA–PBS for 1 h. After washing, 100 µL of samples or standards were added to wells and were incubated for 2 h at room temperature. Afterwards, the plate was washed a further three times and wells were incubated with the secondary antibody, biotinylated anti-human IL-6R, clone Sf 21 (R&D Systems, Minneapolis, USA). After 1 h, the plate was washed three times and HRP was added to the wells for 20 min before washing the plate a further three times. The color was developed by the addition of a substrate (TMB). DS-sIL-6R concentrations were determined using the baculovirus-expressed form of the receptor as a control standard. The optical density was read at 450 nm. Samples were analyzed in triplicate, and there was an inter-assay coefficient of 7.6%.
Statistical analysis
All statistical analysis was performed using SPSS 16.0 software (Statistical Package for the Social Sciences Inc., Chicago, IL, USA), and data are presented as mean (standard deviation (SD)). IL-6, sIL-6R and the IL-6/sIL-6R complex were analyzed using a within-group repeated measures ANOVA. IL-6 and IL-6/sIL-6R data were log-transformed in order to comply with ANOVA assumptions that data are normally distributed. An independent Student’s t test was performed to assess differences in DS-sIL-6R concentration. Statistical significance was accepted at P < 0.05.
Results
Exercise outcomes
The mean 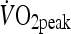 for the participants was 51.0 (6.5) mL·kg−1·min−1 and occurred at a mean power output of 294 (33) W. Table 1 summarizes the exercise descriptors for both HIIT and MOD trials.
for the participants was 51.0 (6.5) mL·kg−1·min−1 and occurred at a mean power output of 294 (33) W. Table 1 summarizes the exercise descriptors for both HIIT and MOD trials.
Table 1.
Summary of exercise descriptors for HIIT and MOD exercise trials
Percentage of 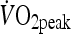 during exercise during exercise |
Heart rate during exercise (beats·min−1) | Power output during exercise (W) | Work done (kJ) | Exercise session duration (min) | |
|---|---|---|---|---|---|
| HIIT | 87.5 (3.4) | 172 (9) | 242 (34) | 582 (82) | 58 (0) |
| MOD | 61.8 (2.6) | 146 (12) | 165 (19) | 582 (82) | 58.7 (1.9) |
Values are means (SD), N = 11
Biomarker concentrations
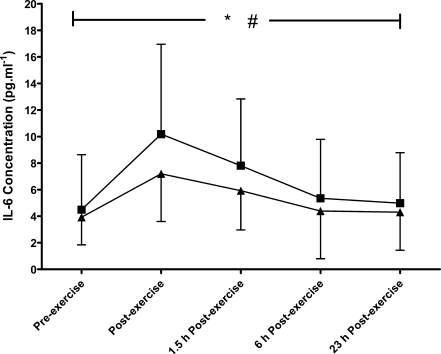
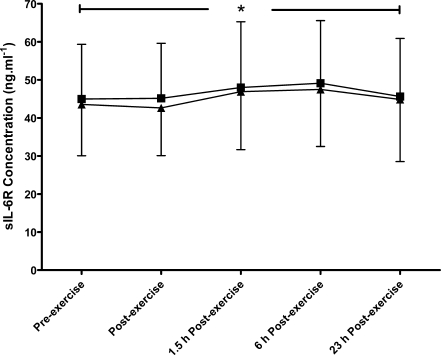
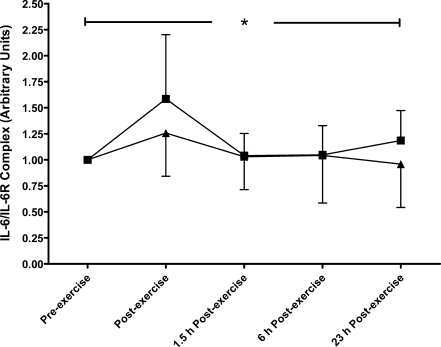
A main trial effect was found (P = 0.018), with higher IL-6 concentrations during the HIIT compared to the MOD trial (Fig. 1). As well as this, a main effect of time was seen (P < 0.001), with peak levels occurring immediately post-exercise in both trials (10.2 (6.8) and 7.2 (3.6) pg·mL−1 in HIIT and MOD, respectively). No differences were found in sIL-6R concentrations between the two trials (P = 0.214, Fig. 2); however, a main effect of time was found (P < 0.001) with peak levels occurring at 6 h after exercise, with concentrations of 49.1 (16.5) ng·mL−1 during HIIT and 47.5 (15.0) ng·mL−1 during MOD. No significant differences were seen in the IL-6/sIL-6R complex concentration between the two trials (P = 0.215), although a main effect of time was found (P < 0.001, Fig. 3). Peak IL-6/sIL-6R complex concentration occurred immediately post-exercise during both trials (159 (61.7)% and 126 (41.5)% increase from pre-exercise in HIIT and MOD, respectively).
