Abstract
The myocardial stress response to exercise is dependent on exercise intensity and thus understanding the molecular responses between various exercise intensity levels might aid in exercise prescription. Nuclear factor kappa B (NF-κB) is a ubiquitous transcription factor that mediates a variety of cellular processes including inflammation, immune responses, apoptosis and cell growth/development. NF-κB can be comprised of homo- and/or heterodimers formed from five distinct proteins: p50 (NF-κB1), p52 (NF-κB2), RelA (p65), c-Rel, and RelB. NF-κB is located in the cytoplasm and kept inactive by inhibitory proteins but following the exposure to a myriad of stimuli, an activated NF-κB dimer translocates to the nucleus and exerts transcriptional effects on upwards of 150 genes. To examine the activation of NF-κB in the myocardium following exercise, male Sprague–Dawley rats (n = 24) were exercised by treadmill running at 20 m/min for 30 min or 30 m/min for 20 min. At 0, 2, or 24 h following exercise, animals were anesthetized, hearts excised and immediately frozen in liquid nitrogen. Portions of hearts were homogenized, protein concentrations determined and extracts assayed for NF-κB activation (DNA binding activity) using electrophoretic mobility shift assays (EMSA). Visual examination of EMSA autoradiographs revealed an enhanced NF-κB activation in the hearts from exercised animals when compared with non-running controls. Subsequent supershift analyses using antibodies specific for NF-κB subunits showed the higher intensity exercise was associated with p65 (RelA) in the activated NF-κB complex while the NF-κB complex in hearts from animals exercised at the lower intensity was comprised primarily of p50. These data suggest exercise is capable of activating myocardial NF-κB and that a threshold for the activation of specific NF-κB subunits may exist.
Keywords: Exercise, NF-κB, Myocardium, Rat
Introduction
The cellular response of the myocardium to exercise varies based on a number of factors, including intensity. In view of this, low-intensity exercise is generally considered a eustress possibly creating a hormesis-like effect, while high-intensity exercise is considered more of a distress and may exacerbate underlying pathologies. Thus, understanding the cellular responses between different exercise intensity levels may be valuable for developing safe exercise prescription.
Nuclear factor kappa B (NF-κB) is a redox sensitive transcription factor involved in a variety of cellular processes including inflammation, immune responses, cytokine/chemokine production, apoptosis, as well as cell growth and development (for review see Gilmore 2006). In skeletal muscle, NF-κB activation plays a key role in mediating muscle fiber atrophy (Cai et al. 2004; Bar-Shai et al. 2008). Thus, determining whether NF-kB is activated in the myocardium during or following exercise may provide insight in to how the myocardium adapts to physiological stressors.
In vertebrates, NF-κB consists of homodimers or heterodimers comprised of various subunits: p50 (NF-κB1), p52 (NF-κB2) p65 (RelA), RelB, and c-Rel. Depending upon the stimulus, NF-κB can be activated by at least three different pathways: a classical pathway (canonical), an alternative pathway (non-canonical), or a third pathway (pathway 3). In the classical pathway, cytoplasmic NF-κB is kept inactive due to its binding to an inhibitory protein, known as I kappa B (IκB). When cells are exposed to a variety of agents, IκB becomes phosphorylated and subsequently degraded by the ubiquitin proteasome pathway (Sun and Ley 2008). This allows the activated NF-κB complex (usually a p50/p65 heterodimer) to translocate to the nucleus where it binds to kappa B sequences and alters the expression of various target genes (Werner et al. 2005). In the alternative pathway, p100 is phosphorylated and processed to create an NF-κB complex consisting of p52/RelB which translocates to the nucleus and influences target genes (Senftleben et al. 2001). A third pathway involving p105 processing results in a p50/p50 homodimer that interacts with a coactivator, Bcl-3 (Hunter et al. 2002; Gilmore 2006). The variations in NF-κB subunit composition and subsequent modification allows for potentially distinct sets of genes to be expressed. For example, Bakkar et al. (2008) showed that NF-κB activation consisting of the p65/p50 dimer (classical pathway) repressed myogenesis during its early stages, while the p52/RelB dimer (alternative pathway) promoted mitochondrial biogenesis during the later stages of myogenesis. Similarly, Hunter et al. (2002) showed that in skeletal muscle, a p50/p50/Bcl-3 complex was involved in regulating disuse atrophy. In view of these results, it seems likely that other dimer combinations may also promote or repress other important cellular pathways or processes.
