Abstract
We have developed a gas chromatography-high resolution mass spectrometry method for measuring pyrethroid, organophosphorus, carbamate and fipronil pesticides and the synergist piperonyl butoxide in human plasma. Plasma samples were extracted using solid phase extraction and were then concentrated for injection and analysis using isotope dilution gas chromatography-high resolution mass spectrometry. The limits of detection ranged from 10 to 158 pg/mL with relative recoveries at concentrations near the LODs (e.g., 25 or 250 pg/mL) ranging from 87% to 156% (9 of the 16 compounds were withing ± 15% of 100%). The extraction recoveries ranged from 20% to 98% and the overall method relative standard deviations were typically less than 20% with some exceptions. Analytical characteristics were determined at 25, 250, and 1000 pg/mL.
Keywords: biomonitoring, insecticide, pyrethroid, plasma, organophosphorus, carbamate, gas chromatography-high resolution mass spectrometry
1. Introduction
Approximately 912 million pounds of conventional pesticides are used annually in the United States. Useage is distributed as approximately 80% agriculture, 8% homes and gardens, and the remainder as government, commercial, or industrial applications. Herbicides comprise the bulk of conventional pesticides (42%) while insecticides (10%), fungicides (6%) and other insecticides (43%) make up the remainder. In 1999, roughly 125 million pounds of organophosphorous (OP) insecticides were for agricultural and residential pest control [2]. Although some agricultural uses of OP insecticides were restricted in 2001, agricultural use is still widespread. Residential uses of chlorpyrifos and diazinon, two common OP insecticides, were eliminated in 2001 and 2003, respectively [3]. Carbamate insecticides are also widely used in both residential and agricultural applications. Reportedly, synthetic pyrethroids have largely replaced residential uses of OP insecticides and are now the dominant class of insecticides used in homes and gardens [4].
While use of pyrethroid insecticides has been documented since the 1970s, preliminary evidence suggests that usage has been increasing and that pyrethroid insecticides are replacing the organophosphorous insecticides for residential control. This conclusion comes in part from; 1) recommendations by the U.S. EPA of alternatives to chlorpyrifos and diazinon for home use [5]; 2) a recent survey by the Attorney General of New York State showing that pyrethroid insecticides were the major class of insecticides used by residents of public housing [6], and 3) point of sales tracking data of residential pesticide sales [7]. By the mid-1990s, pyrethroid insecticides represented 23% of the U.S. dollar value of the world insecticide market [8–10]. However, use of pyrethroid insecticides in the United States has increased substantially since 1998 as a result of the decreased use of organophosphorous pesticides [11]. In 2002, nonagricultural use of pyrethroid insecticides in California (231,000 kg), for example, was five times greater than in the early 1990s, with structural pest control as the principal application [12]. Based on the Toxic Exposure Surveillance System (TESS) statistics for the years 2001–2003 in the United States, the number of human exposures to organophosphorous insecticides decreased, while exposures to pyrethroid insecticides increased [13].
The current extensive use of pesticides in agricultural and residential sectors has resulted in widespread exposure to the U.S. population. Insecticide contamination in residential environments, including air, dust, and surfaces, has been documented in a variety of urban and rural environments [14–16]. Detectable levels of contemporary insecticides were found in approximately 47% of the fruit and vegetable samples tested as part of market-basket surveys by the USDA in 2002 [17]. Pesticide contamination of surface and groundwater has been well-documented [18, 19] and residential exposure is widespread. 3-Phenoxybenzoic acid (3PBA), a pyrethroid metabolite, was detected in 75% of urine samples analyzed for pesticides in the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002 [20]. Although some organophosphorus insecticide metabolites were detected with similar frequency [21, 22], recent evidence shows that these metabolite levels are declining over time (unpublished data). Recent evidence suggests increased use and exposure to pyrethroid insecticides as they replace organophosphorus insecticides for residential pest control [23].
