Abstract
How does the womb determine the future? Scientists have begun to uncover how environmental and maternal factors influence our long-term health prospects.
About two decades ago, David Barker, Professor of Clinical Epidemiology at the University of Southampton, UK, proposed a hypothesis that malnutrition during pregnancy and resultant low birth weight increase the risk of developing cardiovascular disease in adulthood. “The womb may be more important than the home,” remarked Barker in a note about his theory (Barker, 1990). “The old model of adult degenerative disease was based on the interaction between genes and an adverse environment in adult life. The new model that is developing will include programming by the environment in fetal and infant life.”
This new idea about the influence of the environment during prenatal development on adult disease risk comes with a better understanding of epigenetic processes…
The ‘Barker theory' has been increasingly accepted and been expanded to other diseases, prominently diabetes and obesity, but also osteoporosis and allergies. “In the last few years, the evidence [of an extended] range of potential disease phenotypes with a prenatal developmental component to risk […] has become much stronger,” said Peter Gluckman at the University of Auckland, New Zealand. “We also need to give greater attention to the growing evidence of prenatal and early postnatal effects on cognitive and non-cognitive functional development and to variation in life history patterns.” Similarly, Michael Symonds and colleagues from the University Hospital at Nottingham, UK, wrote: “These critical periods occur at times when fetal development is plastic; in other words, when the fetus is experiencing rapid cell proliferation making it sensitive to environmental challenges” (Symonds et al, 2009).
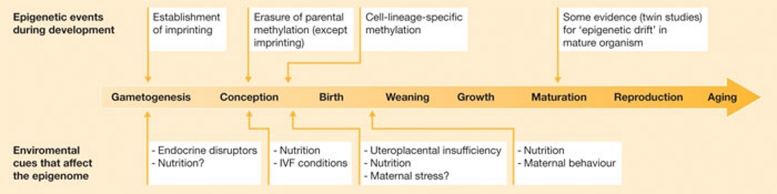
This new idea about the influence of the environment during prenatal development on adult disease risk comes with a better understanding of epigenetic processes—the biological mechanisms that explain how in utero experiences could translate into phenotypic variation and disease susceptibility within, or over several, generations (Gluckman et al, 2009; Fig 1). “I think it has been the combination of good empirical data (experimental and clinical), the appearance of epigenetic data to provide molecular mechanisms and a sound theoretical framework (based on evolutionary biology) that has allowed this field to mature,” said Gluckman. “Having said that, I think it is only as more human molecular data (epigenetic) emerges that this will happen.”
Figure 1.
Environmental sensitivity of the epigenome throughout life. Adapted from Gluckman et al (2009), with permission.
Epidemiological data in support of the Barker theory have come from investigations of the effects of the ‘Dutch famine'. Between November 1944 and May 1945, the western part of The Netherlands suffered a severe food shortage, owing to the ravages of the Second World War. In large cities such as Utrecht, Amsterdam, Rotterdam and The Hague, the average individual daily rations were as low as 400–800 kcal. In 1994, a large study involving hundreds of people born between November 1943 and February 1947 in a major hospital in Amsterdam was initiated to assess whether and to what extent the famine had prenatally affected the health of the subjects in later life. The Dutch Famine Birth Cohort Study (www.hongerwinter.nl) found a strong link between malnutrition and under-nutrition in utero and cardiovascular disease and diabetes in later life, as well as increased susceptibility to pulmonary diseases, altered coagulation, higher incidence of breast cancer and other diseases, although some of these links were only found in a few cases.
More recently, a group led by Bastiaan Heijmans at the Leiden University Medical Centre in The Netherlands and Columbia University (New York, USA) conducted epigenetic studies of individuals who had been exposed to the Dutch famine during gestation. They analysed the level of DNA methylation at several candidate loci in the cohort and found decreased methylation of the imprinted insulin-like growth factor 2 (IGF2) gene—a key factor in human growth and development—compared with the unexposed, same-sex siblings of the cohort (Heijmans et al, 2008). Further studies have identified another six genes implicated in growth, metabolic and cardiovascular phenotypes that show altered methylation statuses associated with prenatal exposure to famine (Heijmans et al, 2009). The overall conclusion from this work is that exposure to certain conditions in the womb can lead to epigenetic marks that can persist throughout life. “It is remarkable to realize that history can leave an imprint on our DNA that is visible up to six decades later. The current challenge is to scale up such studies to the genome,” said Heijmans. His team is now using high-throughput sequencing to see whether there are genomic regions that are more susceptible to prenatal environmental influences. “Genome-scale data may also allow us to observe the hypothesized accumulation of epigenetic changes in specific biological processes, perhaps as a sign of adaptive responses,” he said.
