Cyclic-AMP-dependent protein kinase A regulates apoptosis by stabilizing the BH3-only protein Bim
Phosphorylation of the proapoptotic BH-3 only protein Bim usually leads to Bim degradation by the proteasome. Here, the authors show that phosphorylation of Bim by the cAMP dependent protein kinase A (PKA) instead leads to Bim protein stabilization and to apoptosis.
Keywords: apoptosis, Bim, cAMP, PKA
Abstract
The proapoptotic Bcl2 homology domain 3(BH3)-only protein Bim is controlled by stringent post-translational regulation, predominantly through alterations in phosphorylation status. To identify new kinases involved in its regulation, we carried out a yeast two-hybrid screen using a non-spliceable variant of the predominant isoform—BimEL—as the bait and identified the regulatory subunit of cyclic-AMP-dependent protein kinase A—PRKAR1A—as an interacting partner. We also show that protein kinase A (PKA) is a BimEL isoform-specific kinase that promotes its stabilization. Inhibition of PKA or mutation of the PKA phosphorylation site within BimEL resulted in its accelerated proteasome-dependent degradation. These results might have implications for human diseases that are characterized by abnormally increased PKA activity, such as the Carney complex and dilated cardiomyopathy.
Introduction
The proapoptotic Bcl2 homology domain 3(BH3)-only Bcl2 family protein Bim is an essential initiator of apoptosis in a variety of physiological settings (Wong & Puthalakath, 2008). Downregulation of Bim seems to be common to many cellular survival signalling pathways. This has led to efforts aimed at inducing Bim expression in tumour cells, as a cancer therapy (Kuroda et al, 2006; Cragg et al, 2007). The importance of Bim in tumorigenesis is also highlighted by data showing that the bim gene is deleted or Bim protein is downregulated in many tumours (Tagawa et al, 2005; Zantl et al, 2007; Anderton et al, 2008; Dai et al, 2008).
Bim expression can be regulated by transcriptional or post-translational processes (Puthalakath et al, 2007; Wong & Puthalakath, 2008). At the post-translational level, the two main isoforms of Bim—BimEL and BimL—can be sequestered to the microtubule-associated dynein motor complex through its interaction with the dynein light chain LC8 (Puthalakath et al, 1999). Apoptotic stimuli that activate c-Jun amino-terminal kinase signalling lead to the release of Bim from this complex, allowing it to bind to prosurvival Bcl2 family proteins to initiate cell death (Lei & Davis, 2003). The proapoptotic activity of Bim can also be regulated by phosphorylation of the extracellular-signal-regulated kinases/mitogen-activated protein kinase (ERK/MAPK) pathway (Ley et al, 2006). Five BimEL phosphorylation sites have been described; however, isoelectric focusing two-dimensional (IEF-2D) gel analysis of the endogenously expressed BimEL can be resolved into nine distinct spots (Puthalakath et al, 2007), demonstrating that this BimEL can be phosphorylated at up to eight serine/threonine/tyrosine sites. This indicates that BimEL might be regulated at the post-translational level by additional kinases. To identify these kinases, we carried out a yeast two-hybrid library screen using a non-spliceable mutant of BimEL as the bait. Here, we report the identification of the cyclic AMP (cAMP)-regulated protein kinase A (PKA) regulatory subunit-α (PRKAR1A) as an interaction partner of BimEL. In cells, PRKAR1A exists as a heterotetramer with the PKA catalytic subunit-α(PKACα), cAMP flux results in its release. Our results suggest that the interaction of BimEL with PRKAR1A helps to dock PKACα near by and enables PKA to phosphorylate BimEL. Furthermore, we report that BimEL is a PKA substrate and that BimEL phosphorylation by PKA stabilizes the protein by protecting it from proteasomal degradation, thereby promoting apoptosis.