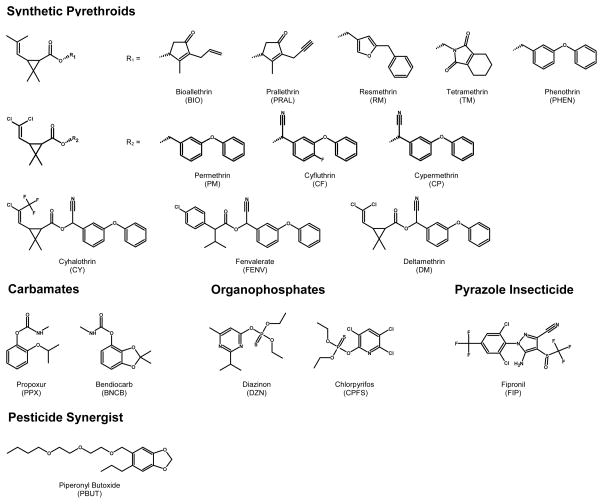
To ascertain internal exposure to most pesticides measurement of the parent pesticide in human blood serum or plasma is preferred [24, 25]. Because levels of pesticides in serum or plasma are typically several orders of magnitude lower than urinary metabolite levels, sensitive and selective methods must be employed [24–26]. In addition, their short biological half-lives make them increasingly difficult to measure. We have developed a sensitive and selective method for measuring a battery of contemporary use insecticides in human plasma (Figure 1). As one application of the method is to determine if pyrethroid pesticides are replacing the organophosphate insecticides following 2001–2002 U.S. EPA regulations, the method focuses primarily on measuring levels of 12 pyrethroid insecticides. It also includes the piperonyl butoxide, (a pyrethroid synergist), two most commonly applied organophosphorous insecticides (i.e., chlorpyrifos and diazinon), two carbamate insecticides (i.e., propoxur and bendiocarb), and fipronil (a phenylpyrazole compounds). Compounds were measured in human plasma using solid phase extraction coupled with gas chromatography-high resolution mass spectrometry.
Figure 1.
Chemical structures of the target insecticide analytes.
2. Experimental
2.1. Chemicals
All organic solvents used were analytical grade with purities greater than 98%. Methanol (MeOH), acetonitrile, toluene, and dichloromethane were purchased from Tedia Company Inc. (Fairfield, OH, USA). Organically and biologically purified deionized water (dI-H2O) was generated in-house with a model D11901 NANOpure Diamondback Analytical ultrapure water purifications system (Barnstead Thermolyne Corporation, Dubuque, IA, USA).
The unlabeled (native) pesticides standards propoxur (PPX; 99%), bendiocarb (BNCB; 99%), diazinon (DZN; 98.7%), chlorpyrifos (CPFS; 99.5%), fipronil (FIP; 98%), bioallethrin (BIO; 97%), prallethrin (PRAL; 98%), piperonyl butoxide (PBUT; 98%), resmethrin (RM; 98%), tetramethrin (TM; 98%), phenothrin (PHEN; 98.3%), cyhalothrin (CY; 98%), cis-permethrin (c-PM; 99%), trans-permethrin (t-PM; 96%), cyfluthrin (CF; 98%; mix of isomers), cypermethrin (CP; 98%; mix of isomers), fenvalerate (FENV; 98%), and deltamethrin (DM; 99%) were all purchased from Chem Service (West Chester, PA, USA). The isotopically labeled internal standards propoxur (isopropyl-D7; 98%), bendiocarb (13C3; 98%), diazinon (diethyl-D10; 98%), chlorpyrifos (diethyl-D10; 99%), cis-permethrin (phenoxy-13C6; 99%), trans-permethrin (phenoxy-13C6; 99%), cyfluthrin (phenoxy-13C6; 99%; mix of isomers), and cypermethrin (phenoxy-13C6; 98%; mix of isomers)were synthesized by Cambridge Isotope Laboratories (Andover, MA, USA) with the exception of isotopically labeled deltamethrin (methyl-D3, 13C4; 99+%) which was synthesized by Los Alamos National Laboratories (Los Alamos, NM, USA).
Gases used by laboratory equipment and instrumentation had a minimum purity of 99.99%. Research grade helium and nitrogen were purchased from Airgas Inc. (Hapeville, GA, USA). Anhydrous ammonia was purchased from Scott Specialty Gases (Plumsteadville, PA, USA).
