Abstract
Co-chaperone HOP (also called stress-inducible protein 1) is a co-chaperone that interacts with the cytosolic 70-kDa heat shock protein (HSP70) and 90-kDa heat shock protein (HSP90) families using different tetratricopeptide repeat domains. HOP plays crucial roles in the productive folding of substrate proteins by controlling the chaperone activities of HSP70 and HSP90. Here, we examined the levels of HOP, HSC70 (cognate of HSP70, also called HSP73), and HSP90 in the tumor tissues from colon cancer patients, in comparison with the non-tumor tissues from the same patients. Expression level of HOP was significantly increased in the tumor tissues (68% of patients, n = 19). Levels of HSC70 and HSP90 were also increased in the tumor tissues (95% and 74% of patients, respectively), and the HOP level was highly correlated with those of HSP90 (r = 0.77, p < 0.001) and HSC70 (r = 0.68, p < 0.01). Immunoprecipitation experiments indicated that HOP complexes with HSC70 or HSP90 in the tumor tissues. These data are consistent with increased formation of co-chaperone complexes in colon tumor specimens compared to adjacent normal tissue and could reflect a role for HOP in this process.
Keywords: Colonic carcinoma, Heat shock protein, HOP, Molecular chaperone, Protein folding
Introduction
Molecular chaperones play essential roles in the folding of newly synthesized proteins and the refolding of denatured proteins (Bukau et al. 2006; Young et al. 2004). The 70-kDa heat shock protein (HSP70) and 90-kDa heat shock protein (HSP90) are molecular chaperones abundant in the cytosol and exert distinct functions in the protein folding pathway (Nollen and Morimoto 2002; Wegele et al. 2004). HSP70 recognizes hydrophobic surfaces of unfolded and partially folded substrate proteins and facilitates productive folding of proteins by preventing aggregation of the folding intermediates, and this function is exerted by concerted actions of the amino-terminal nucleotide binding domain and carboxyl-terminal substrate binding domain upon ATP binding and hydrolysis (Jiang et al. 2005; Vogel et al. 2006). Although HSP90 also assists in the productive folding of proteins, this chaperone appears to have more specialized functions in the folding/assembly pathways (Csermely et al. 1998; Pratt and Toft 2003; Wegele et al. 2004). For example, signaling protein kinases (e.g., Cdk4, Cdk6, and Raf family kinases) are stabilized by HSP90 in the presence of Cdc37 prior to the formation of functional complexes or final enzymatic forms (Grbovic et al. 2006; Vaughan et al. 2006). HSP90 associates with steroid hormone receptors (e.g., glucocorticoid receptor, androgen receptor, and estrogen receptor) and regulates the transport and maturation of the receptors (Carrigan et al. 2005; Fiskus et al. 2007). We have shown that the HSP70 and HSP90 chaperones strongly interact with a number of medical drugs (Ishida et al. 2008; Miyazaki et al. 2004; Otaka et al. 2007).
The HSP90/HSP70-organizing protein (HOP, also called stress-inducible protein 1) is a multi-domain co-chaperone that regulates the chaperone activities of HSP90 and HSP70 (Chen and Smith 1998; Johnson et al. 1998). HOP has three tetratricopeptide (TPR) domains: TPR1, TPR2A, and TPR2B, from the amino terminus to calboxyl terminus. HSP70 and HSP90 bind to HOP through the TPR1 and TPR2A domains, respectively (Brinker et al. 2002; Richter et al. 2003; Scheufler et al. 2000; Wegele et al. 2003). Folding of the client proteins is considered to be assisted by HSP70 and HSP90 in a sequential manner under the control by HOP (Nollen and Morimoto 2002; Pratt and Toft 2003; Wegele et al. 2004; Wegele et al. 2006). The organized action of the two chaperones prevents misfolding and aggregation of proteins, and thus facilitates productive folding of proteins and formation of functional protein complexes (Carrigan et al. 2005). Kamal et al. (2003) indicated that HSP90 forms complexes with co-chaperones HOP and p23 in tumor tissues and cultured tumor (BTB474, MCF7, and Hs578t) cells (Kamal et al. 2003). HSP90-containing complexes purified from tumor (BT474, N87, SKOV3, SKBR3, MCF7, A549, HT29, MDA468, SKMG3, U87, HT1080, and Hs578t) cells showed significantly high affinity to 17-allylaminogeldanamycin (17-AAG), an HSP90-binding anti-cancer drug, when compared with those purified from normal (NDF, RPTEC, HMVEC, HMEC, HUVEC, Hs578Bst, and PBMC) cells. HSP90 complexes from tumor cells had higher ATPase activity than those of normal cells. From these observations, these authors suggested that the HSP90 contained in multi-chaperone complex strongly binds 17-AAG. In the study of Kamal et al., however, no investigation of the expression levels of HSP90 or co-chaperones in clinical samples was reported, although these authors reported by western blot analysis of cultured cells that the total Hsp90 protein levels did not vary greatly between tumor and normal cells and that neither the total levels of p23 and HOP nor the growth rate of the tumor cells differed significantly from those of the normal cells. In other studies, levels of the HSP90 and HSP70 proteins have been reported to be increased in tumor tissues (Ciocca and Calderwood 2005). However, the expression levels of HOP in clinical tumor samples remain to be investigated.