Results
BimEL is a protein kinase A substrate
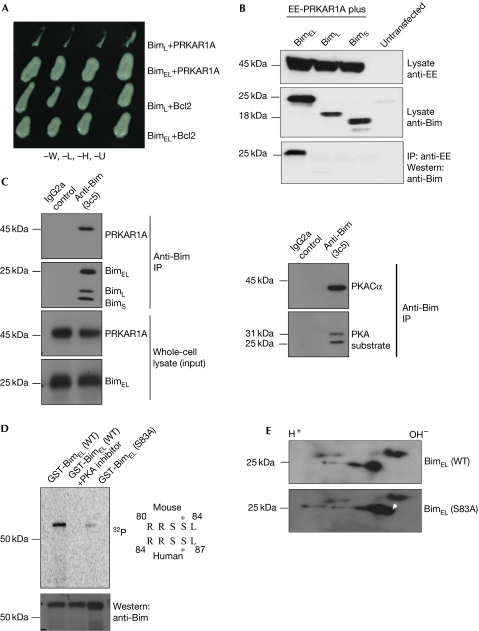
A yeast two-hybrid library screen was carried out to identify the interaction partners of BimEL. The cAMP-dependent PRKAR1A was identified as a specific interaction partner of BimEL. In yeast two-hybrid assays, only the BimEL isoform interacted with PRKAR1A, whereas the other isoforms did not (Fig 1A). This interaction was confirmed in 293T overexpression, as well as by physiological levels of expression of the breast-cancer cell line MCF7 (Fig 1B,C). It was not mediated by the AKAP-binding domain of PRKAR1A (supplementary Fig S1A,B online). This is similar to the interaction of PRKAR1A with activation-induced deaminase, which was found to be responsible for phosphorylation and activation of activation-induced deaminase by PKA (Pasqualucci et al, 2006). Therefore, we hypothesized that BimEL might be a PRKAR1A-dependent substrate for PKA-mediated phosphorylation. Indeed, the primary amino-acid sequence of BimEL (but not that of the other Bim isoforms) contains the signature motif for PKA phosphorylation—K/RK/RXS/T—at amino acids 80–83 in the mouse sequence and 83–87 in the human sequence (Fig 1D). This indicated that Ser83 of mouse BimEL and Ser87 of human BimEL might be phosphorylated by PKA, which we tested in three assays. First, immunoprecipitation of endogenous BimEL from MCF cells was probed with antibodies that recognize motifs phosphorylated by PKA (Fig 1C, right panel), this identified a band similar in size to Bim. The blot also showed an additional band (around 30 kDa), which might be Mcl-1—a known PKA substrate (Ozaki et al, 2008). Second, in vitro kinase assays using recombinant mouse BimEL or the S83A mutant showed that only the wild-type protein can be phosphorylated by PKA (Fig 1D). This mutant was functional, as it retained its ability to interact with anti-apoptotic proteins (supplementary Fig S1C online). Third, when both wild-type and mutant proteins were expressed in 293T cells—together with PKACα—the wild-type protein resolved into two hyperphosphorylated spots on IEF, whereas the mutant resolved into three spots, the third of which was closer to the cathode, indicating that it is less phosphorylated (Fig 1E).
Figure 1.
Cyclic-AMP-dependent protein kinase A can phosphorylate BimEL. (A) BimEL interacts specifically with PRKAR1A in yeast two-hybrid assays. Interaction with Bcl2 is shown as a control for the expression of the shorter Bim isoform BimL. (B) BimEL specifically interacts with PRKAR1A in HEK 293T cells. EE-tagged PRKAR1A was co-expressed with the different isoforms of Bim and the lysates were tested for the expression of proteins using EE or Bim antibodies. The lysates were subjected to immunoprecipitation with EE antibodies and probed with Bim antibodies. (C) BimEL, PRKAR1A and PKACα form a tripartite complex. BimEL was immunoaffinity purified from MCF7 cells, and Bim and PRKAR1A (left panel) and PKACα and PKA substrate (right panel) were probed. (D) The wild-type and the S83A mutant forms of mouse BimEL were purified from Escherichia coli as GST-fusion proteins and subjected to in vitro kinase assays using purified PKA. The middle lane shows the product of a reaction in which a PKA-specific inhibitor was included as a control for specificity. The membrane was exposed to a phosphorimager and then probed with Bim-specific antibodies as a loading control. The PKA consensus sequence within the mouse and human BimEL proteins is shown on the right with the phosphoserine indicated by an asterisk. (E) IEF-2D gel analysis of the wild-type and S83A mutant forms of mouse BimEL. The extra spot (that is, less phosphorylated) in the S83A mutant mouse BimEL is indicated by the white arrowhead. EE, EYMPME; GST, glutathione-S-transferase; IEF-2D, isoelectric focusing two-dimensional; IP, immunoprecipitation; PKA, protein kinase A; PKACα, PKA catalytic subunit-α; PRKAR1A, cyclic-AMP-regulated protein kinase A regulatory subunit-α; WT, wild type.
