Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling
The tyrosine kinase receptor PTK7 implicated in planar cell polarity is shown here to play a role also in the Wnt canonical signaling pathway both during Xenopus embryo development and in mammalian cells.
Keywords: β-catenin, PTK7, Wnt, Xenopus
Abstract
The receptor protein tyrosine kinase 7 (PTK7) was recently shown to participate in noncanonical Wnt/planar cell polarity signalling during mouse and frog embryonic development. In this study, we report that PTK7 interacts with β-catenin in a yeast two-hybrid assay and mammalian cells. PTK7-deficient cells exhibit weakened β-catenin/T-cell factor transcriptional activity on Wnt3a stimulation. Furthermore, Xenopus PTK7 is required for the formation of Spemann's organizer and for Siamois promoter activation, events that require β-catenin transcriptional activity. Using epistatic assays, we demonstrate that PTK7 functions upstream from glycogen synthase kinase 3. Taken together, our data reveal a new and conserved role for PTK7 in the Wnt canonical signalling pathway.
Introduction
Protein tyrosine kinase 7 (PTK7)—also known as colon cancer kinase 4 (CCK4)—is a single transmembrane receptor with a kinase dead domain that was first identified in melanocytes (Lee et al, 1993) and then in fetal colon and colon carcinoma (Mossie et al, 1995; Boudeau et al, 2006). Both mouse and Xenopus PTK7-deficient embryos show developmental defects, supporting its role in noncanonical Wnt signalling or the Wnt/planar cell polarity (PCP) pathway (Lu et al, 2004; Shnitsar & Borchers, 2008; Yen et al, 2009). Consistent with this, mouse ptk7 interacts genetically with vangl2, a core Wnt/PCP gene (Lu et al, 2004).
To gain further insights into the function of PTK7, we screened for PTK7-binding partners by using a yeast two-hybrid assay and identified β-catenin as a protein that interacts with the defective tyrosine kinase domain. In the canonical Wnt pathway, ligands such as Wnt3a bind to Frizzled and low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) receptors, which causes the recruitment of Dishevelled to the cell membrane and inhibits the β-catenin degradation complex that includes the scaffold protein Axin and glycogen synthase kinase 3 (GSK3). Consequently, β-catenin is stabilized and enters the nucleus to regulate gene expression together with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors (Angers & Moon, 2009). In Xenopus embryos, β-catenin activates the expression of Siamois and Spemann's organizer genes (Kodjabachian & Lemaire, 2004). In this study, we show that PTK7 is required for β-catenin-dependent transcriptional events induced by canonical Wnt ligands, in both frog and mammalian cells. This study, together with previous research, indicates that PTK7 is an important conserved modulator of multiple Wnt pathways in normal and possibly pathological conditions, including cancer.
Results And Discussion
Physical interaction between PTK7 and β-catenin
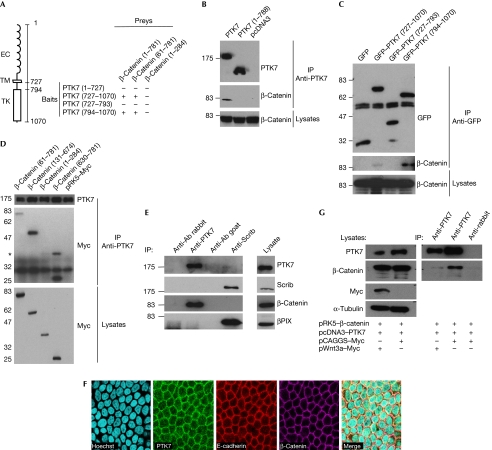