Many HSP90 client proteins are known to be associated with cancer phenotypes. For example, Akt, Src, Raf-1, Bcr-Abl, and ErbB2 are HSP90-dependent protein kinases that are involved in signal transduction pathways required for abnormal cancer cell growth by self-sufficient signals (Neckers 2002). HSP90 regulates the activity of the tumor suppressor protein p53 (Muller et al. 2004), and HSP90 regulates the degradation of hypoxia-inducible factor 1, a transcription factor important for tumor cell survival under hypoxic conditions (Isaacs et al. 2002). These observations indicate that HSP90 plays crucial roles in cancer cell growth and survival by chaperoning tumorigenic proteins.
Here, we quantified the amount of HOP, HSP90, and HSC70 (cognate of HSP70, a major cytosolic HSP70 family protein expressed both in non-stress and stress conditions) in clinical tumor samples from colon cancer patients, along with a comparison to the surrounding normal tissues, by western blotting. HOP level was frequently increased in the tumor tissues concomitant with those of HSP90 and HSC70. Immunoprecipitation experiments indicated that HOP associated with HSP90 or HSC70 in the tumor samples containing abundant HOP and the two chaperones. The increased complex formation is consistent with the co-regulated high expression of HOP and partner chaperones, and the increased HOP may play a role in this process.
Materials and methods
Tissue sampling
Nineteen colon cancer patients (11 males and 8 females in age from 57 to 84) were enrolled in this study at the Department of Gastroenterological Surgery, Akita University Graduate School of Medicine. A written informed consent was obtained from all patients, and the protocol of this study was approved by the ethical committee of Akita University Graduate School of Medicine (No. 10-4). Samples of the primary tumors (one cecal, seven ascending, six transverse, one descending, and four sigmoid colon cancers) and vicinal non-neoplastic tissues were obtained from surgical specimens. Tissue samples were immediately frozen and stored at −80°C until analysis.
Preparation of human recombinant HOP
First strand cDNAs were synthesized from human HL-60 cell RNA by SuperScript III kit (Invitrogen, Carlsbad, USA). HOP cDNA was amplified by the polymerase chain reaction using specific primers (5′-ATTCGATTCAACGGGGTTC-3′ and 5′-CAGCTCCTCTTTCCACATGA-3′). Amplified HOP cDNA was cloned into the PGEM-T Easy vector (Promega, Madison, USA) and subcloned into the KpnI-EcoRI site of the pCold I His-tagged protein expression vector (Takara, Tokyo, Japan). The pCold I-based HOP expression vector was transformed into Eschericha coli BL21, and bacteria were grown in L-broth at 37°C. Expression of His-tagged HOP was induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside for 24 h at 15°C. Bacteria were resuspended in 10 mM Tris–HCl (pH 7.4) and lysed by sonication on ice. Supernatant was recovered after centrifugation at 15,000 rpm for 5 min at 4°C and applied to a Ni-NTA affinity column (GE Healthcare, Amersham Place, UK) equilibrated with buffer A (300 mM NaCl and 10 mM Tris–HCl pH 7.4) supplemented with 20 mM imidazole. After washing the column with 50 mM imidazole/buffer A, proteins were eluted with 300 mM imidazole/buffer A.