PKA activity is necessary for the stability of BimEL
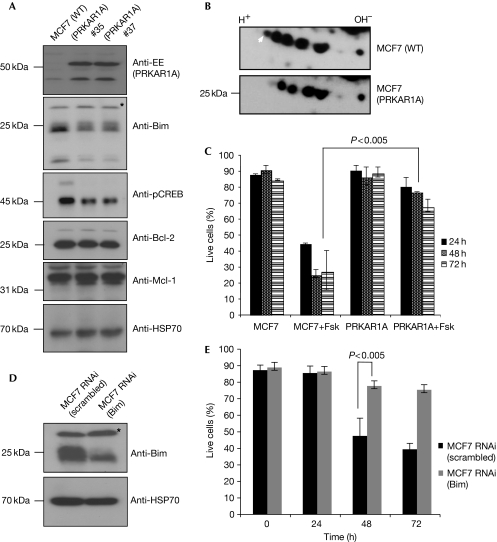
Having established that BimEL is a substrate for PKA, we next investigated the effect of PKA-mediated phosphorylation on the rate of BimEL turnover. Independent MCF7 clones ectopically overexpressing PRKAR1A—which downregulates PKA activity; (Fig 2A)—were generated. These cells had lower levels of endogenous BimEL than parental MCF7 cells, whereas the levels of anti-apoptotic proteins remained unchanged (Fig 2A). Furthermore, PRKAR1A overexpression caused a significant alteration in the phosphorylation status of endogenous BimEL. IEF-2D gel analysis showed that BimEL immunopurified from PRKAR1A-overexpressing MCF7 cells was less phosphorylated than BimEL from parental MCF7 cells (Fig 2B).
Figure 2.
PRKAR1A regulates the phosphorylation status and levels of endogenous BimEL in MCF7 cells. (A) MCF7 cells were stably transfected with a construct encoding EE-tagged PRKAR1A and cell lysates from two independent clones were tested by western blotting for the levels of indicated proteins. The asterisk indicates a nonspecific band. (B) Endogenous BimEL proteins were immunopurified from lysates of PRKAR1A-overexpressing or parental MCF7 cells and subjected to IEF-2D gel analysis. The white arrowhead indicates the differentially phosphorylated spot. (C) The PRKAR1A-overexpressing and parental MCF7 cells were treated with 24-μM forskolin and analysed for apoptosis induction at the indicated time points. (D,E) cAMP-induced apoptosis is Bim dependent. MCF7 cells transduced with constructs encoding scrambled RNAi (control) or Bim RNAi (polyclonal population) were analysed for Bim expression by western blotting (D; asterisk indicates a nonspecific band) and examined for sensitivity to forskolin as described in C. Data in C and E represent means ± s.e.m. of three independent experiments and the P-values were determined by one-tailed, type 1 Student's t-test. cAMP, cyclic AMP; CREB, cAMP response element-binding; EE, EYMPME; Fsk, forskolin; IEF-2D, isoelectric focusing two-dimensional; PKA, protein kinase A; PRKAR1A, cyclic-AMP-regulated protein kinase A regulatory subunit-α; RNAi, RNA interference; WT, wild-type.
As Bim was shown to be crucial for cAMP-induced apoptosis (Zhang & Insel, 2004), we examined whether PRKAR1A overexpression could protect MCF7 cells against forskolin, a potent inducer of adenylyl cyclase. Overexpression of PRKAR1A protected these cells against cAMP-induced apoptosis (Fig 2C), to an extent comparable with RNA-interference-mediated knockdown of Bim (Fig 2D,E; polyclonal population of RNA interference knockdown). These experiments were repeated with physiologically relevant β-adrenergic receptor agonist isoproterenol, with a similar outcome (supplementary Fig S2 online). These results indicate that the stability—and probably the proapoptotic activity—of BimEL can be regulated by PKA-mediated phosphorylation.