Fig. 1.
The response of IL-6 concentration to continuous moderate- (triangle) and intermittent high- (square) intensity exercise trials, mean (SD). *Denotes a significant main effect of time (P < 0.001) and # denotes a significant trial effect (P = 0.018; N = 11)
Fig. 2.
Soluble IL-6 receptor response to continuous moderate- (triangle) and intermittent high- (square) intensity exercise trials, mean (SD). *Denotes a significant main effect of time (P < 0.001; N = 11)
Fig. 3.
Fold change in the IL-6/sIL-6R complex concentration from pre-exercise in continuous moderate- (triangle) and intermittent high- (square) intensity exercise trials, mean (SD). *Denotes a significant time effect (P < 0.001; N = 11)
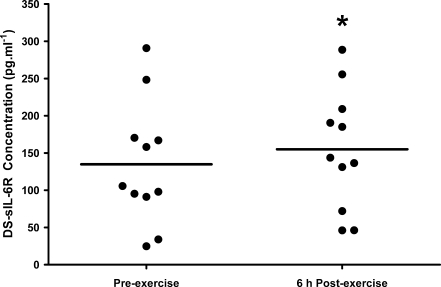
As sIL-6R peaked at 6 h post-exercise, blood samples at pre-exercise and 6 h post-exercise were analyzed from the HIIT trial for DS-sIL-6R concentration in order to determine the source of sIL-6R before and after exercise. DS-sIL-6R concentration was significantly elevated at 6 h post-exercise in comparison to pre-exercise concentration (155.0 (80.4) vs. 134.9 (82.5) pg·mL−1 respectively, P = 0.02); however, this contributed to <1% of total sIL-6R at both time points. There was a large inter-subject variation in DS-sIL-6R concentration, with concentrations ranging from 25 to 290 pg mL−1 in individuals (Fig. 4).
Fig. 4.
Differential IL-6 receptor mRNA spliced concentration during the intermittent high-intensity exercise trial at pre-exercise and 6 h post-exercise in individuals. *Significant difference between time points (P = 0.02; N = 11)
Discussion
The main findings of this study were that increases in IL-6 concentration after HIIT exercise were greater than after MOD when the same external amount of work was undertaken, however, there were no differences in sIL-6R concentration or the IL-6/sIL-6R complex between trials. In addition, the findings of the present study indicate that the increase in sIL-6R after exercise is derived from both proteolytic cleavage and differential IL-6R mRNA splicing, although the latter contributes <1% of the total.
To our knowledge, the present study is the first that has shown a higher IL-6 response during intermittent high-intensity exercise than continuous moderate-intensity exercise. Previous intermittent exercise protocols have shown that IL-6 increased in runners completing 10 × 1,000 m sprints with a 2 min recovery (Niess et al. 2003) and again with 4 × 250 m sprints (Meckel et al. 2009). In addition to these protocols not being applicable to the general population, there was no indication as to whether the IL-6 elevations were comparable to moderate-intensity exercise or not. The elevated levels in the current study may be due to increased glycogen usage with HIIT as circulating IL-6 is increased to a greater extent when muscle glycogen levels are low compared to normal levels (Keller et al. 2001; Steensberg et al. 2001). However, confirmation of this cause would need to be further explored.
Dixon et al. (2009) found IL-6 levels to be significantly greater when an indwelling cannula was used. In the current study, this would apply to our post-exercise and our 1.5 h post-exercise samples and suggest that our values may be slightly high at these time points. However, as cannulae were used in both trials, this should not affect trial differences. In addition, in four subjects, we took both venipuncture and cannulation samples at 1.5 h post-exercise samples and found that samples taken via cannulation were on average 0.88 pg·mL−1 higher than by venipuncture (data not shown). If the cannulation data from the remaining seven subjects are corrected to reflect a theoretical venipuncture value, the results are not altered.
