Yox1 links MBF-dependent transcription to completion of DNA synthesis
This study shows that the transcriptional repressor Yox1 is phosphorylated upon activation of the DNA synthesis checkpoint in fission yeast, which alleviates the Yox1-mediated repression of MBF complex-controlled transcription of S-phase genes. Yox1 therefore couples the DNA synthesis checkpoint with the G1-S transcription machinery.
Keywords: DNA synthesis checkpoint, HU, MBF, S-phase transcription
Abstract
When DNA replication is challenged cells activate a DNA synthesis checkpoint, blocking cell cycle progression until they are able to overcome the replication defects. In fission yeast, Cds1 is the effector kinase of this checkpoint, inhibiting M-phase entry, stabilizing stalled replication forks and triggering transcriptional activation of S-phase genes. The molecular basis of this last effect is largely unknown. The Mlu1 binding factor (MBF) complex controls the transcription of S-phase genes. We purified novel interactors of the MBF complex and identified the repressor Yox1. When the DNA synthesis checkpoint is activated, Yox1 is phosphorylated, which abrogates its binding to MBF. MBF-dependent transcription therefore remains active until cells are able to overcome this challenge.
Introduction
Checkpoints recognize damaged DNA or blocks to replication and delay cell-cycle progression by inhibiting cell-cycle machinery until the problem is resolved (Hartwell & Weinert, 1989; Elledge, 1996; Rhind & Russell, 1998). Fission yeast Cds1 is the effector kinase of the DNA synthesis checkpoint (Murakami & Okayama, 1995; Boddy & Russell, 1999) and has an essential role in arresting cells before M phase through phosphorylation and/or inhibition of the essential phosphatase Cdc25. It also maintains DNA replication fork stability when a replication fork stalls, through phosphorylation of several proteins including Mus81-Eme1, Rqh1 and Rad60 (Boddy et al, 1998; Rhind & Russell, 2000; Kai & Wang, 2003). These processes allow cells to survive replication challenges by preventing stalled replication forks from degenerating into defective DNA structures and blocking cell-cycle progression. Thus, cells are arrested until they are able to overcome the replication defects by inducing a transcriptional induction of S-phase genes that helps to re-start the replication machinery.
The activity of the transcription factor Mlu1 binding factor (MBF)—a multimeric complex containing at least Cdc10, Res1 and Res2—is required for the completion of Start in Schizosaccharomyces pombe (Lowndes et al, 1992; Caligiuri & Beach, 1993). MBF—the functional homologue of mammalian E2F/RB—drives the G1–S wave of transcription, controlling the expression of some genes that are directly or indirectly required for DNA synthesis, such as cdc18 and cdt1—which code for proteins that bind replication origins as well as cdc22 (ribonucleotide reductase; Lowndes et al, 1992; Ayte et al, 2001). Therefore, control of MBF activity is essential to ensure normal cell-cycle progression. MBF is bound to its target promoters throughout the cell cycle (Wuarin et al, 2002), suggesting that MBF activity is not due to modulation of its DNA-binding activity. Interestingly, two MBF targets—the cyclin Cig2 and the co-repressor Nrm1—have been implicated in negative feedback loops that could explain, at least partly, how MBF-dependent transcription is inactivated at the end of S phase (Ayte et al, 2001; de Bruin et al, 2008). In this work, we demonstrate a direct link between the DNA synthesis checkpoint and the S-phase transcriptional programme on a single protein, Yox1, which couples both events. Furthermore, phosphorylation of Yox1 when the checkpoint kinase is activated triggers the transcriptional induction of the S-phase genes.
Results And Discussion
Yox1 is a repressor of the MBF complex
To elucidate the mechanism by which MBF is regulated, we purified the native MBF complex and characterized the proteins that interact physically with this transcription factor. We combined immunoprecipitation with isobaric tags for relative and absolute quantification (Ross et al, 2004) followed by liquid chromatography, tandem mass spectrometry (LC/MS/MS) (supplementary Fig S1A,B online). Cdc10-associated proteins were purified from cells expressing haemagglutinin-tagged Cdc10 from its chromosomal locus using a haemagglutinin antibody. We used an untagged wild-type strain as a control. The most enriched proteins in the purification were Cdc10, Res1, Res2, as expected, and a homeodomain-containing protein encoded by a nonessential open reading frame (SPBC21B10.13c), recently named Yox1 due to its sequence homology to budding yeast YOX1 (Aligianni et al, 2009). By using specific antibodies, we detected a reciprocal in vivo interaction between Yox1 and Cdc10 (supplementary Fig S1C online), which is dependent on intact MBF complex; in the absence of Res1 or Res2, Yox1 was not able to bind to Cdc10 (supplementary Fig S1D online).