Antibodies
HOP purified by Ni-NTA affinity column chromatography was separated by SDS-PAGE using 9% gels and blotted onto nitrocellulose membranes. After membranes were stained with Coomasie Brilliant Blue, HOP bands were excised and eluted with dimethyl sulfoxide. Polyclonal antibody against HOP was prepared by immunization of rabbits with the protein eluted from membranes. Rabbit polyclonal antibody against HSP90 was prepared as described previously (Itoh and Tashima 1990). Rat monoclonal antibody to HSC70/HSP73 (clone 1B5) and mouse monoclonal antibody to HSP90 (AC88) were purchased from StressGen (Victoria, Canada). Mouse monoclonal antibodes to β-actin (AC-15) and Akt1 (B-1) were obtained from Sigma (St Louis, USA) and Santa Cruz Biotechnology (Santa Cruz, USA), respectively. According to the new nomenclature guideline proposed by Kampinga et al. (2009), HSP70 and HSC70 are HSPA1A and HSPA8, respectively. HSP90 is considered to be composed of HSP90α (HSPC1 in the new guideline) and HSP90β (HSPC3)
Western blotting
Tissue specimens were homogenized in lysis buffer containing 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, and 25 mM HEPES-KOH (pH 7.4) on ice, and supernatant was recovered after centrifugation (15,000 rpm, 15 min, 4°C). Protein concentration was determined by the bicinchoninic acid method (Smith et al. 1985) using BCA Protein Assay kit (Pierce, Rockford, USA) and bovine serum albumin as a standard. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using 10% gels and blotted onto polyvinylidene difluoride filters. Detection of proteins with specific antibodies was performed as described previously (Yokota et al. 1999). Digital images of blots were analyzed by the public domain ImageJ program (US Natl. Inst. Health).
Cell culture and viability test
Human colon cancer LS174T cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, in the presence or absence of 17-AAG (Sigma). Cell viability was analyzed by using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (Promega, Madison, USA) as a substrate.
Immunoprecipitation
Tissue extracts were prepared as described above and incubated with the rabbit polyclonal antibody against HOP. Protein A/G Plus-Agarose (Santa Cruz Biotechnology, Santa Cruz, USA) was added to the extracts and washed three times in lysis buffer. Proteins bound to the gel were eluted in SDS-PAGE sample buffer and separated by SDS-PAGE using 10% gels. Eluted proteins were analyzed by western blotting using specific antibodies.
Immunohistochemistry
Tissue samples were fixed in 4% formaldehyde, and immunohistochemical staining of paraffin sections (3 μm) was carried out using iVIEW DAB kit (Roche Diagnostics, Basel, Switzerland) according to manufacturer’s instructions. Briefly, after blocking endogenous peroxidase activity and non-specific protein binding, sections were sequentially incubated with anti-HOP antibody (1:500), biotinylated anti-rabbit immunoglobulin, and peroxidase-conjugated streptavidin. Sections were developed with 3,3′-diaminobenzidine and counterstained with hematoxylin.
Statistical analysis
Significance of difference between two groups was analyzed by the Wilcoxon signed-rank test or Student’s t test. Correlation of the levels of tumor/non-tumor ratios against two proteins was analyzed by Spearman’s rank correlation test.
Results
Increased expression of HOP, HSC70, and HSP90 in tumor tissues
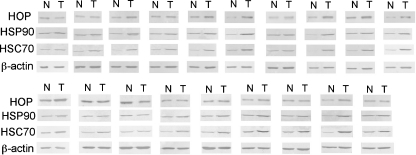
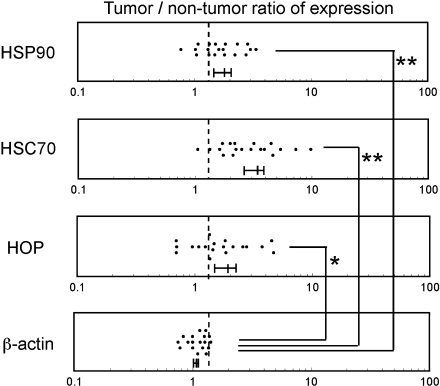
Tumor tissues and surrounding non-tumor tissues were obtained from the same colonic carcinoma patients (n = 19) at the time of surgery, and protein expression level of HOP was analyzed by western blotting (Fig. 1). We also analyzed levels of HSC70 and HSP90, chaperones known to interact with HOP, as well as level of β-actin as a control. Intensity of each band was quantified, and tumor/non-tumor ratios of the individual proteins in the same patients were determined (Fig. 2). The expression level of HOP was frequently increased in the tumor tissues obtained from the colon cancer patients, as 68% of the patients showed tumor/non-tumor ratios greater than 1.3. The levels of HSC70 and HSP90 were also increased (95% and 74% of patients, respectively), whereas the level of β-actin used as a control was mostly unaffected (only increased in 13% of patients). These results indicated that the levels of HOP, HSC70, and HSP90 are frequently increased in the tumor tissue.