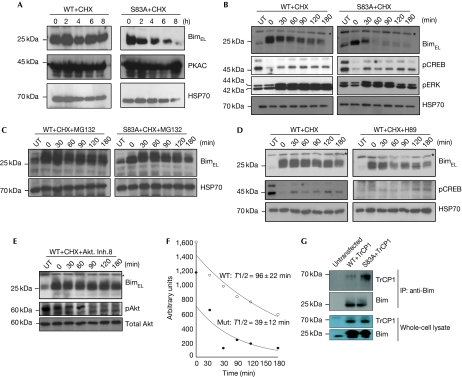
cAMP flux is known to induce bim at the transcriptional level (Zhang & Insel, 2004). Therefore, to distinguish the transcriptional effect from the post-translational effect and also to compare the kinetics of the turnover of wild-type with mutant BimEL proteins, the proteins were overexpressed in transiently transfected 293T cells—together with PKACα—as well as in mouse embryonic fibroblasts (MEFs), by lentiviral infection. Time-course experiments after the addition of cycloheximide showed that mutant BimEL had a higher turnover rate than wild-type BimEL (Fig 3A). In addition, we transduced bim−/− MEFs with constructs that allow inducible expression of wild-type or S83A mutant mouse BimEL, selecting clones with similar levels of expression of the two proteins. Northern and western blot analyses show that the S83A mutant clone is expressed at a higher level and yet protein levels are comparable (Fig 3B; supplementary Fig S3A,B online). Protein turnover analysis showed that the S83A mutant has a significantly shorter half-life (39 min) than wild-type BimEL (96 min; Fig 3B,F). cAMP flux is known to inhibit ERK activation (Cook & McCormick, 1993), but the difference in turnover could not be due to differences in ERK or cAMP activation levels in these cell lines (Fig 3B), but could be attributed to increased proteasomal degradation of the mutant protein, as wild-type and S83A mutant mouse BimEL showed similar turnover when these cells were treated with both cycloheximide and the proteasome inhibitor MG132 (Fig 3C). Conversely, treatment of transfected cells with the PKA-specific inhibitor H89 or PKA inhibitory peptide (as indicated by phospho-CREB levels in these cells) accelerated the degradation of wild-type BimEL (Fig 3D; supplementary Fig S3C online). Ser 83 of BimEL has been reported to be a substrate for Akt phosphorylation, which primes the protein for degradation (Qi et al, 2006). However, Akt inhibition had no effect on protein turnover (Fig 3E), but phosphorylation by ERK1/2 was shown to prime BimEL for ubiquitination and proteasomal degradation. This has been attributed to the recruitment of BimEL by Cullin 1 to the ubiquitin ligase βTrCP1 (Dehan et al, 2009). Co-expression in 293T cells and immunoprecipitation studies showed that the S83A mutant BimEL seems to bind to βTrCP1 more than wild-type BimEL (Fig 3G). However, the mutation had no impact on the binding of BimEL to the anti-apoptotic protein Bcl2 (supplementary Fig S1C online). These results demonstrate that PKA-mediated phosphorylation regulates the turnover of BimEL by controlling its interaction with the ubiquitin ligase βTrCP1 and hence its proteasomal degradation.
Figure 3.