Next, we wanted to determine whether Yox1 had an effect on MBF regulation. We prepared RNA from wild-type and Δyox1 cells, either from asynchronous cultures or from cells arrested in S phase with hydroxyurea. There was an overall increase in the level of MBF-dependent transcription in Δyox1 cells, as measured by the amount of cdc18 mRNA (Fig 1A). The effect of Yox1 on MBF-dependent transcription was confirmed in synchronous cultures, as they progressed from a G2–M block (supplementary Fig S2 online). Thus, whereas a cdc25-22 strain was able to induce the expression of cdc18 immediately after release and in the subsequent G1–S phase, a cdc25-22 Δyox1 strain had high levels of cdc18 expression throughout the time-course. yox1 expression is also dependent on MBF (Aligianni et al, 2009; Dutta & Rhind, 2009). Despite having a cell-cycle-dependent regulated transcription, Yox1 protein was detected during two complete cell cycles, with minor changes at the level of protein concentration and with constitutive nuclear localization (supplementary Fig S3A–C online), indicating that post-translational modification of Yox1 could have a role in the regulation of its activity. In fact, despite its presence in the nucleus throughout the cell cycle, the timing of Yox1 association with cdc18 promoter during the cell cycle fits with inactivation of MBF-dependent transcription (supplementary Fig S3D online), indicating that Yox1 has a negative role in the regulation of MBF-dependent transcription.
Figure 1.
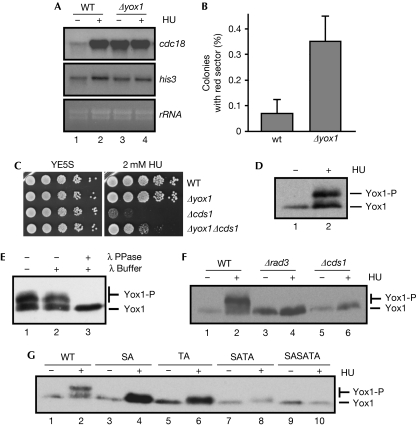
Yox1 is a substrate of the DNA synthesis checkpoint. (A) Total RNA was prepared from untreated (−) or HU-treated (+) cultures (3 h at 30°C) of wild-type and Δyox1 cells, and analysed by hybridization with the indicated probes. his3 and rRNA are shown as loading control. (B) Mitotic chromosome stability assay (minichromosome Ch16 loss) of wild-type and Δyox1 strains were measured as the percentage of colonies with a red sector (Ade-). The average (±s.d.) of four experiments is shown. (C) Serial dilutions of wild-type, Δyox1, Δcds1 and Δyox1 Δcds1 strains were spotted on agar plates without (YE5S) or with 2 mM HU, and grown for 3 days at 30°C. (D) Extracts prepared from untreated (−) or HU-treated (+) cultures of a strain expressing Yox1-13Myc were resolved in an 8% SDS–PAGE and western blotted to detect Yox1. (E) Extracts from cells treated with HU were incubated with λ-phosphatase where indicated, resolved on an 8% SDS–PAGE, transferred and blotted with Myc antibodies to detect Yox1. (F) Extracts prepared from untreated (−) or HU-treated (+) cultures of wild-type, Δrad3 and Δcds1 strains expressing Yox1-13Myc were analysed to detect Yox1 phosphorylation. (G) Extracts from untreated (−) or HU-treated (+) cultures from strains expressing Yox1-13Myc (wild-type) or the mutants S114A (SA), T115A (TA), S114AT115A (SATA) or S6AS114AT115A (SASATA), were analysed to detect Yox1 phosphorylation. HA, haemagglutinin; HU, hydroxyurea; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis; λ PPase, λ-phosphatase; WT, wild-type.