2.2. Standard preparation
2.2.1. Isotopically labeled internal standards
Individual labeled internal standard (ISTD) stock solutions for PPX, BNCB, DZN, CP, and DM were prepared in acetonitrile to give concentrations ranging from 100 mg/L to 200 mg/L. CPFS, c-PM, t-PM, and CF were purchased as nonane solutions at concentrations of 100 mg/L, 50 mg/L, 50 mg/L, and 100 mg/L, respectively. Appropriate volumes of each individual stock solution were combined in a 10-mL volumetric flask and diluted with dichloromethane to produce a multi-analyte internal standard stock solution of 2 μg/mL for each isotopically labeled analyte. Dichloromethane was used as the diluting solvent since acetonitrile and nonane (immiscible solvents) were both miscible in this medium. This 2 μg/mL ISTD stock solution was used to prepare a 50 ng/mL ISTD spiking solution by aliquoting 5 mL of the stock solution into a 200 mL volumetric flask and diluting to the mark with acetonitrile. This solution was used as an ISTD spiked in all unknown samples, quality control (QC) materials, and calibration standards. All concentrations were corrected for chemical purity as well as isomeric ratios (if applicable). The individual and multi-analyte stock solutions and the spiking solution were stored at −20°C until used. They remained stable under these conditions throughout the conduct of the study.
2.2.2. Native standards
Individual stock solutions of each of the unlabeled (native) analytes were prepared in acetonitrile to yield concentrations of ~200 μg/mL. A 5μg/mL multi-analyte stock solution was prepared by aliquoting the appropriate volumes of each individual stock solution to a 25-mL volumetric flask and diluting to the mark with acetonitrile. The multi-analyte stock solution was used to prepare a serial dilution of 10 different concentrations (0.4, 0.8, 1.6, 4.0, 8.0, 16.0, 40.0, 80.0, 160.0, and 320 ng/mL) in acetonitrile to be used as calibration standard spiking solutions. Three quality control (QC) spiking solutions (1.25, 12.5, and 50 ng/mL) were also prepared using the 320 ng/mL calibration standard spiking solution. All concentrations were corrected for chemical purity as well as isomeric ratios, if applicable. Individual and multi-analyte stock solutions, calibration standard spiking solutions, and QC spiking solutions were stored at −20°C until used. Calibration standards and QC samples were prepared daily by spiking “blank” plasma with 40 μL of the appropriate spiking solution. The calibration standards and spiked quality control samples were prepared according to the sample preparation procedure described below.
2.2.3. QC materials
Pooled plasma containing sodium heparin as the anticoagulant, purchased from Interstate Blood Bank, Inc. (Memphis, TN, USA), was used for method development and QC materials. No further filtration or preparation of the plasma pools was performed prior to their use. The pooled plasma was aliquoted into vials, capped, and stored at −20°C. QC materials were prepared daily at three different concentrations (0.025, 0.250, and 1.0 ng/mL) by spiking plasma with the appropriate quality control spiking solution. All three QC concentration levels were characterized to determine the mean concentrations and the 95th (1.96σ) and 99th (2.96σ) control limits by consecutive analysis of 36 samples of each QC level. QC data within each analytical run were compared to the control limits to evaluate the validity of analyses using the Westgard rules [27].