An acute bout of exercise has been shown to activate a number of cellular pathways and several studies have investigated the effects of acute exercise on the activation of NF-κB in skeletal muscle (Hollander et al. 2001; Durham et al. 2004; Ji et al. 2004; Ho et al. 2005; Kramer and Goodyear 2007). Hollander et al. (2001) and Ji et al. (2004) showed a single bout of exercise increased NF-κB activation in the deep portion of rat vastus lateralis muscles and activation peaked at 2 h after the cessation of exercise. In contrast, Durham et al. (2004) showed exercise decreased NF-κB activation in both mouse and human skeletal muscles. Investigations of NF-κB activation in rat cardiac muscle following exercise are limited. Veneroso et al. (2009) has shown an enhanced NF-κB activation in the heart 2 h after exercise; however, specific subunits were not identified. Given that the effects of acute exercise on NF-κB activation in cardiac muscle have not been thoroughly investigated and that NF-κB plays a key role in mediating skeletal muscle atrophy, the aim of this study was to determine whether exercise varying in intensity and duration alters NF-κB activation in the myocardium and identify any subunits involved.
Materials and methods
Animals and exercise
Twenty-eight male Sprague–Dawley rats (Charles River, Quebec) were housed in pairs and maintained on a 12-h dark/light cycle, at 20 ±1°C, 50% relative humidity and provided food and water ad libitum. All animals were habituated to a motorized treadmill by walking or slowly running at less than 20 m/min (m/min) for 4–5 days. Following at least 2 days of rest, the rats were randomly divided into groups: non-running controls (n = 4), exercised at 20 m/min for 30 min (low intensity, n = 12), exercised at 30 m/min for 20 min (high intensity, n = 12). Each of the exercised groups was further subdivided based on three different recovery times, 0, 2, and 24 h post-exercise. Rectal temperatures were recorded prior to, and directly after exercise. Animals were anesthetized using pentobarbital through an intraperitoneal injection (65 mg/kg), hearts removed, frozen in liquid nitrogen and stored at −70°C until further processing.
Electrophoretic mobility shift assay
Portions of heart tissue (50 mg) were homogenized in 15 volumes of extraction buffer (25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA (pH 8.0), 20 mM HEPES (pH 7.9), 0.5 mM phenylmethylsulfonylflouride) in an ice bath. Homogenates were centrifuged at 14,000 rpm for 20 min at room temperature. The supernatant was removed, and protein concentration was determined (Lowry et al. 1951). Analyses of NF-κB or AP-1 DNA binding ability was determined using the procedure described by Frier et al. (2008). Extracts of 50 μg, were incubated with a 32P-labeled, NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; E3291, Promega or AP-1 5′-CGC TTG ATG AGT CAG CCG GAA-3′; E3201, Promega) in binding buffer (10% glycerol, 50 mM NaCl, 1.0 mM EDTA (pH 8.0), 20 mM Tris (pH 8.0), 1.0 mM DDT, 0.3 mg/ml BSA) with approximately 0.1 ng (50 000 cpm) of 32P-labeled oligonucleotide and 2.0 ug poly dI dC (Pharmacia Fine Chemicals, Piscataway, NJ, USA) for 30 min at room temperature, prior to electrophoresis on 4% acrylamide gel at 200 V for 2–3 h. Gels were dried with a BioRad Slab dryer (Model 433) and exposed to radiographic film (Bioflex MSI film, Clonex Corp, Markham, Canada) at −70°C. Films were scanned using Agfa Arcus II scanner and bands were quantified using Kodak 1D 2.0. Positive identification and location of NF-κB binding was carried out using recombinant NF-κB (p50; Promega, E3770) to confirm the presence and location of the NF-κB-DNA oligonucletide complex. To determine the specific subunit composition of the activated NF-κB complexes electrophoretic mobility shift assays (EMSA) supershifts were performed. Thirty minutes prior to electrophoresis, antibody to the NF-κB subunit (p50, RelA/p65, p52, RelB, c-Rel), (p50 (NLS; sc-114X), RelA/p65 (C-20; sc-372X), p52 (K-27; sc-298X), RelB (C-19; sc-226X), C-Rel (C; sc-71X), and Bcl-3 (C-14; sc-185X); Santa Cruz Biotechnology, CA, USA) was added to the labeled oligonucleotide-sample extract. Presence of the specific NF-κB subunit was identified with an upward shift of the visible protein-DNA complex.