Fig. 1.
Protein expression levels of HOP, HSC70, HSP90, and actin in tumor and non-tumor tissues from colon cancer patients. Soluble proteins were extracted from the tumor and non-tumor tissues of the same patients. These proteins (2 μg/lane for HSP90 and HSC70 or 1 μg/lane for HOP and β-actin) were separated by SDS-PAGE and analyzed by western blotting using the rabbit antibody to HSP90, rat antibody to HSC70, rabbit antibody to HOP, or mouse antibody to β-actin. Tissue samples from 19 colon cancer patients were tested. N non-tumor tissue, T tumor tissue
Fig. 2.
Relative expression levels of HOP, HSC70, HSP90, and actin in tumor tissues. Expression levels of proteins in tumor and non-tumor tissues were analyzed by western blotting as described in Fig. 1 and quantified by digital image analysis after scanning the blots. Tumor/non-tumor ratios were calculated from the quantified data. Dotted lines indicate a tumor/non-tumor ratio of 1.3. Mean value with standard errors for each group is indicated under the plots. Significance of difference was analyzed by the Wilcoxon signed-rank test. *p < 0.01, **p < 0.001
Although we examined whether the degree of upregulation of HOP, HSC70, or HSP90 in the colonic cancer tissues showed correlation with the degree of tumor differentiation, disease stage, metastatic phenotype, or location, no significant correlation was observed (data not shown). Investigation of a larger number of tumors is probably required to determine the exact correlation between the protein levels and tumor phenotypes.
Increased expression of HOP in colonic carcinoma cells in vivo
We next employed immunohistochemical analysis to examine whether the increased expression of HOP in tumor tissues is caused by upregulation in carcinoma cells. Immunostaining of the tumor tissue samples indicated that the expression level of HOP in colonic carcinoma cells is significantly higher than those in normal cells that comprise neighboring epithelial and connective tissues (Fig. 3). These results clearly indicated that the expression of HOP is upregulated in colon carcinoma cells in vivo.
Fig. 3.
Immunohistochemical staining of HOP in colonic carcimoma cells. Sections of a colon cancer and b surrounding normal colon tissue from the same patient were stained with the rabbit antibody against HOP. HOP proteins are stained in brown. Bar = 200 μm
Correlated expression of HOP, HSP90, and HSC70
To examine whether the levels of HOP and interacting chaperones are simultaneously upregulated in the tumor tissues of the same colon cancer patients, the tumor/non-tumor ratio of the HOP expression level was plotted against those of the HSP90 or HSC70 (Fig. 4). The degree of upregulation of HOP in the tumor tissue strongly correlated with that of HSP90 (r = 0.77, p < 0.001) and to a lower but significant degree with that of HSC70 (r = 0.67, p < 0.01). The degree of upregulation of HSC70 also showed a strong correlation to that of HSP90 (r = 0.73, p < 0.001). These observations indicate that HOP and interacting chaperones, HSP90 and HSC70, are frequently co-upregulated in colon cancers in vivo.
Fig. 4.