The literature is variable as to how it reports IL-6 concentration following exercise. In this study, all data have been corrected for plasma volume changes in order to accurately reflect alterations in the amount of protein present. However, a substantial amount of the IL-6 literature has not corrected for plasma volume; therefore, we also analyzed the data before correction for plasma volume changes, and the main outcome is not altered (data not shown). Interestingly, correcting for plasma volume alters the timing of peak levels of the sIL-6R due to hemoconcentration associated with exercise and the subsequent hemodilution (Kargotich et al. 1998). Before correction, peak sIL-6R levels occur immediately post-exercise in both trials compared to 6 h post-exercise when corrected for plasma volume changes.
In a previous study, our laboratory identified plasma volume-corrected peak sIL-6R levels immediately after exercise (Gray et al. 2009), although there was no significant difference between this point and that taken at 1.5 h after exercise - the only other time point to be sampled. Similarly, in the current study, although peak sIL-6R is at 6 h post-exercise, there is no significant difference between the different time points. Keller et al. (2005) reported peak IL-6R mRNA levels at 6 h after the cessation of exercise, however were unable to identify any significant differences at the protein levels, possibly due to a small sample size (N = 6). Robson-Ansley et al. (2009) identified significantly elevated levels of sIL-6R the morning following strenuous exercise. We were unable to identify an elevation in sIL-6R approximately 24 h from the start of exercise. Discrepancies between this study and that of Robson-Ansley et al. (2009) could be explained by the extremely large volume of exercise (468 km cycled over 6 days) completed in comparison to the current study and that repeated exercise may extend the elevations of the receptor. Following eccentric exercise, Walshe et al. (2009) have reported a decrease in sIL-6R after 48 and 72 h. If our data are not corrected for plasma volume and are analyzed similarly to that of Walshe et al., then our data show that during HIIT, sIL-6R was significantly decreased at 23 h post-exercise when compared to pre-exercise levels (P = 0.034).
Although there are no differences between trials in IL-6/sIL-6R complex levels, both trials in the current study are consistent with our previous report of elevations in the IL-6/sIL-6R complex after exercise (Gray et al. 2009), which will prolong the half-life of IL-6 (Peters et al. 1996).
To determine the contribution of each isoform of the exercise-dependent elevation in sIL-6R, we quantified DS-sIL-6R levels. At rest, DS-sIL-6R represents <1% of the total, which is consistent with other previous studies (Dimitrov et al. 2006). The novel finding is that DS-sIL-6R increases significantly with exercise, although it still remains <1% of the total. As the increase in DS-sIL-6R does not account for the total increase in sIL-6R, then PC-sIL-6R must also increase There remains the possibility that proportionally, the contributions of the two sIL-6R isoforms varied at different time points, as previously shown with sleeping patterns (Dimitrov et al. 2006). A more detailed study would be needed to comprehensively answer this question. Functionally, the two receptor isoforms are similar in that they appear to mediate IL-6 signaling in a similar fashion (McLoughlin et al. 2004; Nowell et al. 2003); however, they are produced by different cell types. DS-sIL-6R has been shown to be produced by a defined subset of monocytic cells (McLoughlin et al. 2004) as well as T cells (Horiuchi et al. 1994), whereas PC-sIL-6R appears to be shed from all cells expressing the membrane-bound IL-6R. PC-sIL-6R activation is rapid and is shed by a number of activators, including C-reactive protein (Jones et al. 1999), whereas few activators have been found to regulate DS-sIL-6R (reviewed in Jones et al. 2008).
In conclusion, the present study is the first to compare the effects of high-intensity intermittent exercise and continuous moderate-intensity exercise on inflammatory markers in the circulation. This is the first study to show that sIL-6R produced by differentially spliced mRNA IL-6R increases in response to exercise. The more general benefits of intermittent exercise over continuous exercise are reviewed elsewhere (Laursen and Jenkins 2002), and this study lends support to advocating intermittent exercise as a physical activity intervention when health benefits are the main outcome.