Epigenetic modification of genes involved in key regulatory pathways is central to the mechanisms of nutritional programming of disease, but other factors also seem to have a role including altered cell number or cell type, precocious activation of the hypothalamic–pituitary–adrenal axis, increased local glucocorticoid and endocrine sensitivity, impaired mitochondrial function and reduced oxidative capacity. “The particular type of mechanism invoked seems to vary between tissues according to the duration and timing of the nutritional intervention through pregnancy and/or lactation,” commented Symonds et al (2009).
“If we just focus on metabolic, cardiovascular and body compositional outcome, I think the data is now overwhelming that there is an important life-long early developmental contribution. The emergent data would suggest that the underpinning epigenetic processes are likely to be at least as important as genetic variation in contributing to disease risk,” commented Gluckman. His research in animal models has shown that epigenetic changes are potentially reversible in mammals through intervention during development, when the growing organism still has sufficient plasticity (Gluckman et al, 2007). For instance, the neonatal administration of leptin has a bidirectional effect on gene expression and the epigenetic status of key genes involved in metabolic regulation in adult rats; an effect that is dependent on prenatal nutrition and unaffected by post-weaning nutrition (normal compared with high-fat diet). In rats that were manipulated in utero by maternal under-nutrition and fed a hypercaloric diet after weaning, leptin treatment normalized adiposity and hepatic gene expression of proteins that are central to lipid metabolism and glucose homeostasis. “The experimental data showing that programming is reversible is a critical proof of concept. I think there is still confusion as to the role of catch-up growth—its effect may be dependent on its timing and this may have implications for infant nutrition,” Gluckman said.
The Dutch Famine Birth Cohort Study […] found a strong link between malnutrition and under-nutrition in utero and cardiovascular disease and diabetes in later life…
Central to this view of the link between the developing fetus and its later risk of metabolic disease is the idea of ‘developmental mismatch'. The fetus is programmed, largely through epigenetic changes, to match its environment. However, if the environment in childhood and adult life differs sharply from that during prenatal and early postnatal development, ill adaptation can occur and bring disease in its wake (Gluckman & Hanson, 2006). Poor nutrition during fetal development, for example, would lead the organism to expect a hostile future environment, adversely affecting its ability to cope with a richer environment. “Developmental factors do not cause disease in this context, rather they create a situation where the individual becomes more (or less) sensitive in an obesogenic postnatal environment,” said Gluckman. “The experimental and early clinical data point to both central and peripheral effects and this may explain why lifestyle intervention is so hard in some individuals.”
Yet there is another nutrition-related pathway that goes beyond mismatch. According to a recent, large population-based study published in The Lancet, maternal weight gain during pregnancy increases birth weight independently of genetic factors, which increases the long-term risk of obesity-related disease in offspring (Ludwig & Currie, 2010). To reduce or eliminate potential confounds such as genetics, sociodemographic factors or other individual characteristics, the researchers examined the association between maternal weight gain—as a measure of over-nutrition during pregnancy—and birth weight using State-based birth registry data in Michigan and New Jersey, allowing them to compare outcomes from several pregnancies in the same mother. “During pregnancy, insulin resistance develops in the mother to shunt vital nutrients to the growing fetus. Excessive weight or weight gain during pregnancy exaggerates this normal process by further increasing insulin resistance and possibly also by affecting other maternal hormones that regulate placental nutrient transporters. The resulting high rate of nutrient transfer stimulates fetal insulin secretion, overgrowth, and increased adiposity,” the authors speculated (Ludwig & Currie, 2010).