Cells in which yox1 has been deleted show minor cell-cycle defects, such as a slight delay in cytokinesis, producing larger cells than wild type during septation (Aligianni et al, 2009). As Δyox1 cells have an increased transcription of all the MBF-dependent genes and deregulated transcription has an effect on the functionality of yeast centromeres (Kagansky et al, 2009), we aimed to determine the effect of the lack of Yox1 on chromosome segregation. We noticed that Δyox1 cells were genomically unstable, shown by an increased rate (six-fold) of chromosome loss (0.35% in Δyox1 cells compared with 0.06% in wild-type cells; Fig 1B). Δcds1 cells are hypersensitive to hydroxyurea, even in low doses (Fig 1C). We hypothesized that cells with a mutation on yox1 (Δyox1) could compensate for the absence of a DNA synthesis checkpoint in Δcds1 cells by maintaining upregulated MBF-dependent transcription. Thus, when Δyox1 Δcds1 cells were challenged in the presence of low doses of hydroxyurea, they were more resistant to the drug than Δcds1 cells (Fig 1C).
Yox1 is a target of the DNA synthesis checkpoint
While treating cells with hydroxyurea, we noticed that Yox1 changed its mobility (Fig 1D). To determine whether this was due to phosphorylation, extracts were treated with λ-phosphatase, resulting in a shift to the faster migrating form (Fig 1E). These observations led us to investigate whether Yox1 is a substrate of the effector kinase Cds1 (Boddy et al, 1998). In support of this, we observed no change in the mobility of Yox1 in Δrad3 or in Δcds1 cells after hydroxyurea treatment (Fig 1F). Yox1 has two putative Cds1 phosphorylation sites (LXRXXS/T) on Ser 114 and Thr 115 (Seo et al, 2003; Xu & Kelly, 2009). When both sites were mutated to alanine (SATA), the Yox1 mobility shift of hydroxyurea-treated extracts was almost abolished (Fig 1G, lanes 2 and 8). Conversely, Yox1 also has a CDK phosphorylation site on Ser 6. Substitution of this serine with alanine (S6A) resulted in a substrate that could not be phosphorylated in vitro by Cdc2 or Cdc13 (supplementary Fig S4 online). Further inclusion of the Ser 6 mutation in Yox1.SATA (SASATA) completely inhibited the mobility shift observed in hydroxyurea-treated cells (Fig 1G, lanes 9 and 10). We concluded that while Ser 6 is the single target of CDK, Ser 114 and Thr 115 are the direct or indirect targets of the Cds1 pathway. We demonstrated a physical interaction between MBF and Yox1 in asynchronously growing cells (supplementary Fig S1C,D online), but when Yox1 was phosphorylated as a consequence of the activation of the DNA synthesis checkpoint after hydroxyurea treatment, this interaction was lost (Fig 2A, lanes 5 and 6; Fig 2B). Nevertheless, this interaction was preserved in cells in which this Cds1-dependent phosphorylation was abrogated, because they expressed a mutant Yox1 that cannot be phosphorylated (Yox1.SATA; Fig 2A, lanes 7 and 8).
Figure 2.
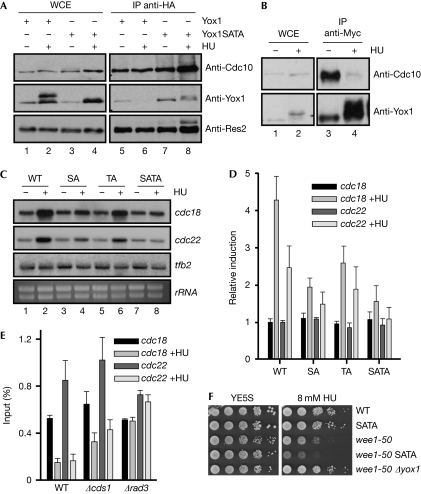
Yox1 phosphorylation abrogates its binding to MBF and induces MBF-dependent transcription. (A) Extracts from a Cdc10-HA strain expressing Yox1-13Myc or Yox1.SATA-13Myc were immunoprecipitated with haemagglutinin antibodies (IP anti-HA) and proteins were detected by western blotting. Anti-Res2 is shown as a control of the Cdc10 (anti-HA) immunoprecipitation. (B) Extracts from a Cdc10-HA Yox1-Myc strain were prepared from cells treated (+HU) or untreated (−HU) with 10 mM HU and were immunoprecipitated (1 mg) with Myc antibodies. Proteins were detected by western blotting with HA (to detect Cdc10) or Myc (to detect Yox1) antibodies. (C) Total RNA was prepared from untreated (−) or HU-treated (+) cultures of wild-type, Yox1.SA (SA), Yox1.TA (TA) and Yox1.SATA (SATA) cells and analysed by hybridization to the probes indicated on the right. tfb2 and rRNA are shown as loading control. A representative experiment is shown. (D) The fold induction of the previous experiment is plotted as the average induction of three experiments (±s.d.), over the untreated wild-type strain. (E) Loading of Yox1 on cdc22 and cdc18 promoters was measured by chromatin immunoprecipitation in untreated or HU-treated (+HU) cultures of wild-type, Δcds1 and Δrad3 cells. The average of three experiments (±s.d.) is plotted. (F) Serial dilutions of a wild-type, Yox1.SATA, wee1-50, wee1-50 Yox1.SATA and wee1-50 Δyox1 strains were spotted on agar plates without (YE5S) or with 8 mM HU, and grown for 3 days at 30°C. HA, haemagglutinin; HU, hydroxyurea; IP, immunoprecipitation; MBF, Mlu1 binding factor; WCE, whole-cell extracts; WT, wild-type.