References
- Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:2174–2176. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Dixon NC, Hurst TL, Talbot DCS, Tyrrell RM, Thompson D. Active middle-aged men have lower fasting inflammatory markers but the postprandial inflammatory response is minimal and unaffected by physical activity status. J Appl Physiol. 2009;107:63–68. doi: 10.1152/japplphysiol.91532.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- Gray SR, Robinson M, Nimmo MA. Response of plasma IL-6 and its soluble receptors during submaximal exercise to fatigue in sedentary middle-aged men. Cell Stress Chaperones. 2008;13:247–251. doi: 10.1007/s12192-008-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SR, Clifford M, Lancaster R, Leggate M, Davies M, Nimmo MA. The response of circulating levels of the interleukin-6/interleukin-6 receptor complex to exercise in young men. Cytokine. 2009;47:98–102. doi: 10.1016/j.cyto.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol. 2003;546:299–305. doi: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S, Koyanagi Y, Zhou Y, Miyamoto H, Tanaka Y, Waki M, Matsumoto A, Yamamoto M, Yamamoto N. Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur J Immunol. 1994;24:1945–1948. doi: 10.1002/eji.1830240837. [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Ampofo W, Koyanagi Y, Yamashita A, Waki M, Matsumoto A, Yamamoto M, Yamamoto N. High-level production of alternatively spliced soluble interleukin-6 receptor in serum of patients with adult T-cell leukaemia/HTLV-I-associated myelopathy. Immunol. 1998;95:360–369. doi: 10.1046/j.1365-2567.1998.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Novick D, Horiuchi S, Yamamoto N, Szalai AJ, Fuller GM. C-reactive protein: a physiological activator of interleukin 6 receptor shedding. J Exp Med. 1999;189:599–604. doi: 10.1084/jem.189.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Jones SA, Jones GW, Williams AS, Nowell MA. Appreciating the balance between classical Interleukin (IL)-6 receptor signaling and IL-6 trans-signaling: implications for arthritis progression. IEMAMC. 2008;8:235–246. [Google Scholar]
- Kargotich S, Goodman C, Keast D, Morton AR. The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med. 1998;26:101–117. doi: 10.2165/00007256-199826020-00004. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, Hidalgo J, Pedersen BK. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J. 2005;19:1181–1183. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002;32:53–73. doi: 10.2165/00007256-200232010-00003. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Hurst SM, Nowell MA, Harris DA, Horiuchi S, Morgan LW, Wilkinson TS, Yamamoto N, Topley N, Jones SA. Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. J Immunol. 2004;172:5676–5683. doi: 10.4049/jimmunol.172.9.5676. [DOI] [PubMed] [Google Scholar]
- Meckel Y, Eliakim A, Seraev M, Zaldivar F, Cooper DM, Sagiv M, Nemet D. The effect of a brief sprint interval exercise on growth factors and inflammatory mediators. J Strength Cond Res. 2009;23:225–230. doi: 10.1519/JSC.0b013e3181876a9a. [DOI] [PubMed] [Google Scholar]
- Mullberg J, Oberthur W, Lottspeich F, Mehl E, Dittrich E, Graeve L, Heinrich PC, Rose-John S. The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site. J Immunol. 1994;152:4958–4968. [PubMed] [Google Scholar]
- Niess AM, Fehrenbach E, Strobel G, Roecker K, Schneider EM, Buergler J, Fuss S, Lehmann R, Northoff H, Dickhuth HH. Evaluation of stress responses to interval training at low and moderate altitudes. Med Sci Sports Exerc. 2003;35:263–269. doi: 10.1249/01.MSS.0000048834.68889.81. [DOI] [PubMed] [Google Scholar]
- Nowell MA, Richards PJ, Yamamoto N, Rose-John S, Topley N, Horiuchi S, Williams AS, Jones SA. Soluble IL-6R receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by glycoprotein 130. J Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans—effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009;107:1006–1014. doi: 10.1152/japplphysiol.00734.2009. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Scherling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Keller C, Keller P, Jauffred S, Pedersen BK. Immunohistochemical detection of interleukin-6 in human skeletal muscle fibers following exercise. FASEB J. 2003;17:2166–2168. doi: 10.1096/fj.03-0311fje. [DOI] [PubMed] [Google Scholar]
- Peters M, Jacobs S, Ehlers M, Vollmer P, Mullberg J, Wolf E, Brem G, Meyer zum Büschenfelde KH, Rose-John S. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson-Ansley P, Barwood M, Canavan J, Hack S, Eglin C, Davey S, Hewitt J, Hull J, Ansley L. The effect of repeated endurance exercise on IL-6 and sIL-6R and their relationship with sensations of fatigue at rest. Cytokine. 2009;45:111–116. doi: 10.1016/j.cyto.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300:281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain D, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2007;102:1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- Walshe I, Ansley L, Robson-Ansley P (2009) A reduction in plasma soluble IL-6R concentration during recovery from eccentric exercise. 9th Symposium of the International Society of Exercise and Immunology 9:85