To gain insight into the signalling pathways associated with PTK7, we fused its cytoplasmic region (PTK7727–1070) to the Gal4 DNA-binding domain and screened a human colon complementary DNA (cDNA) library with yeast two-hybrid assay. Among the positive clones recovered, we focused on two that encompassed most of the peptide sequence of β-catenin (data not shown). In yeast two-hybrid assays, the whole intracellular region of PTK7 (PTK7727–1070) and the isolated tyrosine kinase domain (PTK7794–1070) interacted with full-length β-catenin (β-catenin1–781), whereas the extracellular (PTK71–727) and juxtamembrane (PTK7727–793) regions did not (Fig 1A). Mapping experiments showed that deletion of the first 60 residues of β-catenin (β-catenin61–781) did not impair PTK7 binding, whereas the amino-terminal fragment (β-catenin1–284) had no affinity for PTK7 (Fig 1A). Next, we asked whether PTK7 and β-catenin also interact in human cells. For this, Myc–β-catenin was transiently expressed in COS7 cells together with Flag–PTK7 or Flag–PTK71–788, a construct containing only the extracellular and transmembrane regions of PTK7. Immunoprecipitation using a PTK7 antibody demonstrated that Flag–PTK7, but not Flag–PTK71–788, co-immunoprecipitated with β-catenin (Fig 1B). We also transiently expressed green fluorescent protein (GFP) N-terminally fused to the cytoplasmic regions of PTK7 in COS7 cells together with Myc–β-catenin, and immunoprecipitated the protein complexes with GFP antibody. Similarly to yeast, GFP–PTK7727–1070 and GFP–PTK7794–1070 but not GFP–PTK7727–793 or GFP alone interacted with β-catenin (Fig 1C). As GFP–PTK7727–1070 interacted more weakly than GFP–PTK7794–1070, we suspect that sequence 727–794 might destabilize the interaction. In the reverse experiment, Myc-tagged β-catenin constructs were coexpressed with PTK7 in COS7 cells. Myc–β-catenin61–781, armadillo repeats (β-catenin131–674) and the carboxy terminal (β-catenin630–781) interacted with PTK7 (Fig 1D, upper panel). To reveal the endogenous PTK7–β-catenin interaction, we used protein extracts from epithelial Caco2 cells. Immunoprecipitation with PTK7 antibody pulled down endogenous β-catenin, but not β-PAK-interacting exchange factor (PIX), which was used as control (Fig 1E). In both Madin–Darby canine kidney (MDCK) and Caco2 polarized epithelial cells, PTK7 colocalized at the cell–cell junctions with β-catenin and E-cadherin (Fig 1F and data not shown, respectively). However, immunoprecipitation assays revealed that PTK7 interacts with β-catenin but not E-cadherin in MDCK cells (supplementary Fig S1 online), suggesting the existence of distinct pools of β-catenin at the cell membrane. To explore the possible modulation of the PTK7–β-catenin interaction by Wnt canonical ligands, Flag–PTK7 and Myc–β-catenin were transiently expressed in MDCK cells together with Wnt3a–Myc or its backbone vector (pCAGGS-Myc). Immunoprecipitation with PTK7 antibody demonstrated that Wnt3a stimulation reduces the amount of co-immunoprecipitated β-catenin (Fig 1G). Together, these data reveal that the tyrosine kinase domain of PTK7 is able to interact dynamically with β-catenin, under the control of Wnt ligands.
Figure 1.