Although single mutations (Yox1.SA or Yox1.TA) had a strong effect on Yox1 mobility (Fig 1G), the effect on MBF-dependent transcription after hydroxyurea treatment was minor. However, Yox1.SATA cells had severely impaired MBF-dependent induction of transcription when treated with hydroxyurea (Fig 2C,D), without affecting the normal cell-cycle regulation of MBF-dependent genes (supplementary Fig S5 online). Thus, Yox1 phosphorylation on Ser 114 and Thr 115 was the single event required to activate MBF-dependent transcription when the checkpoint was activated until DNA synthesis machinery was replenished with deoxynucleotides. Concomitantly with the loss of interaction between Yox1 and MBF after treatment with hydroxyurea, Yox1 is evicted from cdc18 and cdc22 promoters, as measured by chromatin immunoprecipitation (ChIP) experiments (Fig 2E). This release from chromatin was diminished in Δcds1 cells and absent in Δrad3 cells, in which the checkpoint pathway is completely abolished. Next, we wanted to know whether fission yeast cells that expressed a mutant Yox1 that cannot be phosphorylated (Yox1.SATA) were sensitive to hydroxyurea. Surprisingly, Yox1.SATA cells behaved exactly as wild-type cells (Fig 2F and data not shown), indicating that, at least in wild-type cells, maintenance of MBF gene expression by Cds1 is not crucial to the replication checkpoint response. This led us to investigate what would happen in cells with a shorter G2 phase that were growing in the presence of hydroxyurea. Wild-type S. pombe cells spend most of their time in G2 phase, which could compensate for the lack of induction of MBF-dependent transcription observed in the Yox1.SATA cells, preventing aberrant mitosis. Consequently, in a wee1-50 background—which has a shorter G2 phase as they have an advanced G2–M transition—Yox1.SATA cells showed hypersensitivity to hydroxyurea (Fig 2F, compare wee1-50 with wee1-50 SATA). By contrast, deleting yox1 restored wild-type sensitivity to wee1-50 cells (Fig 2F, compare wild type with wee1-50 Δyox1).