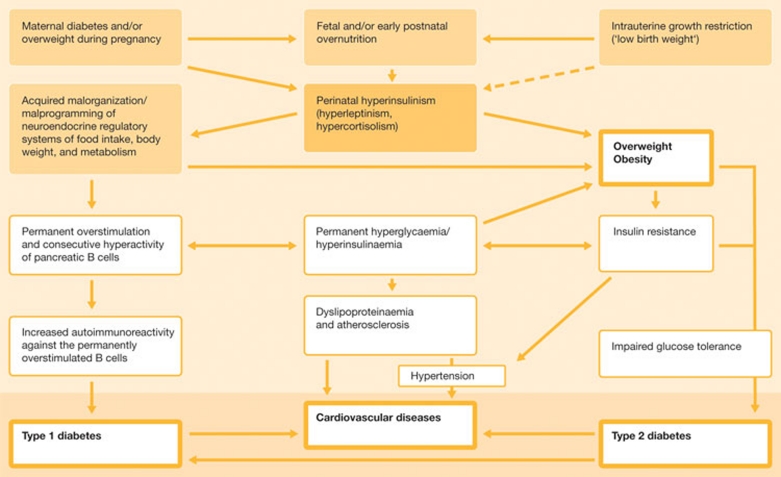
It could be that epigenetic malprogramming is also involved in these cases. The group of Andreas Plagemann at the Charitè–University Medicine in Berlin, Germany, analysed acquired alterations of DNA methylation patterns of the hypothalamic insulin receptor promoter (IRP) in neonatally overfed rats. They found that altered nutrition during the critical developmental period of perinatal life induced IRP hypermethylation in a seemingly glucose-dependent manner. This revealed an epigenetic mechanism that could affect the function of a promoter that codes for a receptor involved in the life-long regulation of food intake, body weight and metabolism (Plagemann et al, 2010). “In parallel with the general ‘diabesity' epidemics, diabetes during pregnancy and overweight in pregnant women meanwhile reach dramatic prevalences. Consequently, mean birth weight and frequencies of ‘fat babies' rise,” said Plagemann. “Taking together epidemiological, clinical and experimental observations, it seems obvious that fetal hyperinsulinism induced by maternal hyperglycaemia/overweight has ‘functionally teratogenic' significance for a permanently increased disposition to obesity, diabetes, the metabolic syndrome, and subsequent cardiovascular diseases in the offspring” (Fig 2).
Figure 2.
Pathogenetic framework, mechanisms and consequences of perinatal malprogramming, showing the etiological significance of perinatal overfeeding and hyperinsulinism for excess weight gain, obesity, diabetes mellitus and cardiovascular diseases in later life. Credit: Andreas Plagemann.
Added to the mix is the ‘endocrine-disruptor hypothesis', one nuance of which proposes that prenatal—as well as postnatal—exposure to environmental chemicals contributes to adipogenesis and the development of obesity by interfering with homeostatic mechanisms that control weight. Several environmental pollutants, nutritional components and pharmaceuticals have been suggested to have ‘obesogenic' properties—the best known are tributyltin, bisphenol and phthalates (Grün & Blumberg, 2009). “While one cannot presently estimate the degree to which obesogen exposure contributes to the observed increases in obesity, the main conclusion to be drawn from research in our laboratory is that obesogens exist and that prenatal obesogen exposure can predispose an exposed individual to become fatter, later in life,” said Bruce Blumberg at the University of California at Irvine, USA, who is also credited with coining the term ‘obesogen'. “The existence of such chemicals was not even suspected as recently as seven years ago when we began this research.”
Several environmental pollutants, nutritional components and pharmaceuticals have been suggested to have ‘obesogenic' properties…
It is clear that diet and exercise are important contributors to the body weight of an individual. However, weight maintenance is not as simple as balancing a ‘caloric checkbook', or fewer people would be obese, Blumberg commented. Early nutrition and chemical exposure could alter the metabolic set-point of an individual, making their subsequent fight against weight gain more difficult. “We do not currently know how many chemicals are obesogenic or the entire spectrum of mechanisms through which obesogens act,” Blumberg said. “Our data suggest that prenatal obesogen exposure alters the fate of a type of stem cells in the body to favour the development of fat cells at the expense of other cell types (such as bone). In turn, this is likely to increase one's weight with time.”
Obesogen exposure in utero and/or during the first stages of postnatal growth could therefore predispose a child to obesity by influencing all aspects of adipose tissue growth, starting from multipotent stem cells and ending with mature adipocytes (Janesick & Blumberg, 2011). “Epigenetics may also allow us to have a clearer view of the role of xenobiotics, such as bisphenol A, where traditional teratogenetic approaches to analysis seem inappropriate,” Gluckman said. “I expect the potential for either direct or indirect epigenetic inheritance will get much focus in human studies over the next few years.”