Protein tyrosine kinase 7 interacts with β-catenin. (A) Schematic representation of PTK7 and results of two-hybrid analysis in yeast. Interactions were positive (+) when β-galactosidase activity and auxotrophy for histidine were detected in the presence of 10 mM 3-aminotriazole. (B) Myc–β-catenin was coexpressed with Flag-tagged PTK7–Flag or Flag–PTK71–788 in COS7 cells. Proteins were immunoprecipitated using PTK7 antibody and revealed with PTK7 (upper panel) and β-catenin (middle panel) antibodies. Equal amounts of β-catenin or PTK7 (data not shown) were present in the lysates (bottom panel). (C) GFP or indicated PTK7 regions fused to GFP were coexpressed with Myc–β-catenin in COS7 cells. After immunoprecipitation with GFP antibody (upper panel), bound partners were detected by β-catenin antibody (middle panel). Comparable amounts of β-catenin and GFP proteins (data not shown) were detected in the total lysates (bottom panel). (D) Indicated Myc-tagged β-catenin constructs and Flag–PTK7 were coexpressed in COS7 cells. Proteins were immunoprecipitated with PTK7 antibody (upper panel). Bound partners were then detected using Myc antibody (middle panel), asterisk indicates a nonspecific band. Total lysates were blotted using Myc and PTK7 (bottom panel and not shown) antibodies. (E) Proteins extracted from Caco2 cells were immunoprecipitated with PTK7 or Scrib antibodies (or control antibodies of same species) and bound proteins were revealed by western blot with the mentioned antibodies (PTK7, Scrib, β-catenin or βPIX). (F) Subcellular localization of endogenous proteins was revealed by using immunofluorescence and confocal analysis in polarized MDCK cells. PTK7 is shown in green, E-cadherin is shown in red and β-catenin is shown in purple. Nuclei were stained with Hoechst blue dye. (G) After transfection of Wnt3a–Myc-, Myc–β-catenin- and Flag–PTK7-expressing plasmids in MDCK cells, total proteins were extracted and immunoprecipitated with PTK7 antibody; bound proteins were revealed by western blotting, as indicated. Total lysates were blotted using PTK7, β-catenin, Myc and α-tubulin (right panel) antibodies. Ab, antibody; β-PIX, β-PAK-interacting exchange factor; EC, extracellular; GFP, green fluorescent protein; IP, immunoprecipitation; MDCK, Madin–Darby canine kidney; PTK7, protein tyrosine kinase 7; TK, tyrosine kinase domains; TM, transmembrane.
PTK7 affects β-catenin transcriptional activity
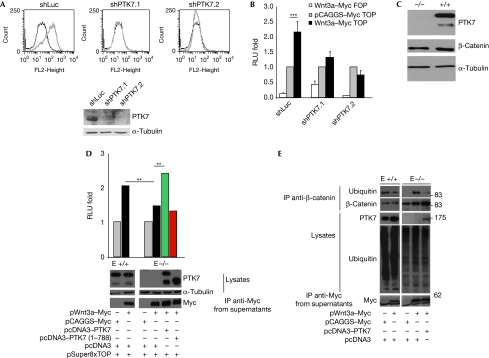
To test the potential implications of PTK7 for Wnt canonical signalling, we measured β-catenin transcriptional activity by the TOP-Flash/luciferase reporter construct, which has several TCF/LEF-binding sites. We used the human colon cancer cell line HCT116, as it expresses PTK7 endogenously. We established two HCT116 clones in which PTK7 expression was stably knocked down by specific short hairpin RNA (shRNA), as confirmed by fluorescence-activated cell sorting (FACS) and western blotting analysis (Fig 2A). In HCT116 control cells (shLuc), we observed a basal TCF/LEF transcriptional activity that was upregulated by twofold on Wnt3a stimulation. By contrast, decreased expression of PTK7 in the two shPTK7 clones prevented Wnt3a-induced TCF/LEF transcriptional activity (Fig 2B).
Figure 2.