Yox1 binding to MBF is dependent on Nrm1
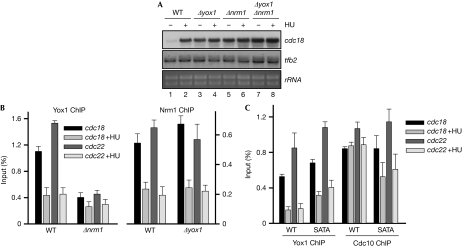
Yox1 was responsible for the repression of MBF-dependent transcription at the end of S phase (Aligianni et al, 2009). A similar role has previously been shown for Nrm1 (de Bruin et al, 2008). To determine whether these proteins had an overlapping role in the regulation of MBF-dependent transcription, we prepared RNA from wild-type, Δyox1, Δnrm1 and Δyox1 Δnrm1 cells, either from asynchronous cultures or from cells treated with hydroxyurea. As shown in Fig 3A, we found similar constitutive expression of MBF-dependent genes in Δyox1, Δnrm1 and Δyox1 Δnrm1 cells, measured by the amount of cdc18 mRNA. Next, we wanted to determine whether this effect correlated with the release of Yox1 and/or Nrm1 from chromatin, as measured by ChIP experiments. As shown in Fig 3B, both Yox1 and Nrm1 are cleared from cdc18 and cdc22 promoters when cells are treated with hydroxyurea. However, whereas Yox1 is dispensable for Nrm1 loading onto chromatin, Nrm1 is essential for Yox1 loading onto its MBF-target promoters (Fig 3A, compare Yox1 ChIP in Δnrm1 cells with Nrm1 ChIP in Δyox1 cells). Thus, in Δyox1 cells, Nrm1 is normally loaded onto MBF-target promoters and cleared on hydroxyurea treatment, but MBF-dependent transcription remains active in both the presence and absence of hydroxyurea (compare Fig 3A lanes 3–4 with Fig 3B). Finally, we measured Yox1 and Yox1.SATA release from chromatin when cells were treated with hydroxyurea (Fig 3C). Although cells expressing Yox1.SATA showed an impaired induction of cdc18 and cdc22 compared with wild-type cells (Fig 2C,D), we only detected a minor effect on Yox1.SATA retention in chromatin. However, Cdc10—and, probably, other components of the MBF complex—was partly evicted from chromatin when Yox1.SATA cells were treated with hydroxyurea (Fig 3C), which could account for the lack of induction of MBF-dependent genes observed in these cells (Fig 2C,D). All of these experiments indicate that the regulation of MBF-dependent transcription is highly complicated, with several layers of control including retention and/or release of the repressor (Yox1) or the transcription factor itself (Cdc10).
Figure 3.
Nrm1 loads Yox1 onto MBF-dependent genes. (A) Total RNA was prepared from untreated (−) or HU-treated (+) cultures of wild-type (wt) and Δyox1, Δnrm1 and Δyox1 Δnrm1 cells, and analysed by hybridization to the probes indicated on the right. tfb2 and rRNA are shown as loading control. (B) Loading of Yox1 (left panel) or Nrm1 (right panel) on cdc22 and cdc18 promoters was measured by ChIP in untreated or HU-treated (+HU) cultures of wt Δnrm1 and Δyox1 cells. The average of four experiments (±s.d.) is plotted. (C) Loading of Yox1 or Cdc10 on cdc22 and cdc18 promoters was measured in untreated or HU-treated (+HU) cultures of wt and Yox1.SATA (SATA) cells by ChIP. The average of three experiments (±s.d.) is plotted. ChIP, chromatin immunoprecipitation; HU, hydroxyurea; MBF, Mlu1 binding factor.
Previous reports have shown a link in mammalian cells between the DNA damage checkpoint and E2F/retinoblastoma, the functional homologue of yeast MBF (Stevens et al, 2003; Inoue et al, 2007; Zalmas et al, 2008). Here, we report a new mechanism that couples cell-cycle regulation and the DNA replication checkpoint, focusing on a single protein, Yox1. In a normal cell cycle, Nrm1 (de Bruin et al, 2008) loads the repressor Yox1 onto MBF-target genes and, together with Cig2 (Ayte et al, 2001), they are part of the mechanism by which MBF-dependent genes are repressed at the end of each S phase of the cell cycle (Fig 4). It remains to be clarified what is the role, if any, of the CDK phosphorylation of Yox1 on MBF-dependent transcription; so far, we have been unable to find any in vivo effect of Ser 6 phosphorylation on MBF-dependent transcription. However, when DNA synthesis is compromised and the checkpoint is activated, Yox1 is phosphorylated by the effector kinase Cds1. As a result, transcription of MBF-dependent genes is active until DNA synthesis can be completed. Other reports have shown that Cds1 is able to phosphorylate other components of MBF, such as Cdc10 and Nrm1 (Dutta et al, 2008; de Bruin et al, 2008). However, Cdc10 and Nrm1 mutants that cannot be phosphorylated by Cds1 showed no significant defect in checkpoint regulation of transcription, whereas Yox1.SATA abolished this checkpoint regulation (Fig 2C,D), pointing to Yox1 as the main target of the Cds1 pathway. Interestingly, both the G1–S transition of a normal cell cycle and the DNA synthesis checkpoint (Cds1-dependent phosphorylation of Yox1) manage to activate MBF-dependent transcription, although to a different extent: the former induces a transient activation of transcription and the latter maintains the induction of MBF-dependent genes for as long as the checkpoint is active. A different question is whether Δyox1 cells have an advantage compared with wild-type cells in stress situations, why have they not been selected during evolution? The answer could be related to the increased genomic instability that we observed in Δyox1 cells (Fig 1B). Thus, Yox1 would be the nexus of a robust system to ensure that cells can respond to stress situations in which DNA synthesis is compromised, by using the same machinery that normally regulates cell-cycle-dependent transcription.