Statistical analyses
Analysis of variance was used to compare differences in animal body mass, between control, low- and high-intensity exercised groups while a Student's t tests were used to evaluate differences in body temperature. For all tests, differences were considered to be statistically significant at a level of p ≤ 0.05.
Results
Body mass
Control and exercised (low and high intensity) animals were compared based on body mass. Control animals were heaviest (318.8 ± 16.3 g), followed by animals that were exercised at 20 m/min (313.7 ± 20.0 g) and animals exercised at 30 m/min (312.9 ± 19.2 g), respectively. There were no significant differences between the groups.
Rectal temperature
Prior to exercise, mean rectal temperatures were similar for animals exercised at either 20 m/min (37.6 ± 0.2°C) or 30 m/min (37.9 ± 0.1°C). The control (non-running) group had a mean rectal temperature of 37.1 ± 0.3°C, which was significantly lower (p < 0.5) than the animals to be exercised at 30 m/min. Following exercise, animals showed an increase in mean rectal temperatures of 2.3°C and 2.4°C for the 20 m/min and 30 m/min groups, respectively. In both low- (39.9 ± 0.6°C) and high-intensity (40.4 ± 0.3°C) groups, exercise resulted in a significant increase in rectal temperature (p < 0.001) when compared with pre-exercise temperatures (37.6 ± 0.5°C and 38.0 ± 0.5°C, respectively). In addition, the post-exercise temperatures of the low- (20 m/min) and high-intensity (30 m/min) groups were also significantly different (p > 0.01) indicating that the high-intensity group (30 m/min) was subjected to a greater exercise stress.
NF-κB activation and subunit composition
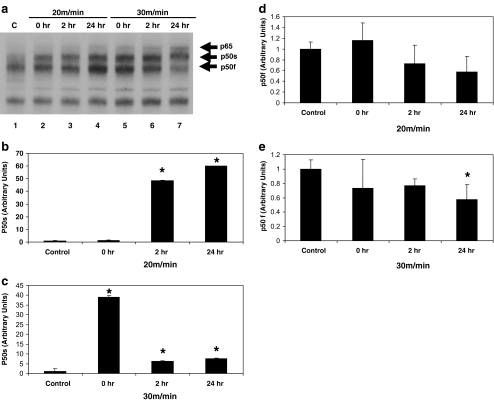
Visual analyses of autoradiographs from EMSAs revealed NF-κB-DNA binding in myocardial extracts from all animals (Fig. 1). When compared with non-exercised controls (Fig. 1a—lane 1), the hearts from exercised animals (Fig. 1a—lanes 2–7) showed the enhancement of a slow-migrating NF-κB band, labeled p50s. A fast-migrating p50 band, labeled p50f, was also detected and varied in intensity but was diminished in the hearts from animals exercised at 30 m/min and allowed 24 h of recovery (Fig. 1a—lane 7). In some cases, a new and even slower migrating band, labeled p65, was detected in hearts from exercised animals. This slower migrating band was detected to a greater extent in the hearts from animals that were exercised at the higher intensity (30 m/min; Fig. 1a—lanes 5–7) than at the lower intensity or in controls.
Fig. 1.