Protein kinase A-mediated phosphorylation controls the stability of BimEL protein. (A) HEK 293T cells were transfected with constructs encoding either the wild type or BimEL (S83A) and after 48 h were treated with 10 μM CHX to inhibit protein synthesis. Samples were collected at the indicated time points and subjected to western blot analysis for the indicated proteins. (B) MEFs expressing either the WT or the mutant protein were treated with CHX and samples were collected at the indicated time points and subjected to western blot analysis for the indicated proteins. (C) The experiment was carried out as in B, but with the addition of the proteasomal inhibitor MG132 (40 μM). (D,E) MEFs expressing wild-type mouse BimEL were treated with CHX ± the PKA inhibitor H89 (20 μM), or with the Akt inhibitor VIII (5 μM, Calbiochem) and lysates were analysed by western blotting for the indicated proteins. Asterisks indicate a nonspecific band. (F) Half-life measurement of the wild-type and PKA phosphorylation site mutant S83A mouse BimEL proteins in MEFs, using the same experimental setting as in B. (G) HEK 293T cells were co-transfected with constructs encoding TrCP1 and with either the WT or the S83A mutant form of BimEL. Lysates were immunoprecipitated with Bim-specific antibodies and probed for βTrCP (upper panel). Total protein expression is shown in the lower panel. cAMP, cyclic AMP; CHX, cycloheximide; CREB, cAMP response element-binding; Fsk, forskolin; MEF, mouse embryonic fibroblast; RNAi, RNA interference; UT, cells not treated with 4-OHT; WT, wild-type.
S83A mutant BimEL has reduced apoptotic activity
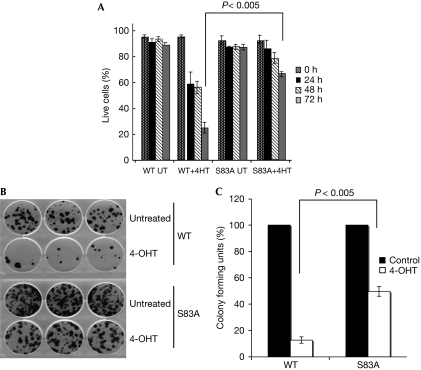
Finally, we examined whether PKA-mediated phosphorylation could have an effect on the proapoptotic activity of BimEL. Cell survival analysis in MEFs showed that wild-type BimEL was more potent in short-term killing (Fig 4A), reduction of clonogenicity (Fig 4B,C) and in caspase activation (supplementary Fig S3 online) compared with the mutant. Together, these results demonstrate that the cAMP-dependent PKA has a role in the control of the turnover of BimEL and thereby regulates cAMP-induced apoptosis.
Figure 4.
Mutation of the protein kinase A phosphorylation site reduces the proapoptotic activity of BimEL. (A) MEFs were treated with 4-OHT (5 nM) to induce either the WT or mutant BimEL, and induction of apoptosis was measured at the indicated time points. (B,C) The impact of induced expression of WT or mutant BimEL on clonogenic survival was examined in the MEFs described in A. Data in A and C represent the mean±s.e.m. of three independent experiments and P-values were determined by one-tailed, type 1 Student's t-test. MEF, mouse embryonic fibroblast; UT, untransfected; WT, wild type.
Discussion
It is thought that phosphorylation primes Bim for degradation, but some findings have challenged this idea (Häcker et al, 2006; Ley et al, 2006). For example, ERK1/2 mediation not only targets Bim for ubiquitination but also lowers its affinity for Bcl-xL and Mcl1, which promote survival (Ewings et al, 2007). Phosphorylation of Bim by c-Jun N-terminal kinase mediation reportedly increases the proapoptotic activity of Bim by causing its release from the dynein motor complex (Lei & Davis, 2003), although phosphorylation occurs at the same site that is targeted by ERK1/2. These discordant results could be reconciled if Bim is not controlled by a single phosphorylation event, but by a complex code of phosphorylations on many residues. Our previous study supports this hypothesis, showing that BimEL is phosphorylated at several sites (Puthalakath et al, 2007). The identification here of the BimEL–PRKAR1A interaction is intriguing because the function of the regulatory subunit—PRKAR1A—is to bind to PKACα and retain it in an inactive complex. Elevation of cellular cAMP levels results in the release and activation of the catalytic subunit (Bossis & Stratakis, 2004). Binding of PRKAR1A to BimEL might thus ensure that PKA and its substrate are brought together so that BimEL can be phosphorylated efficiently in response to extracellular cues. Our experiments demonstrate that BimEL is a PKA substrate in a variety of cell types, suggesting that this is a general process.