Figure 4.
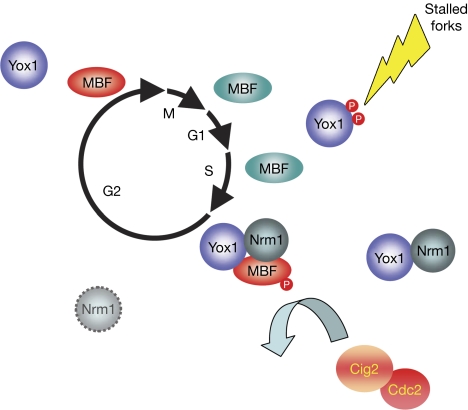
Model for the role of Yox1, integrating cell-cycle-dependent transcription and the DNA synthesis checkpoint. MBF-dependent transcription is high (MBF in turquoise) until the end of S phase, when it is repressed by a double-negative feedback loop that includes loading of Nrm1/Yox1 onto MBF and Cig2-dependent phosphorylation of Res1 (MBF is shown in red; Ayte et al, 2001; de Bruin et al, 2008). MBF-dependent transcription is repressed until Nrm1 is degraded later in G2 (dashed Nrm1; de Bruin et al, 2008). However, when DNA replication is challenged, the DNA-synthesis checkpoint is activated and, as a consequence, Yox1 is phosphorylated on Ser 114 and Thr 115. These phosphorylations disrupt the interaction between Yox1 and MBF, and MBF-dependent transcription is fully activated until cells can overcome the arrest and finish DNA synthesis. MBF, Mlu1 binding factor.
Methods
Strains and media. All S. pombe strains are isogenic to wild-type 972h- and are listed in the supplementary information online. Media were prepared as described previously (Moreno et al, 1991). Hydroxyurea treatment (10 mM) was performed with midlog grown cultures (3–4 × 106 cells/ml) for 3 h at 30°C. To analyse sensitivity to hydroxyurea on plates, S. pombe strains were grown in liquid YE5S media to an OD600 of 0.5. Cells were then diluted in YE5S and spotted onto YE5S media agar plates. Plates were incubated at 30°C for 3–4 days.
Protein extraction and immunoprecipitation. Extracts were prepared in NET-N buffer (20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP40, 1 mM dithiothreitol, 1 mM phenylmethyl sulphonyl fluoride, 5 μg/ml aprotinin, protease inhibitor cocktail (Sigma), 2 mM sodium fluoride (NaF), 0.2 mM sodium orthovanadate (Na3VO4), 2 mM β-glycerophosphate). Cells were broken with glass beads in a BioSpec Minibeadbeater. Immunoprecipitation analyses (1–3 mg of cleared whole-cell lysate) were performed with 10 μl of protein G sepharose and 100 μl of tissue culture supernatant from monoclonal hybridoma Myc antibody. For HA antibody immunoprecipitations, antibody was previously crosslinked to protein G sepharose. After 1 h of incubation, immunoprecipitates were washed three times with the same buffer and resolved on an 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with the indicated antibody.
Gene expression analysis. RNA extraction was performed as described (Moldon et al, 2008) and 10 μg of extracted RNA was loaded. cdc18, cig2, tfb2 and his3 probes contained the complete open reading frames of the genes.
ChIP. ChIP experiments were performed as described previously (Moldon et al, 2008). All the experiments were plotted as the average of at least three replicates±s.d.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Paul Nurse, Paul Russell and Hiroshi Murakami for strains, Jim DeCaprio for monoclonal antibodies, Concha Gil for the isobaric tags for relative and absolute quantification analysis, Alberto Moldón, Gabriel Gil, Manuel Mendoza and Luciano Di Croce for critical reading of the paper, and Pilar Pérez and members of the Oxidative Stress and Cell Cycle Group at UPF for suggestions and comments. We acknowledge the technical support of Mercè Carmona. This work was supported by grants from the Ministerio de Ciencia e Innovación (BFU2009-07453), PLAN E and FEDER, the Consolider-Ingenio (CSD 2007-0020), and by SGR2009-196 from Generalitat de Catalunya. J.A. is the recipient of an ICREA Academia Award (Generalitat de Catalunya).