Myocardial activation of NF-κB subunit composition varies with exercise intensity and recovery from exercise. a Protein extracts were incubated with a 32P-labeled κB binding sequence and analyzed by EMSA and quantified as described in “Materials and methods”. Shown here is a portion of a representative autoradiogram. Lane 1, control (no running). Lane 2, 20 m/min, no recovery. Lane 3, 20 m/min, 2 h recover. Lane 4, 20 m/min, 24 h recovery. Lane 5, 30 m/min, no recovery. Lane 6, 30 m/min, 2 h recovery. Lane 7, 30 m/min, 24 h recovery. p65 and the fast and slow-migrating p50 NF-κB subunits are identified. b NF-κB (p50s) activation in heart extracts from animals exercised at 20 m/min. c NF-κB (p50s) activation in heart extracts from animals exercised at 30 m/min. d NF-κB (p50f) activation in heart extracts from animals exercised at 20 m/min. e NF-κB (p50f) activation in heart extracts from animals exercised at 30 m/min. Data are expressed as mean ± SD. An asterisk denotes a significant difference from control (p ≤ 0.05)
Quantification of p50 bands revealed significant (p < 0.05) increases in p50s in the hearts from five of six exercised groups when compared with the hearts from non-running animals (Fig. 1b, c). Only in hearts from animals that exercised for 20 m/min with no recovery was there no change in p50s activation. In hearts from animals that were exercised at the low intensity and allowed 2 or 4 h of recovery, a significant increase (p < 0.05) in p50s activation was observed. In addition, p50s activation was significantly increased (p < 0.05) in the animals that exercised at a high intensity (30 m/min) regardless of recovery time when compared with controls, (Fig. 1c). Quantification of p50f activation showed a significant decrease (p < 0.05) in hearts 24 h after exercising at the high intensity (Fig. 1e) but no change in hearts from the other five exercise groups (Fig. 1d, e).
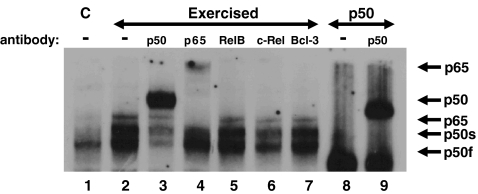
To determine the subunit composition of activated NF-κB detected, EMSA supershifts were performed. Visual analyses showed the NF-κB activation observed in hearts from exercised animals was primarily comprised of p50 and to a lesser extent p65 (Fig. 2—lanes 3 and 4) when compared with controls (Fig. 2—lane 1). Following the addition of antibodies specific for RelB, c-Rel and Bcl-3 no changes in mobility of the NF-κB complexes were detected (Fig. 2—lanes 5–7). However, following the addition of the p50 antibody, the two bands previously described as p50f and p50s were shifted suggesting two p50 subunits (Fig. 2—lane 3). As mentioned previously, p50f was predominantly present in controls, whereas both (the fast and the slow-migrating bands, p50s) appeared in the hearts from exercised animals (Figs. 1 and 2). The shifted p50 bands corresponded to shifts observed after p50 antibody was added to purified p50 protein (Fig. 2—lane 9).
Fig. 2.
The NF-κB complex activated following exercise is composed of p50 and p65. Rats were exercised at 30 m/min for 20 min, hearts removed and processed for EMSA supershifts as described in “Materials and methods”. Shown here is a portion of an autoradiogram. Lane 1, control (no running). Lane 2, 30 m/min, no recovery. Lane 3, 30 m/min, no recovery plus p50 antibody. Lane 4, 30 m/min, no recovery plus p65 antibody. Lane 5, 30 m/min, no recovery plus RelB antibody. Lane 6, 30 m/min, no recovery plus c-Rel antibody. Lane 7, 30 m/min, no recovery plus Bcl-3 antibody. Lane 8, purified p50 protein. Lane 9, purified p50 protein plus p50 antibody. p65 and the fast and slow-migrating p50 NF-κB subunits are identified
Following the addition of the p65 antibody, a shift in the migration of the slowest NF-κB migrating band was primarily detected in hearts from animals exercised at the higher intensity (Fig. 2—lane 4). Since this band was not detectable in the hearts from non-exercised (controls) animals it suggests that exercise is capable of inducing activation of the p65 NF-κB subunit. Taken together, these data suggest that exercise above a specific intensity is capable of causing NF-κB activation possibly by a p50/p65 dimer (classical pathway).
AP-1 activation following exercise
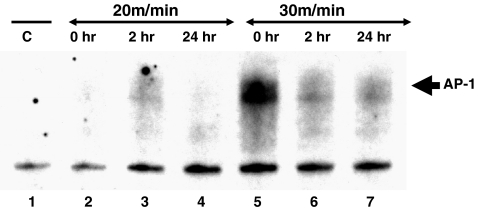
Given that the p65 NF-κB subunit was observed to be activated at the higher exercise intensity and that the p65 NF-κB subunit is known to play a role in a variety of cellular processes, including inflammation (Karin et al. 1997; Adcock and Caramori 2001), we tested hearts from exercised animals for the presence of AP-1, a transcription factor known to be activated during inflammation. Similar to p65, AP-1 activation was observed primarily in hearts from animals exercised at the higher intensity (Fig. 3—lanes 5–7) but not readily detectable in hearts from controls or animals that exercised at the lower intensity (Fig. 3—Lanes 1–4). These data suggest that exercise intensity may mediate specific NF-κB and/or AP-1 cellular responses.