Protein tyrosine kinase 7 is involved in Wnt/β-catenin canonical signalling. (A) HCT116 cells were stably transfected with shRNAs directed against PTK7 (shPTK7.1 or shPTK7.2) or luciferase (shLuc). PTK7 expression was assessed by FACS analysis and western blotting. (B) pSuper8xTOP or FOPFlash firefly luciferase reporter plasmids and pBeta–Renilla vector were transfected into HCT116 shPTK7 or shLuc cells along with Wnt3a–Myc-expressing plasmid or a relative empty vector (pCAGGS–Myc). Relative luciferase units normalized to pCAGGS–Myc TOP values (RLU fold) are represented. Error bars represent s.e. values and statistical analyses were performed by using a Student's t-test in at least three independent experiments. (C) Indicated proteins were revealed by western blotting in protein extracts of MEFs deficient in (−/−) or expressing (+/+) PTK7. (D) MEFs were nucleofected with pSuper8xTOP/FOPFlash (pSuper8xFOPFlash not shown) reporter plasmids, pBeta–Renilla vector and the indicated expression vector combinations. Relative luciferase units normalized to TOP-empty vectors values (RLU fold) are represented. Expression of Wnt3a–Myc and PTK7 was revealed by western blotting in protein extracts and cell culture supernatants (Wnt3a). Error bars represent s.e. values and statistical analyses were performed by using a Student's t-test in at least three independent experiments. (E) After nucleofection of Wnt3a–Myc-expressing plasmid and proteasome inhibition treatment, by MG132 and NEM, proteins extracted from MEF cells were immunoprecipitated with β-catenin mouse antibody and bound proteins were revealed by western blot with the mentioned antibodies (ubiquitin, PTK7 and β-catenin). Expression of Wnt3a–Myc was revealed by western blotting in cell culture supernatants (Myc). Blots are representative of at least three independent experiments. FACS, fluorescence-activated cell sorting; MEF, mouse embryonic fibroblast; MG132, N-(benzyloxycarbonyl)leucinylleucinylleucinal; NEM, N-ethylmaleimide; PTK7, protein tyrosine kinase 7; RLU, relative luciferase unit; shRNA, short hairpin RNA.
To confirm the role of PTK7 in Wnt canonical signalling, we generated primary mouse embryonic fibroblasts (MEFs) from 15-day-old littermate wild-type (+/+) or PTK7-deficient (−/−) embryos (Lu et al, 2004). The expression of PTK7 in MEFs, according to their genotype, was confirmed by western blotting (Fig 2C). Plasmid expressing Wnt3a–Myc was nucleofected in MEFs along with the luciferase reporters. Wnt3a–Myc activated the TOP-flash reporter more robustly in wild type than in PTK7 null MEFs (Fig 2D). We were able to rescue the Wnt3a-induced TCF/LEF transcriptional activity by re-expression of Flag–PTK7, but not Flag–PTK71–788 lacking the kinase domain (Fig 2D). To test whether this reduced transcriptional response involved destabilization of β-catenin in the absence of PTK7, we measured levels of ubiquitinylated β-catenin, a form tagged for degradation. Whereas Wnt3a lowered β-catenin ubiquitinylation in wild-type MEFs, it increased it in PTK7 null MEFs. Re-expression of PTK7 reversed this effect (Fig 2E). In summary, PTK7 seems to favour β-catenin stabilization and transcriptional activity on Wnt3a ligand stimulation.
PTK7 is required for organizer gene expression
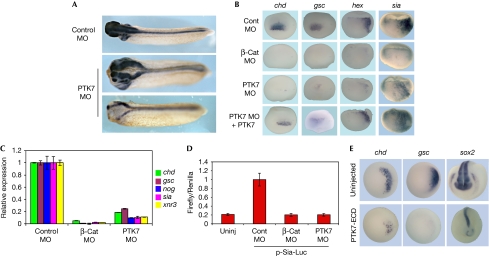
In the Xenopus embryo, the Wnt/β-catenin pathway triggers the formation of Spemann's organizer, an embryonic structure required for axis formation (Kodjabachian & Lemaire, 2004). We used published morpholinos (MOs) targeting PTK7 to address its function in organizer formation. As expected, PTK7 morphant embryos showed neural-tube closure defects at tailbud stage (Lu et al, 2004). However, they also often displayed incomplete blastopore closure and a reduced nervous system, as shown by sox2 staining, particularly in the brain region (Fig 3A), suggesting reduced organizer activity. To address this possibility, we analysed organizer gene expression before gastrulation movements, thus excluding indirect consequences of aberrant morphogenesis. Both whole-mount in situ hybridization (WISH; 81%, n=82) and reverse transcriptase–quantitative PCR (RT–qPCR) analyses showed that the organizer genes chordin (chd), goosecoid (gsc), hex, noggin (nog), siamois (sia) and xnr3 were downregulated in the absence of PTK7 (Fig 3B,C). The extent of this repression was comparable to, although weaker than, that caused by β-catenin knockdown (Heasman et al, 2000; Fig 3B,C). This repression was partly reverted (38%, n=87) in PTK7 morphant embryos that received a separate injection of messenger RNAs (mRNAs) encoding PTK7 or PTK7–Venus, both of which lacked the MO-binding sites (Fig 3B). By contrast, the isolated cytosolic or extracellular domains of PTK7 were not able to rescue PTK7 morphants (data not shown). Of the organizer genes studied here, siamois is known to be directly controlled by Wnt canonical signalling, as its promoter contains binding elements for the TCF3–β-catenin complex (Fan et al, 1998). We found that both β-catenin and PTK7 MOs resulted in a fivefold reduction in the level of expression of the co-injected siamois-luciferase reporter plasmid (p-Sia-Luc, Fig 3D). To confirm the specificity of PTK7 knockdown, we injected embryos with a dominant-negative form of PTK7 that lacks the intracellular domain (Lu et al, 2004). We found that this mutant could also suppress organizer and neural gene expression (Fig 3E). Taken together, these results suggest that, similarly to that in mammalian cells, β-catenin transcriptional activity is compromised in embryonic cells depleted of PTK7 activity.