One unusual finding of our work is the stabilization of BimEL as opposed to proteasomal degradation by phosphorylation. Our results also demonstrate that this degradation occurs irrespective of the ERK activation status (Fig 3B). This accelerated degradation is associated with increased affinity of BimEL for the ubiquitin ligase βTrCP1. This is in contrast to the observation that Ser 83 of mouse BimEL (or Ser 87 of human BimEL) can be phosphorylated by PI3K/Akt and that this promotes its degradation (Qi et al, 2006). Although the same residue can be phosphorylated by two different kinases, it is difficult to reconcile the two opposite outcomes. In this study we demonstrate that phosphorylation of Ser 83 on BimEL is crucial for its stabilization, by showing that the S83A mutant protein has an abnormally shortened half-life by protein turnover measurement, increased avidity for the ubiquitin ligase βTrCP1 by coimmunoprecipitation studies and decreased apoptotic potential by short-term as well as clonogenic survival assays. Such detailed analyses were not carried out in the previous study (Qi et al, 2006), in which apoptosis was measured by DNA laddering, which is qualitative at best, and by transient transfection without normalization for transfection efficiency. This might account for the observed discrepancies.
Our findings might have implications for human diseases that have been attributed to mutations in PRKAR1A, such as Carney's complex or multiple endocrinal neoplasia (Bossis & Stratakis, 2004; Nadella & Kirschner, 2005; Sasaki et al, 2008). Mutations in PRKAR1A are expected to lead to increased PKA activity, which has been suggested to be crucial for the pathophysiology of these disorders. Our results demonstrate that in addition to the reported increase in bim transcription (Zhang & Insel, 2004), enhanced PKA activity can also lead to increased BimEL stabilization. These processes are thought to act together to promote apoptosis. This indicates that the development of endocrinal tumours associated with PRKAR1A mutation can only occur if the cell undergoing neoplastic transformation is able to overcome the apoptotic effect of BimEL, through mutations in bim, by another crucial regulator of Bim or by increased expression of anti-apoptotic Bcl2 proteins. Understanding the molecular mechanisms involved in cAMP-induced apoptosis might help the development of cAMP-based therapies for the treatment of lymphoid malignancies (Lerner et al, 2000).
Methods
Library construction and yeast two-hybrid screening. The cDNA libraries from day 17 mouse embryos or from mouse embryos from embryonic day 9 to day 1 postpartum were prepared in pAD-GAL4-2.1 (HybriZAP-2.1 kit, Stratagene). The primary library consisted of approximately two million independent clones. A non-spliceable form of BimEL was used as the bait (GGT to GGC for Gly 42, and AGA to CGC for Arg 97). The two-hybrid screen was carried out as described previously (Puthalakath et al, 1999). Interaction between BimEL and PRKAR1A was confirmed by β-galactosidase staining as described previously (Puthalakath et al, 2001).
PKA assays. In vitro PKA assays were carried out using the PKA assay kit (Upstate, NY). Each assay contained 5–10 ng of the recombinant glutathione-S-transferase-tagged protein in a total volume of 60 μl reaction buffer that contained the following: 10 μl of 1 × assay dilution buffer, 5 μl of 20 μM cAMP, 10 μl of nonspecific kinase inhibitor cocktail, 1 μl of PKA catalytic subunit (2.5–10 U) and 10 μl of Mg-ATP cocktail. A volume of 10 μl of PKA inhibitor peptide was used as a negative control. The reaction tubes were incubated at 30° C for 30 min. Reactions were stopped by mixing with equal volumes of 2 × Laemmli loading buffer and boiled before separating on precast SDS–polyacrylamide gel electrophoresis gels (Novex), followed by transfer onto PVDF membrane. The radioactive fusion proteins were detected in a Typhoon 9410 phosphorimager (GE Healthcare).
IEF-2D gel analysis. IEF electrophoresis was performed as described previously (Ninnis et al, 2009). Endogenous BimEL was immunoaffinity purified from Dithiobis(succinimidyl propronate)-crosslinked MCF7 (2 μM) cells before IEF separation (Puthalakath et al, 2007).