2.3. Sample preparation
All plasma samples (blanks, QC samples, and unknowns) and spiking standard solutions were brought to room temperature and vortex mixed to ensure homogeneity of the sample. A 2-mL aliquot of plasma sample was pipetted into a 15 mL screw-cap test tube and spiked with 40μL of the ISTD spiking solution using an automatic Gilson 215 liquid handler (Middleton, WI, USA), resulting in a concentration of approximately 1 ng/mL for each analyte. Varian ABS ELUT-Nexus 60mg 3mL SPE cartridges (Palo Alto, CA, USA) were conditioned with 2 mL MeOH followed by equilibration with 2 mL dI-H2O. Plasma samples were loaded onto their respective cartridges and allowed to pass through without vacuum. The cartridges were washed with 4 mL (2 × 2mL) dI-H2O followed by 4 mL (2 × 2mL) 40% MeOH in water. Cartridges were dried by applying full vacuum for 5 min before elution with 2mL (2 × 1mL) of toluene. The extracts were collected in 15-mL conical centrifuge tubes and placed in a Zymark TurboVap LV Evaporator (Milford, MA, USA) for 30 min at 40°C and 15 psi of nitrogen. A 1-mL aliquot of acetonitrile was then added to the sample to remove any residual traces of water via azeotropic evaporation, vortex mixed, and blown down to dryness at 40°C and 15 psi for 30 min. Samples were reconstituted in 100 μL toluene, vortex mixed, and transferred to GC autosampler vials. Sample extracts were finally concentrated with heated nitrogen gas (40°C) to a final volume of 10 μL using a Glas-Col 96-well evaporator system (Terre Haute, IN, USA), resulting in a 200-fold concentration of the original 2-mL plasma sample. Samples were then capped and stored at −70°C until analysis on the gas chromatograph-high resolution mass spectrometer (GC-HRMS).
2.4 Instrumental analysis
2.4.1. GC conditions
One microliter of the concentrated extract was injected into an Agilent Technologies Hewlett-Packard 6890N GC (Atlanta, GA, USA) by splitless injection using a CTC A200S autosampler (Carrboro, NC, USA) with an injection purge time of 1 min. Chromatographic separation was achieved on a 30-m J&W Scientific (Folsom, CA, USA) DB-5MS ([5%-phenyl]-methylpolysiloxane, 0.25μm film thickness, 0.25mm i.d.) capillary column. Helium was used as the carrier gas at a constant flow of 1 mL/min. The injector and transfer line temperatures were both set isothermally at 300°C. The initial column temperature, 90°C, was held for 1 min, increased to 180°C at 30°C/min, held for 1 min, increased to 200°C at 5°C/min, held for 5 min, and finally increased to 300°C at 5°C/min and held for 2 min.
2.4.2. High resolution mass spectrometer conditions
A ThermoFinnigan MAT 900 XL high resolution mass spectrometer (Bremen, Germany) was operated in multiple ion detection (MID) mode for mass analysis of positive ions generated using electron ionization (EI+). A full manual or auto-tune of the MS was performed prior to sample analysis to obtain optimum sensitivity. Perfluorokerosene (PFK) was used as the calibration gas for lock and calibration ions. MS parameters were as follows: electron energy 45 eV, ion source temperature 250°C, initial accelerating voltage 5000 V, the electron multiplier voltage varied depending on multiplier lifetime, resolution 10,000 as defined at 10% valley, and filament 0.70 mA.
Quantification and confirmation ions were monitored for each analyte and its respective isotopically labeled internal standard. Masses for each ion monitored, ion type (i.e. fragment or molecular ion), and time segment for analysis are shown in Table 1.
Table 1.
Mass spectrometric multiple ion detection specifications of the described analytical method.
| Analyte | Quantification Ion | Ion Type | Ion Composition | Conformation Ion | Ion Type | Ion Composition | Time Segment |