Figure 3.
Protein tyrosine kinase 7 is required for organizer gene expression. (A) Four-cell embryos were injected with 10 ng per cell control MO in the two dorsal cells, or with a mixture of 5 ng each per cell of PTK7 MOb and MOc and stained for sox2 expression at the tailbud stage. (B) Embryos injected as in (A) or with 2.5 ng per cell β-catenin MO were processed for WISH analysis at gastrula stage 10. For the rescue assay, four-cell PTK7 MO-injected embryos received a second injection of 10 ng PTK7–Venus mRNA in the two dorsal cells. A similar rescue resulted from injection of 1 ng PTK7 mRNA. (C) Embryos injected as in (A) and (B) were collected at stage 10 and processed for RT–qPCR. (D) Embryos were injected with 1 pg per cell pRL-TK, 40 pg per cell 833pSia-Luc plasmids and MOs, as indicated, collected at stage 10 and processed for luciferase assays. (E) Four-cell embryos were injected dorsally with 625 pg per cell PTK7-ECD mRNA and processed for WISH analysis at stage 10 (chd, gsc) and at tailbud stage (sox2, front view). For all qPCR graphs, error bars represent s.e.m. values of three independent experiments with two technical duplicates. For all luciferase reporter assays, error bars represent s.e.m. values of three independent experiments with three technical replicates. MO, morpholino; PTK7, protein tyrosine kinase 7; RT–qPCR, reverse transcriptase–quantitative PCR; WISH, whole-mount in situ hybridization.
PTK7 functions upstream from GSK3
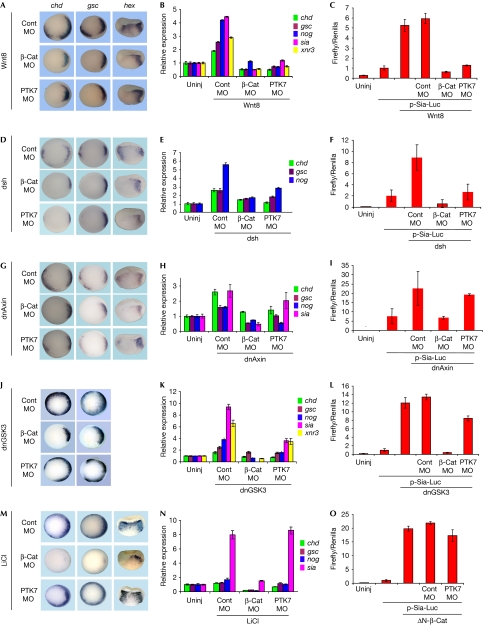
To assess directly the requirement of PTK7 in activation of the canonical Wnt pathway, Xenopus embryos were injected with Wnt8 mRNA in a ventral position to induce the formation of an ectopic organizer (Fig 4A). When Wnt8 was injected into embryos pre-loaded with PTK7 or β-catenin MOs, ectopic organizer gene expression and siamois reporter activation were prevented (Fig 4A–C). As PTK7 protein is normally found at the plasma membrane, it might act upstream from the cytosolic components of the canonical Wnt pathway. Thus, we activated Wnt signalling at the level of Axin and GSK3—two proteins that control β-catenin availability in the cell. For this, we injected dominant-negative forms of Axin (dnAxin; Zeng et al, 1997) or GSK3 (dnGSK3; He et al, 1995) and treated embryos with lithium chloride, a potent inhibitor of GSK3 (Klein & Melton, 1996). The WISH and RT–qPCR analyses and the luciferase assays with the siamois reporter revealed that PTK7 was largely dispensable for organizer gene activation caused by all three reagents. By contrast and as expected, β-catenin knockdown suppressed gene activation induced by such treatments (Fig 4G–N). It was proposed recently