Footnotes
The authors declare that they have no conflict of interest.
References
- Aligianni S, Lackner DH, Klier S, Rustici G, Wilhelm BT, Marguerat S, Codlin S, Brazma A, de Bruin RA, Bahler J (2009) The fission yeast homeodomain protein Yox1p binds to MBF and confines MBF-dependent cell-cycle transcription to G1–S via negative feedback. PLoS Genet 5: e1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayte J, Schweitzer C, Zarzov P, Nurse P, DeCaprio JA (2001) Feedback regulation of the MBF transcription factor by cyclin Cig2. Nat Cell Biol 3: 1043–1050 [DOI] [PubMed] [Google Scholar]
- Boddy MN, Russell P (1999) DNA replication checkpoint control. Front Biosci 4: D841–D848 [DOI] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P (1998) Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280: 909–912 [DOI] [PubMed] [Google Scholar]
- Caligiuri M, Beach D (1993) Sct1 functions in partnership with Cdc10 in a transcription complex that activates cell cycle START and inhibits differentiation. Cell 72: 607–619 [DOI] [PubMed] [Google Scholar]
- de Bruin RA, Kalashnikova TI, Aslanian A, Wohlschlegel J, Chahwan C, Yates JR III, Russell P, Wittenberg C (2008) DNA replication checkpoint promotes G1–S transcription by inactivating the MBF repressor Nrm1. Proc Natl Acad Sci USA 105: 11230–11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta C, Rhind N (2009) The role of specific checkpoint-induced S-phase transcripts in resistance to replicative stress. PLoS ONE 4: e6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta C, Patel PK, Rosebrock A, Oliva A, Leatherwood J, Rhind N (2008) The DNA replication checkpoint directly regulates MBF-dependent G1/S transcription. Mol Cell Biol 28: 5977–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274: 1664–1672 [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Kitagawa M, Taya Y (2007) Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J 26: 2083–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagansky A, Folco HD, Almeida R, Pidoux AL, Boukaba A, Simmer F, Urano T, Hamilton GL, Allshire RC (2009) Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 324: 1716–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M, Wang TS (2003) Checkpoint responses to replication stalling: inducing tolerance and preventing mutagenesis. Mutat Res 532: 59–73 [DOI] [PubMed] [Google Scholar]
- Lowndes NF, McInerny CJ, Johnson AL, Fantes PA, Johnston LH (1992) Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+. Nature 355: 449–453 [DOI] [PubMed] [Google Scholar]
- Moldon A, Malapeira J, Gabrielli N, Gogol M, Gomez-Escoda B, Ivanova T, Seidel C, Ayte J (2008) Promoter-driven splicing regulation in fission yeast. Nature 455: 997–1000 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Meth Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H (1995) A kinase from fission yeast responsible for blocking mitosis in S phase. Nature 374: 817–819 [DOI] [PubMed] [Google Scholar]
- Rhind N, Russell P (1998) Mitotic DNA damage and replication checkpoints in yeast. Curr Opin Cell Biol 10: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind N, Russell P (2000) Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci 113: 3889–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PL et al. (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3: 1154–1169 [DOI] [PubMed] [Google Scholar]
- Seo GJ, Kim SE, Lee YM, Lee JW, Lee JR, Hahn MJ, Kim ST (2003) Determination of substrate specificity and putative substrates of Chk2 kinase. Biochem Biophys Res Commun 304: 339–343 [DOI] [PubMed] [Google Scholar]
- Stevens C, Smith L, La Thangue NB (2003) Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 5: 401–409 [DOI] [PubMed] [Google Scholar]
- Wuarin J, Buck V, Nurse P, Millar JB (2002) Stable association of mitotic cyclin B/Cdc2 to replication origins prevents endoreduplication. Cell 111: 419–431 [DOI] [PubMed] [Google Scholar]
- Xu YJ, Kelly TJ (2009) Autoinhibition and autoactivation of the DNA replication checkpoint kinase Cds1. J Biol Chem 284: 16016–16027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalmas LP, Zhao X, Graham AL, Fisher R, Reilly C, Coutts AS, La Thangue NB (2008) DNA-damage response control of E2F7 and E2F8. EMBO Rep 9: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.