HOP, HSC70, and HSP90 expression levels are significantly correlated in the tumor tissues of colon cancer patients. Correlation of expression levels for a HOP vs HSP90, b HOP vs HSC70, and c HSC70 vs HSP90 was analyzed by using the tumor/non-tumor ratio shown in Fig. 2
Increased formation of HOP-HSC70 and HOP-HSP90 complexes in tumor tissues
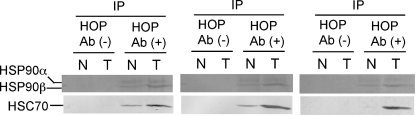
The significantly correlated expression of HOP and partner chaperones, HSP90 and HSC70, suggested that the simultaneous upregulation of these proteins may stimulate the formation of HOP-chaperone complexes in tumor tissues. We examined whether HOP associated with HSP90 or HSC70 in colon cancer tissues by immunoprecipitation. After soluble proteins were extracted from tumor and non-tumor tissues, HOP and associating proteins were recovered using an antibody against HOP. Western blotting analysis of the HOP-binding proteins indicated that the amount of HOP-HSP90 complex is significantly higher in the tumor tissues than in the non-tumor tissues (Fig. 5). Moreover, a very high level of HOP-HSC70 complex was detected in the tumor tissues. These results indicate that colon cancer tissues contain a high level of HOP-chaperone complexes probably due to the simultaneous upregulation of HOP and partner chaperones.
Fig. 5.
Amounts of HOP-HSC70 and HOP-HSP90 complexes are significantly increased in tumor tissues of colon cancer patients. Protein complexes containing HOP were recovered from tissue extracts by immunoprecipitation using the rabbit antibody to HOP and analyzed by western blotting using the mouse antibody to HSP90 or rat antibody to HSC70. HOP Ab (+) and Ab (−) denote the presence and absence of HOP antibody for immunoprecipitation, respectively. Data from three representative patients are shown. N non-tumor tissue, T tumor tissue
HOP-chaperone complexes are formed in cultured colon cancer cells
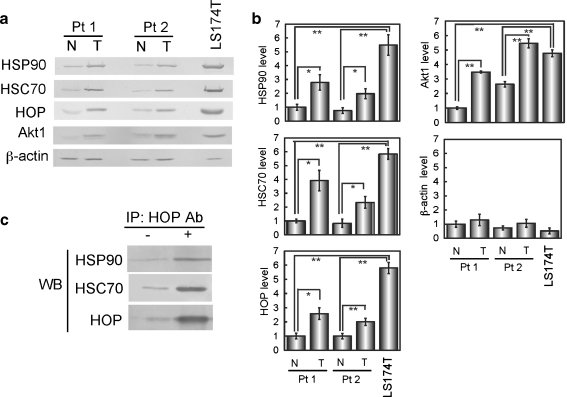
To confirm that HOP-HSC70 and HOP-HSP90 complexes are formed in colon cancer cells, we used LS174T, a colon cancer cell line. Western blotting of HSC70, HSP90, and HOP indicated that these proteins are significantly upregulated in the cultured cell line relative to normal colon tissues of patients (Fig. 6a,b). Immunoprecipitation followed by western blotting confirmed that HOP-HSC70 and HOP-HSP90 complexes are detectable in the cultured colon cancer cells (Fig. 6c). These results support the notion that upregulation of the chaperone and co-chaperone proteins plays a role in complex formation. In addition, level of Akt1, a client of HSP90, was significantly increased in LS174T cells as well as in colon tumor tissues, consistent with the fact that Akt1 is a protein kinase that inhibits apoptosis and stimulates cell growth.
Fig. 6.
HOP is highly expressed and complexes with HSC70 or HSP90 in cultured colon cancer cells. a Increased expression of HOP, HSC70, and HSP in colon cancer LS174T cells. Levels of indicated proteins in LS174T cells were analyzed by western blotting in comparison with those of tumor and normal tissues of two colon cancer patients. b Band intensity was quantified from three independent blots. Significance of difference was analyzed by Student’s t test. *p < 0.05, **p < 0.01. c HOP-containing complexes were recovered from LS174T cells by immunoprecipitation with anti-HOP antibody and analyzed by western blotting
Growth inhibition of colon cancer cells by 17-AAG
To test whether inhibition of HSP90 is useful to block colon cancer cell growth, LS174T cells were treated with 17-AAG. Cell growth of LS174T was significantly inhibited by 0.1–1 μM 17-AAG (Fig. 7a). Expression level of Akt1 was strongly decreased by 1 μM 17-AAG (Fig. 7b,c). In addition, expression of HSP70 is stimulated by 17-AAG, suggesting that the colon cancer cells underwent cytosolic protein-misfolding stress due to inhibition of chaperone activity by 17-AAG. These results suggest that inhibition of HSP90 by 17-AAG may be useful to treat colon cancers, although examination in vivo is required to test the possibility.