The impact of the mother's emotional state during pregnancy on the child's behaviour and cognitive development of the child is also fertile ground for research. “It has been known from over 50 years of research in animals that stress during pregnancy can have long-term effects on the behavioural and cognitive outcome for the offspring. Over the last ten years many studies, including our own, have shown that the same is true in humans,” said Vivette Glover, a leading expert in the field from Imperial College (London, UK). “If the mother is stressed or anxious while she is pregnant, her child is more likely to have a range of problems such as symptoms of anxiety or depression, [attention deficit hyperactivity disorder] ADHD or conduct disorder, and to be slower at learning, even after allowing for postnatal influences.” Most children are not affected, but if the anxiety level of the mother is in the top 15% of the general population, the risk of her child having these problems increases from about 5% to 10%, Glover explained.
Early nutrition and chemical exposure could alter the metabolic set-point of an individual, making their subsequent fight against weight gain more difficult
Focusing on the mechanisms that underlie this, Glover's team has shown that the cognitive development of the child is slower if the fetus is exposed to higher levels of the stress hormone cortisol in the womb (Bergman et al, 2010). Cortisol in fetal circulation is a combination of that produced endogenously by the fetus and that derived from the mother, through the placenta. Glover's hypothesis is that the placenta might have a key role as a programming vector: if the mother is stressed and more anxious, the placenta becomes a less effective barrier and allows more cortisol to pass from the mother to the fetus (O'Donnell et al, 2009). “Our most recent research has studied how these prenatal effects can be altered by the later quality of the mothering, and we have found that the effects can be exacerbated if the child is insecure and buffered if the child is securely attached to the mother. So the effects are not all over at birth. There are both prenatal and postnatal effects,” Glover said. “There are large public health implications of all this. If we, as a society, cared better for the emotional wellbeing of our pregnant women we would also improve the behavioural, emotional and cognitive outcome for the next generation,” she concluded (Sidebar A).
A more integrated view of the developmental ontogeny of a human from embryo to adult is needed…
Sidebar A | Focus on fetal life to help the next generation.
“The global burden of death, disability, and loss of human capital as a result of impaired fetal development is huge and affects both developed and developing countries,” concludes a recent World Health Organization technical consultation (WHO, 2006). It advocates moving away from a focus on birth weight to embrace more factors to ensure an optimal environment for the fetus, to maximize its potential for a healthy life. As our knowledge of developmental biology expands, there is progressively greater awareness that events early in human development can have effects in later stages of life, and even inter-generational consequences in terms of non-communicable diseases, such as cardiovascular disease and diabetes.
Calling for a radical change in medical attitudes—which they say are responsible for not giving enough credit to “the concept that environmental factors acting early in life (usually in fetal life) have profound effects on vulnerability to disease later in life”—Peter Gluckman, Mark Hanson and Murray Mitchell have recently proposed several prevention and intervention initiatives that could reduce the burden of chronic disease in the next generation (Gluckman et al., 2010). These include limitation of adolescent pregnancy, possibly delaying the age of first pregnancy until four years after menarche; promotion of a healthy diet and lifestyle among women becoming pregnant to avoid the long-term effects of both excessive and deficient maternal nutrition, smoking, or drug and alcohol abuse; and encouraging breastfeeding for optimal growth, resistance to infection, cardiovascular health and neurocognitive development. Clearly, such actions would face a mix of educational, political and social issues, depending on the geographical or cultural area.
“None of these solutions seems sophisticated, although it may have taken the recent insights into underlying developmental epigenetic mechanisms to emphasize them. But, when viewed in terms of their potential impact, especially in developing societies and in lower socioeconomic groups in developed countries, it is clear that their importance has been underestimated” (Gluckman et al., 2010).
A more integrated view of the developmental ontogeny of a human from embryo to adult is needed, grounded by appreciation of the fact that the developmental trajectory of the fetus is influenced by factors such as maternal nutrition, body composition and maternal age (Fig 3). This must not be limited to the offspring of gestational diabetics and obese mothers. “While these are more extreme influences on the fetus and will lead to immediate consequences (blurring the boundary between what is physiological and pathophysiological), I think the most important observations and conceptual advances will emerge from understanding the long-term implications and underpinning mechanisms of relatively normal early development still having plastic consequences,” Gluckman said. “Thus, what seem to be unremarkable pregnancies still have important influences on the destiny of the offspring.” Though this might be easy to say, the regulatory mechanisms that underlie the complex journey of development await further clarification.