Fig. 3.
High-intensity exercise activates AP-1. Protein extracts from hearts from animals exercised at 30 m/min for 20 min were incubated with a 32P-labeled AP-1 binding sequence and analyzed by EMSA as described in “Materials and methods”. Shown here is a portion of a representative autoradiogram. Lane 1, control, (no running). Lane 2, 20 m/min, no recovery. Lane 3, 20 m/min, 2 h recovery. Lane 4, 20 m/min, 24 h recovery. Lane 5, 30 m/min, no recovery. Lane 6, 30 m/min, 2 h recovery. Lane 7, 30 m/min, 24 h recovery. AP-1 is identified
Discussion
This study examined alterations in myocardial NF-κB activation (DNA binding ability) and subunit composition following acute bouts of exercise that differed in intensity and duration. The novel features of this paper are: a) acute exercise is capable of activating NF-κB in the myocardium, b) the NF-κB complex activated by exercise consists primarily of p50 and p65 subunits, and c) a threshold for the activation of specific NF-κB subunits exists. NF-κB is known to mediate a wide variety of cellular responses including inflammation, immune responses, cytokine/chemokine production, apoptosis, as well as cell growth and development (Werner et al. 2005; Gilmore 2006). The data presented herein suggest that depending upon the intensity of exercise, specific NF-κB subunits are activated which may possibly enhance or repress the expression of specific NF-κB-mediated genes. This may ultimately result in distinct NF-κB-mediated cellular responses.
In the present study, an increased NF-κB activation consisting of a slow-migrating p50 band (p50s) was observed in the hearts from all animals exercised except for animals exercised at the low intensity with no recovery. Given that the volume of exercise was the same between the two groups (20 m/min for 30 min or 30 m/min for 20 min), yet the exercise was varied in intensity (20 vs. 30 m/min) and duration (30 vs. 20 min) it suggests that a specific intensity is required before p50s is activated and that the stress during recovery from exercise at low intensities may also play a role in the activation of the p50s NF-κB subunit. In agreement with this, Ji et al. (2004) showed that in skeletal muscle, NF-κB was maximally activated at 2 h following an acute bout (60 min) of exercise.
In addition to the p50s band, a fast-migrating p50 band (p50f) was also observed in the hearts from all animals. However, when compared with controls, only the high intensity, 24-h recovery group showed a significant difference in p50f activation. All other groups showed no significant alterations in p50f activation despite a trend of decreased activation in five of the six exercise groups. Interestingly and somewhat in contrast to p50s, the low-intensity group with no recovery, showed a slight increase in p50f activation when compared with controls. While the exact relationship between the two NF-κB p50 subunits cannot be determined from the present study, the altered mobility of the p50 subunits observed is likely the result of modification. Perkins (2006) has outlined the various post-translational modifications of the NF-κB family proteins which include phosphorylations, acetylations, cysteine S-nitrosylations, and cysteine oxidations. In agreement with this, Pérez et al. (2000) found that following exposure to ultraviolet radiation, p50 becomes phosphorylated in mouse skin cells. To our knowledge, no studies have examined NF-κB modifications in the myocardium following exercise and it remains to be determined whether these same modifications or others occur following acute exercise. However, modifications of p50 might explain the apparent antithetical relationship observed between the two p50 subunits identified.