Fig. 7.
Growth inhibition of cultured colon cancer cells by 17-AAG. a LS174T cells were cultured in the presence of 1–1,000 nM 17-AAG for 24 h, and cell viability was analyzed by MTT assay (n = 4). Cell viability in the absence of 17-AAG is set as 100%. b LS174T cells were cultured in the presence of 10–1,000 nM 17-AAG for 24 h, and cell lysates were analyzed by western blotting. c Band intensity was quantified from three independent blots. Significance of difference was analyzed by Student’s t test. *p < 0.05, **p < 0.01
Discussion
HOP plays crucial roles in protein folding by regulating chaperone activities of HSP90 and HSP70 (HSC70) (Nollen and Morimoto 2002; Pratt and Toft 2003; Wegele et al. 2004). In the present study, we have shown that the expression levels of HOP and partner chaperones, HSP90 and HSC70, are frequently upregulated in colon cancer tissues relative to normal colon tissues (Figs. 1 and 2). Upregulation of HOP is evident in colon carcinoma cells in vivo (Fig. 3). The expression level of HOP is significantly correlated with those of HSP90 and HSC70 (Fig. 4). These results are consistent with previous observations that HOP is associated with HSP70 proteins in colon cancer cell lines under stress conditions (Noonan et al. 2008, 2007). Taken together, these observations indicate that HOP and the partner chaperones are simultaneously upregulated in colon cancers in vivo in a significant number of patients.
HSP90 plays crucial roles in the production of tumor-promoting signal transducer proteins (Neckers 2002). For example, Akt (Sato et al. 2000), Bcr-Abl (An et al. 2000), Raf-1 (Schulte et al. 1996), and mutant p53 (Blagosklonny et al. 1996) require HSP90 association for molecular maturation. Inhibition of HSP90 activity by geldanamycin or 17-AAG abrogates the maturation process (Sreedhar et al. 2004), which results in degradation of the client proteins. These observations are consistent with the fact that 17-AAG is a useful drug to treat cancers (Workman et al. 2007). Kamal et al. (2003) reported that HSP90 formed complexes with HOP and p23 in tumor tissues and that the complex-forming HSP90 showed a very high affinity to 17-AAG (Kamal et al. 2003). These observations suggest that the HSP90-containing chaperone complex is a potential target for anti-cancer therapies.
HOP is known to bind HSC70 and HSP90 using different TPR domains (TPR1 and TPR2A domains, respectively) (Brinker et al. 2002; Richter et al. 2003; Scheufler et al. 2000; Wegele et al. 2003). In the present study, we confirmed by immunoprecipitation that HOP forms complexes with HSC70 or HSP90 in tumor tissues (Fig. 5) and cultured colon cancer cells (Fig. 6). However, immunoprecipitation of HSP90 followed by western blotting of HSC70 indicated that HSP90 and HSC70 rarely exist in the same complex even in the tumor tissues abundantly containing the three proteins (data not shown). Similarly, HSC70-HOP-HSP90 complex was hardly detected in cultured colon cancer cells (data not shown). As HOP has been suggested to mediate substrate transfer from HSP70 (HSC70) to HSP90 (Wegele et al. 2006), these proteins may only transiently form an HSC70-HOP-HSP90 complex for substrate transfer in tumor cells in vivo and cultured cells. Alternatively, HOP-HSC70 and HOP-HSP90 complexes play different roles in colon cancer cells.
Although we cannot conclude whether HSP90-containing chaperone complexes are specific target of 17-AAG in colon cancers, growth inhibition of cultured colon cancer cells by 17-AAG concomitant with decreased expression of Akt-1, an HSP90 client protein (Fig. 7), suggests that this drug may be useful to treat colon cancers.
Acknowledgments
SY was supported by a Sasakawa Scientific Research Grant from the Japan Science Society, and HK was supported by a grant from Suzuken Memorial Foundation.