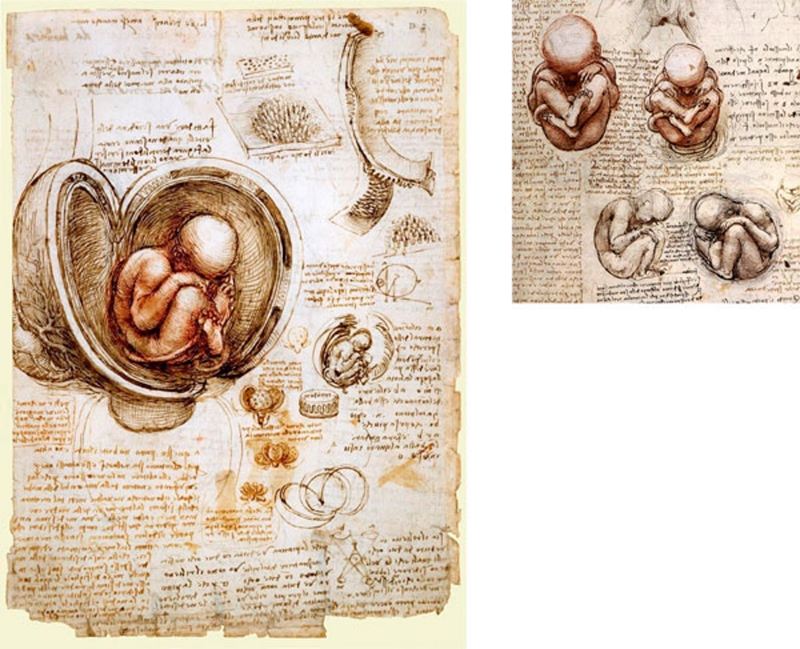
Figure 3.
Leonardo Da Vinci: Studies of the fetus in the womb, circa 1510–1513. In Da Vinci's words, referring to his treatise on anatomy, for which these drawings were made: “This work must begin with the conception of man, and describe the nature of the womb and how the fetus lives in it, up to what stage it resides there, and in what way it quickens into life and feeds. Also its growth and what interval there is between one stage of growth and another. What it is that forces it out from the body of the mother, and for what reasons it sometimes comes out of the mother's womb before the due time” (Dunn, 1997).
References
- Barker DJ (1990) The fetal and infant origins of adult disease. BMJ 301: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O'Connor TG (2010) Maternal prenatal cortisol and infant cognitive development: moderation by infant–mother attachment. Biol Psychiatry 67: 1026–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM (1997) Leonardo Da Vinci (1452–1519) and reproductive anatomy. Arch Dis Child Fetal Neonatal Ed 77: F249–F251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P, Hanson M (2006) Mismatch The Lifestyle Diseases Timebomb. Oxford, UK: Oxford University [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA (2007) Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA 104: 12796–12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS (2009) Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol 5: 401–408 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Mitchell MD (2010) Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, Blumberg B (2009) Endocrine disrupters as obesogens. Mol Cell Endocrinol 304: 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 105: 17046–17049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE (2009) The epigenome: archive of the prenatal environment. Epigenetics 4: 526–531 [DOI] [PubMed] [Google Scholar]
- Janesick A, Blumberg B (2011) Adipocytes as target cells for endocrine disruption. In Endocrine Disrupters and Puberty, Diamanti-Kandarakis E, Gore AC (eds). New York, NY, USA: Humana/Springer (in press) [Google Scholar]
- Ludwig DS, Currie J (2010) The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet 376: 984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K, O'Connor TG, Glover V (2009) Prenatal stress and neurodevelopment of the child: focus on the HPA axis and the role of the placenta. Dev Neurosci 31: 285–292 [DOI] [PubMed] [Google Scholar]
- Plagemann A et al. (2010) Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med 38: 393–400 [DOI] [PubMed] [Google Scholar]
- Symonds ME, Sebert SP, Hyatt MA, Budge H (2009) Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 5: 604–610 [DOI] [PubMed] [Google Scholar]
- WHO (2006) Promoting optimal fetal development: report of a technical consultation. Geneva, Switzerland, WHO. http://www.who.int/nutrition/topics/fetal_dev_report_EN.pdf