The p50 protein is normally inactive in the cytoplasm due to binding with p105 but following stimulation, p105 is processed to form the p50/p50 homodimer (Gilmore 2006). During disuse atrophy of skeletal muscle, the p50/p50 homodimer requires Bcl-3 as a coactivator (Hunter et al. 2002; Hunter and Kandarian 2004). However, our results showed that heart extracts from exercised animals demonstrated no shift in the mobility of the activated NF-κB complex following the addition of an antibody specific for Bcl-3 suggesting Bcl-3 is not involved in the activated NF-κB complex induced by acute exercise. Similarly, the addition of RelB or c-Rel specific antibodies to heart extracts from exercised animals also showed no shift in mobility of the activated NF-κB complex suggesting these subunits are also not likely involved in the NF-κB complex activated by exercise. Given that these proteins (RelB, c-Rel and Bcl-3) were not detected following EMSA supershifts, it suggests that the p50/p65 heterodimer, or p50/p50, p65/p65 homodimers are the major subunit combinations activated by exercise. Furthermore, given that p65 activation was primarily observed after high-intensity exercise, p65 likely combines with either itself, or more likely, with p50, which suggests exercise activates NF-κB via the classical (canonical) pathway. In contrast, the p50/p50 homodimer appears to be altered after a single bout of low-intensity exercise.
In its homodimeric form, the p50 subunit appears to act as a repressor (Grimm and Baeuerle 1993; Baldwin 1996; Brigelius-Flohé et al. 1996) and thus it follows that any exercise induced alteration in the activation of p50 subunits might decrease the expression of the genes controlled by this transcription factor. Thus, it may be the case that p50 homodimers repress specific genes, possibly leading to a decreased response following this type of NF-κB activation. In support of this, exercise training is known to provide systemic anti-inflammatory effects (Lira et al. 2009), although the mechanism for this remains unclear. It should also be noted that although exercise at both intensities appeared to activate p50, following high-intensity exercise any repressive effects of p50 may be diminished due to competitive and/or preferential p65 and p50 heterodimer formation (Hoffmann et al. 2006). In contrast, at the lower exercise intensity, p65 may not be activated allowing the purportedly repressive p50 homodimers to form and attenuate specific responses. Whether this is actually the case remains to be determined.
Visual examination of EMSA autoradiographs also showed the p65 (RelA) subunit of NF-κB was detected in hearts from animals that were exercised, yet p65 was not detected in hearts from controls. Interestingly, the p65 subunit was predominantly detected in the hearts from animals that were exercised at the higher intensity suggesting that a particular exercise intensity or threshold may be required to active this NF-κB subunit. NF-κB in the form of the p65/p50 heterodimer tends to act a transcriptional activator and is known to mediate several responses including remodeling, oxidative stress, inflammation, immune responses, cytokine/chemokine production, apoptosis, as well as cell growth and development (Flohé et al. 1997; Werner et al. 2005; Gilmore 2006). While the exact significance of the p65 activation induced by exercise remains unclear, the higher post-exercise temperature observed with the animals that exercised at the higher intensity (30 m/min) suggests the level of stress experienced by these animals was indeed greater. It is well established that increased heart rate, body temperature, oxidative stress, as well as altered levels of certain hormones or cytokines occurs in conjunction with increased exercise intensity. Thus, the differences in myocardial NF-κB activation observed with exercise may result in specific biochemical responses that may ultimately influence myocardial adaptation.
While the exact significance of the exercise induced p65 NF-κB activation cannot be determined from the present study, it is interesting to note that in skeletal muscle, p65 NF-κB activation plays a key role in mediating muscle fiber atrophy (Cai et al. 2004; Bar-Shai et al. 2008). In view of this, it remains possible that exercise above a specific intensity or threshold may activate an inflammatory and/or remodeling response. In support of this, AP-1, a transcription factor known to be activated during inflammation (Karin et al. 1997; Adcock and Caramori 2001), was also activated to a greater extent in the hearts of animals exercised at 30 m/min compared with controls and the low-intensity exercise group. This suggests that exercise above a specific intensity, may cause activation of the p50/p65 heterodimer or possibly the p65/p65 homodimer which may lead to vastly different cellular responses than the p50–p50 homodimer activated with low-intensity exercise. Indeed, when p65 is heterodimerized with p50, the effects are largely pro-inflammatory (Aoi et al. 2004; Monaco et al. 2004; Wang et al. 2007; Son et al. 2008). For example, Aoi et al. (2004) reported that following oxidative stress, the rat gastrocnemius muscle showed nuclear translocation of p65 and increases in cytokine-induced neutrophil chemoattractant-1 and monocyte chemoattractant protein-1.