Abbreviations
- HSP
heat shock protein
- HOP
HSP70/HSP90 organizing protein
References
- An WG, Schulte TW, Neckers LM. The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 2000;11:355–360. [PubMed] [Google Scholar]
- Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker A, Scheufler C, Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 × Hop × Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Carrigan PE, Riggs DL, Chinkers M, Smith DF. Functional comparison of human and Drosophila Hop reveals novel role in steroid receptor maturation. J Biol Chem. 2005;280:8906–8911. doi: 10.1074/jbc.M414245200. [DOI] [PubMed] [Google Scholar]
- Chen S, Smith DF. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem. 1998;273:35194–35200. doi: 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, Bhalla K. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res. 2007;13:4882–4890. doi: 10.1158/1078-0432.CCR-06-3093. [DOI] [PubMed] [Google Scholar]
- Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, Rosen N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci USA. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- Ishida R, Takaoka Y, Yamamoto S, Miyazaki T, Otaka M, Watanabe S, Komatsuda A, Wakui H, Sawada K, Kubota H, Itoh H. Cisplatin differently affects amino terminal and carboxyl terminal domains of HSP90. FEBS Lett. 2008;582:3879–3883. doi: 10.1016/j.febslet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Itoh H, Tashima Y. A novel testis-specific 105-kDa protein related to the 90-kDa heat-shock protein. Eur J Biochem. 1990;193:429–435. doi: 10.1111/j.1432-1033.1990.tb19356.x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Prasad K, Lafer EM, Sousa R. Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell. 2005;20:513–524. doi: 10.1016/j.molcel.2005.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Kampinga H, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111 [DOI] [PMC free article] [PubMed]
- Miyazaki T, Sagawa R, Honma T, Noguchi S, Harada T, Komatsuda A, Ohtani H, Wakui H, Sawada K, Otaka M, Watanabe S, Jikei M, Ogawa N, Hamada F, Itoh H. 73-kDa molecular chaperone HSP73 is a direct target of antibiotic gentamicin. J Biol Chem. 2004;279:17295–17300. doi: 10.1074/jbc.M312217200. [DOI] [PubMed] [Google Scholar]
- Muller L, Schaupp A, Walerych D, Wegele H, Buchner J. Hsp90 regulates the activity of wild type p53 under physiological and elevated temperatures. J Biol Chem. 2004;279:48846–48854. doi: 10.1074/jbc.M407687200. [DOI] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–61. doi: 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B' regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan E, Giardina C, Hightower L. Hsp70B' and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–2476. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Otaka M, Yamamoto S, Ogasawara K, Takaoka Y, Noguchi S, Miyazaki T, Nakai A, Odashima M, Matsuhashi T, Watanabe S, Itoh H. The induction mechanism of the molecular chaperone HSP70 in the gastric mucosa by Geranylgeranylacetone (HSP-inducer) Biochem Biophys Res Commun. 2007;353:399–404. doi: 10.1016/j.bbrc.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J Biol Chem. 2003;278:10328–10333. doi: 10.1074/jbc.M213094200. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF, Nguyen P, Trepel J, Neckers LM. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–5845. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sreedhar AS, Soti C, Csermely P. Inhibition of Hsp90: a new strategy for inhibiting protein kinases. Biochim Biophys Acta. 2004;1697:233–242. doi: 10.1016/j.bbapap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, Robinson CV, Saibil HR, Pearl LH. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M, Bukau B, Mayer MP. Allosteric regulation of Hsp70 chaperones by a proline switch. Mol Cell. 2006;21:359–367. doi: 10.1016/j.molcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Wegele H, Haslbeck M, Reinstein J, Buchner J. Sti1 is a novel activator of the Ssa proteins. J Biol Chem. 2003;278:25970–25976. doi: 10.1074/jbc.M301548200. [DOI] [PubMed] [Google Scholar]
- Wegele H, Muller L, Buchner J. Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- Wegele H, Wandinger SK, Schmid AB, Reinstein J, Buchner J. Substrate transfer from the chaperone Hsp70 to Hsp90. J Mol Biol. 2006;356:802–811. doi: 10.1016/j.jmb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth. Preferential expression and binding to tubulin at G(1)/S transition through early S phase. J Biol Chem. 1999;274:37070–37078. doi: 10.1074/jbc.274.52.37070. [DOI] [PubMed] [Google Scholar]
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]