Cell death assays. Cell death was quantified by staining with Annexin V-fluorescein isothiocyanate plus propidium iodide (2.5 μg/ml), followed by flow cytometric analysis in a FACScan (Becton Dickinson). For clonogenic survival analysis, cells were treated with different stimuli and dilutions were plated on six-well plates. After the incubation period, cells were fixed with paraformaldehyde (1% in PBS) and stained with 0.1% crystal violet solution (10% in ethanol) at 25°C for 15 min. Excess stain was poured off and plates were rinsed in water and dried at room temperature.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank L. O'Connor, N. Hoogenraad and D.L. Vaux for helpful discussion; L.A. O'Reilly, P. Bouillet, D. Chau, J. Silke and P. Ekert for reagents and cell lines and I. Wertz for the βTrCP1 construct. H.P. is supported by Australian Research Council Future Fellowship and National Health and Medical Research Council project grant (487311). A.S. is supported by the Leukemia and Lymphoma Society (7413) and the National Health and Medical Research Council (461221, 461299). D.M. is supported by a La Trobe Postgraduate Award and Cooperative Research Centre for Biomarker Translation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Anderton E, Yee J, Smith P, Crook T, White RE, Allday MJ (2008) Two Epstein–Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt′s lymphoma. Oncogene 27: 421–433 [DOI] [PubMed] [Google Scholar]
- Bossis I, Stratakis CA (2004) PRKAR1A: normal and abnormal functions. Endocrinology 145: 5452–5458 [DOI] [PubMed] [Google Scholar]
- Cook SJ, McCormick F (1993) Inhibition by cAMP of Ras-dependent activation of Raf. Science 262: 1069–1072 [DOI] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A (2007) Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM. PLoS Med 4: 1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DL, Wang Y, Liu M, Martinka M, Li G (2008) Bim expression is reduced in human cutaneous melanomas. J Invest Dermatol 128: 403–407 [DOI] [PubMed] [Google Scholar]
- Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN, Dustin M, Huang DC, Taunton J, Pagano M (2009) Beta TrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol Cell 33: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ (2007) ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J 26: 2856–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker G, Suttner K, Harada H, Kirschnek S (2006) TLR-dependent Bim phosphorylation in macrophages is mediated by ERK and is connected to proteasomal degradation of the protein. Int Immunol 18: 1749–1757 [DOI] [PubMed] [Google Scholar]
- Kuroda J et al. (2006) Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA 103: 14907–14912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA 100: 2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Kim DH, Lee R (2000) The cAMP signalling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma 37: 39–51 [DOI] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ (2006) Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ 12: 1008–1014 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS (2005) Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65: 10307–10315 [DOI] [PubMed] [Google Scholar]
- Ninnis RL, Spall SK, Talbo GH, Truscott KN, Dougan DA (2009) Modification of PATase by L/F-transferase generates a ClpS-dependent N-end rule substrate in Escherichia coli. EMBO J 28: 1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y, Kato T, Kitagawa M, Fujita H, Kitagawa S (2008) Calpain inhibition delays neutrophil apoptosis via cyclic AMP-independentactivation of protein kinase A and protein kinase A-mediated stabilization of Mcl-1 and X-linked inhibitor of apoptosis (XIAP). Arch Biochem Biophys 477: 227–231 [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Kitaura Y, Gu H, Dalla-Favera R (2006) PKA-mediated phosphorylation regulates the function of activation-induced deaminase (AID) in B cells. Proc Natl Acad Sci USA 103: 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by. Mol Cell 3: 287–296 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A, Huang DC (2001) Rapid selection against truncation mutants in yeast reverse two-hybrid screens. Biotechniques 3: 984–988 [DOI] [PubMed] [Google Scholar]
- Puthalakath H et al. (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349 [DOI] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH (2006) Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem 281: 813–823 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Horikawa Y, Suwa T, Enya M, Kawachi SI, Takeda J (2008) Case report of familial Carney complex due to novel frame shift mutation c.597del C (p.Phe200LeufsX6) in PRKAR1A. Mol Genet Metab 95: 182–187 [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, Morishima Y, Nakamura S, Seto M (2005) Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 24: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Wong WW, Puthalakath H (2008) Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life 60: 390–397 [DOI] [PubMed] [Google Scholar]
- Zantl N, Weirich G, Zall H, Seiffert BM (2007) Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene 26: 7038–7048 [DOI] [PubMed] [Google Scholar]
- Zhang L, Insel PA (2004) The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem 279: 20858–20865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.