This study confirms the findings of previous reports showing that exercise induces NF-κB activation (Hollander et al. 2001; Durham et al. 2004; Ji et al. 2004; Ho et al. 2005) and is in contrast to Durham et al. (2004) that showed a decreased NF-κB following exercise. These discrepancies might be explained by differences in intensity and possibly duration as this study shows they may be factors in NF-κB activation. In the present study, high-intensity exercise induced p65 and p50 activation while low-intensity exercise primarily altered p50 activation. The exact signal(s) from exercise that activate NF-κB could be one or many, as a myriad of agents are known to activate NF-κB (Perkins 2006). It is likely that the exercise induced NF-κB activation observed represents an initial response to the perturbation of cellular homeostasis. However, given the broad range of processes mediated by NF-κB, it is difficult to determine if the NF-κB activation observed represents a beneficial response that may aid in the processes of myocardial adaptation or whether it represents a detrimental response and possibly leads to a pathological condition. Determining the significance of the exercise induced NF-κB activation may prove useful in designing programs that use exercise as a therapeutic or preventative agent, such as during cardiac rehabilitation.
In conclusion, this study showed that myocardial NF-κB activation increased during and following exercise in an intensity dependent manner. The exercise induced activation consisted of primarily p50 and above a certain threshold, p65, suggesting exercise may activate myocardial NF-κB via the classical pathway. Additional characterization of the NF-κB activation pathways will enhance our understanding of NF-κB in the heart and hopefully allow more precise preventative/therapeutic interventions that may aid in disease prevention.
References
- Adcock IM, Caramori G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 2001;79:376–384. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- Aoi W, Naito Y, Takanami Y, Kawai Y, Sakuma K, Ichikawa H, Yoshida N, Yoshikawa T. Oxidative stress and delayed-onset muscle damage after exercise. Free Rad Biol Med. 2004;4:480–487. doi: 10.1016/j.freeradbiomed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:1649–1681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-κB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Bilgin B, Eickemeier S, Hipskind R, Singh M, Szamel M, Resch K. The NF-κB heterodimer/homodimer balance and IL-1 stimulated IL-2 production in murine T lymphocytes. Biofactors. 1996;5:169–174. [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HGW, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKb/NF-kB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Durham W, Li YP, Gerken E, Farid M, Arbogast S, Wolfe R, Reid M. Fatiguing exercise reduces DNA binding activity of NF-κB in skeletal muscle nuclei. J Appl Physiol. 2004;97:1740–1745. doi: 10.1152/japplphysiol.00088.2004. [DOI] [PubMed] [Google Scholar]
- Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–1126. doi: 10.1016/S0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- Frier BC, Noble EG, Locke M. Diabetes-induced atrophy is associated with a muscle-specific alteration in NF-kappa B activation and expression. Cell Stress Chaperones. 2008;13:287–296. doi: 10.1007/s12192-008-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Grimm S, Baeuerle PA. The inducible transcription factor NF-κB: structure-function relationship of its protein subunits. Biochem Journal. 1993;290:297–308. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RC, Hirshman MF, Li Y, Cai D, Farmer JR, Aschenbach WG, Witczak CA, Shoelson SE, Goodyear LJ. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C794–C801. doi: 10.1152/ajpcell.00632.2004. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-κB signalling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Arch. 2001;442:426–434. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig D, Kandarian S. Activation of an alternative NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-κB signalling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kramer H, Goodyear L. Exercise, MAPK, and NF-κB signalling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- Lira FS, Koyama CH, Yamashita AS, Rosa JC, Zanchi NE, Batista ML, Jr, Seelaender MC. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem Funct. 2009;27:458–461. doi: 10.1002/cbf.1594. [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis in human atherosclerosis. PNAS. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez P, Page A, Jorcano JL. Role of phosphorylated p50- NF-κB in the ultraviolet response of mouse skin. Mol Carcinog. 2000;27:272–279. doi: 10.1002/(SICI)1098-2744(200004)27:4<272::AID-MC5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, Kim K. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:71–77. doi: 10.1097/FJC.0b013e31815bd23d. [DOI] [PubMed] [Google Scholar]
- Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneroso CT, Tuñón MJ, Gonzales-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J Pineal Res. 2009;47:184–191. doi: 10.1111/j.1600-079X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, Beg AA. Distinct roles of different NF-kB subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J Immunol. 2007;178:6777–6788. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- Werner S, Barken D, Hoffmann A. Stimulus specificity of gene specifity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]