that PTK7 in association with the Frizzled 7 receptor initiates PCP signalling through recruitment of Dishevelled at the plasma membrane (Shnitsar & Borchers, 2008). We therefore tested the requirement for PTK7 in organizer gene expression induced by Dishevelled (Sokol et al, 1995). As shown in Fig 4D–F, PTK7 knockdown suppressed the response induced by Dishevelled mRNA injection, suggesting that these proteins might also be partners in canonical Wnt signalling. Interestingly, we found that overexpression of Dishevelled in MDCK cells, competes for the binding of PTK7 to β-catenin (supplementary Fig S2 online). Together with the observed reduction in PTK7–β-catenin interaction induced by Wnt3A (Fig 1G), we suggest that PTK7 might act as a platform to deliver stabilized β-catenin upon receptor activation. Finally, we confirmed that a stabilized form of β-catenin that cannot be phosphorylated by GSK3 (ΔN-βcat; Yost et al, 1996) could activate the siamois promoter in the absence of PTK7 (Fig 4O). Taken together, these data indicate that PTK7 functions upstream from the β-catenin destruction complex in the Wnt canonical signalling pathway.
Figure 4.
Protein tyrosine kinase 7 is required for Wnt canonical signalling, upstream from glycogen synthase kinase 3. (A) Four-cell embryos were first injected with MOs in the two ventral cells, followed by a second injection of 10 pg per cell wnt8 mRNA in the same cells. (B) Embryos were injected as in (A), but in all four cells. (C) Embryos were injected as in (A), together with pRL-TK and -833pSia-Luc plasmids. (D–F) Four-cell embryos were injected with MOs first and then with 500 pg per cell dsh mRNA in the two ventral cells. (G–I) Four-cell embryos were injected with MOs first and then with 500 pg per cell dnAxin mRNA in the two ventral cells. (J,K) Four-cell embryos were injected in all four cells with MOs first and then with 500 pg per cell dnGSK3 mRNA. (L) Four-cell embryos were injected with MOs and dnGSK3 mRNA in the two ventral cells. (M,N) Four-cell embryos were injected in all cells with MOs and incubated in 0.3 M LiCl in 5% Ficoll for 10 min at 64-cell stage, washed in 0.1 × Modified Barth's solution (MBS) and further cultured to stage 10. (O) Four-cell embryos were first injected in the two ventral cells with MOs and then with 500 pg per cell ΔN-βcat mRNA. WISH analysis: (A,D,G,J,M); RT–qPCR: (B,E,H,K,N); luciferase assay: (C,F,I,L,O). β-cat, β-catenin; Cont, control; dnAxin, dominant-negative Axin; dnGSK3, dominant-negative glycogen synthase kinase 3; MOs, morpholinos; mRNA, messenger RNA; RT–qPCR, reverse transcriptase–quantitative PCR; Uninj, uninjected; WISH, whole-mount in situ hybridization.
Future studies should address the mechanism of action of PTK7 in the Wnt–β-catenin pathway. Overexpression of full-length PTK7 or its intracellular domain is not sufficient to activate the siamois reporter and organizer gene expression, suggesting that PTK7 acts as a permissive factor in this pathway (data not shown). Pseudo-tyrosine kinase receptors, such as PTK7, might participate with enzymatically active complexes by interacting with functional kinases at the plasma membrane (Boudeau et al, 2006). Previous studies have shown that the enzymatically active Ror2 tyrosine kinase receptor can bind to distinct Wnt ligands and stimulate canonical and noncanonical responses (Angers & Moon, 2009). Thus, Ror2 is a potential partner for PTK7. Overall, identification of additional PTK7 partners might shed more light on the mechanism of action of this important modulator of Wnt pathways.
Methods
Plasmids and short hairpin RNA, antibodies, cell culture, transfection, luciferase assay, FACS analysis, yeast two-hybrid screens, immunofluorescence and protein procedures. See supplementary information online and references.
Xenopus procedures. Eggs commercially obtained from NASCO (California, USA) females were fertilized in vitro, dejellied, cultured and injected as described (Marchal et al, 2009). PTK7 MOs were purchased from GeneTools LLC (Oregon, USA), resuspended in sterile water to a concentration of 10 mg/ml and further diluted before injection. Among the three published MOs targeting Xenopus PTK7 (Lu et al, 2004), we found that oligonucleotide A was toxic, so we used the combination of oligonucleotides B and C. These two MOs target the 5′ UTR of PTK7 messengers, a region that is not present in the mRNA used for the rescue assay. The MO targeting Xenopus β-catenin was reported by Heasman et al (2000). Synthetic capped mRNAs were produced with Ambion (Applied Biosystems, France) mMessage mMachine kit. Wnt8, ΔN-βcat, PTK7, PTK7-Venus and PTK7-ECD mRNAs were synthesized with T3 polymerase after plasmid linearization with SfiI. References for Dsh, dnAxin, dnGSK3 and ΔN-βcat expression constructs are included in the results section. Embryos were processed for RT–qPCR and WISH with digoxygenin-labelled probes (Roche) as described previously (Marchal et al, 2009).
Quantitative RT–PCR. See supplementary information online and references.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank J. Muster, R. Moon, H. Sasaki, S. Sokol, P. Lemaire and H. Yasuo for plasmids. We also thank A. Zurubrica for helpful discussion. This study was supported by Centre National de la Recherche Scientifique, Inserm, Institut Paoli-Calmettes, Association pour la Recherche contre le Cancer, Agence Nationale de la Recherche (V.T. & L.K.), Ligue Nationale Contre le Cancer (Label 2010 J.-P.B.), Fondation de France (T.P.), European Consortium for Anticancer Antibody Development and Infrastrutures en Biologie Sante et Agronomie (Marseille Proteomic Platform).
Footnotes
The authors declare that they have no conflict of interest.
References
- Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477 [DOI] [PubMed] [Google Scholar]
- Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR (2006) Emerging roles of pseudokinases. Trends Cell Biol 16: 443–452 [DOI] [PubMed] [Google Scholar]
- Fan MJ, Gruning W, Walz G, Sokol SY (1998) Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc Natl Acad Sci USA 95: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB (1995) Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374: 617–622 [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C (2000) Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol 222: 124–134 [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA (1996) A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 93: 8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodjabachian L, Lemaire P (2004) Role of Siamois before and during gastrulation. In Gastrulation: From Cells to Embryo, Stern CD (ed.), pp 609–617. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Lee ST, Strunk KM, Spritz RA (1993) A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene 8: 3403–3410 [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M (2004) PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430: 93–98 [DOI] [PubMed] [Google Scholar]
- Marchal L, Luxardi G, Thome V, Kodjabachian L (2009) BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci USA 106: 17437–17442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossie K, Jallal B, Alves F, Sures I, Plowman GD, Ullrich A (1995) Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene 11: 2179–2184 [PubMed] [Google Scholar]
- Shnitsar I, Borchers A (2008) PTK7 recruits dsh to regulate neural crest migration. Development 135: 4015–4024 [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K (1995) Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121: 1637–1647 [DOI] [PubMed] [Google Scholar]
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A (2009) PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 136: 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT (1996) The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454 [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.