|---|---|---|---|---|---|---|---|
| Propoxur | 152.0832 | F | C9H12O2 | 153.0865 | F+1 | C813C1H12O2 | 1 |
| Propoxur-isopropyl D7 | 159.1271 | F (D7) | C9D7H5O2 | 160.1305 | F+1(D7) | C813C1D7H5O2 | 1 |
| Bendiocarb | 151.0390 | FI | C8H7O3 | 166.0624 | FII | C9H10O3 | 2 |
| Bendiocarb-13C3 | 153.0457 | FI (13C2) | 13C2C6H7O3 | 169.0725 | FII(13C3) | 13C3C6H10O3 | 2 |
| Diazinon | 304.1005 | M | C12H21N2O3PS | 276.0692 | F | C10H17N2O3PS | 3 |
| Diazinon-diethyl D10 | 314.1633 | M (D10) | C12D10H11N2O3PS | 381.1006 | F(D5) | C10D5H12N2O3PS | 3 |
| Chlorpyrifos | 313.9569 | F | C9H1135Cl2NO3PS | 315.9539 | F+2 | C9H1135Cl37ClNO3PS | 4 |
| Chlorpyrifos-diethyl ester D10 | 324.0196 | F (D10) | C9D10H35Cl2NO3PS | 326.0167 | F+2(D10) | C9D10H35Cl37ClNO3PS | 4 |
| Fipronil | 366.9429 | F | C11H435Cl2F3N4OS | 368.9400 | F+2 | C11H435Cl37ClF3N4OS | 5 |
| Bioallethrin | 136.0883 | FI | C9H12O | 123.1168 | FII | C9H15 | 6 |
| Prallethrin | 123.1168 | F | C9H15 | 124.1202 | F+1 | C813C1H15 | 6 |
| Piperonyl Butoxide | 176.0832 | F | C11H12O2 | 177.0865 | F+1 | C1013C1H12O2 | 7 |
| Resmethrin | 171.0804 | FI | C12H11O | 123.1168 | FII | C9H15 | 7 |
| Tetramethrin | 164.0706 | FI | C9H10NO2 | 123.1168 | FII | C9H15 | 7 |
| Phenothrin | 183.0804 | FI | C13H11O | 123.1168 | FII | C9H15 | 8 |
| Cyhalothrin | 197.0339 | FI | C8H935ClF3 | 181.0648 | FII | C13H9O | 9 |
| cis-Permethrin | 183.0804 | F | C13H11O | 184.0838 | F+1 | C1213C1H11O | 10 |
| cis-Permethrin-phenoxy 13C6 | 189.1006 | F (13C6) | 13C6C7H11O | 190.1040 | F+1(13C6) | 13C7C6H11O | 10 |
| trans-Permethrin | 183.0804 | F | C13H11O | 184.0838 | F+1 | C1213C1H11O | 10 |
| trans-Permethrin-phenoxy 13C6 | 189.1006 | F (13C6) | 13C6C7H11O | 190.1040 | F+1(13C6) | 13C7C6H11O | 10 |
| Cyfluthrin | 206.0600 | FI | C14H8NO | 226.0663 | FII | C14H9FNO | 11 |
| Cyfluthrin-phenoxy 13C6 | 212.0802 | FI (13C6) | 13C6C8H8NO | 232.0864 | FII (13C6) | 13C6C8H9FNO | 11 |
| Cypermethrin | 181.0648 | FI | C13H9O | 208.0757 | FII | C14H10NO | 11 |
| Cypermethrin-phenoxy 13C6 | 187.0849 | FI (13C6) | 13C6C7H9O | 214.0958 | FII (13C6) | 13C6C8H10NO | 11 |
| Fenvalerate | 125.0153 | FI | C7H635Cl | 167.0622 | FII | C10H1235Cl | 12 |
| Deltamethrin | 252.9045 | F+2 | C7H979Br81Br | 250.9066 | F | C7H979Br2 | 13 |
| Deltamethrin-methyl D3, 13C4 | 259.9368 | F+2 (13C4D3) | 13C4C3D3H679Br81Br | 257.9388 | F (13C4D3) | 13C4C3D3H679Br2 | 13 |
M=Molecular Ion; F=Fragment Ion; F+1(+2)=Fragment Ion Isotope; FI /FII=2 Structurally Different Fragment Ions
2.5. Quantification
Each analytical run consisted of a blank plasma sample, a matrix-based calibration curve, three QC material samples (one at each concentration level), and 16 unknown plasma samples prepared using the described extraction method. The matrix-based calibration plot was constructed with 10 different analyte concentrations, ranging between 0.008 ng/mL and 6.4 ng/mL, plotted against the area of the native pesticide quantification ion divided by the area of the internal standard quantification ion. For BIO, PRAL, PBUT, RM, TM, PHEN, and CY, labeled c-PM was used as the ISTD to determine these area ratios. Labeled CPFS standard was used for FIP and labeled DM standard was used for FENV. Calibration standard concentrations encompassed the entire linear range of the analysis. The lowest standard concentrations were at or below the limits of detection (LOD) to ensure linearity and accuracy at the low concentration end. A linear regression analysis of the calibration plot provided a slope and an intercept from which unknown sample concentrations could be calculated.
2.6. Method validation
2.6.1. Extraction recoveries
Homogenized, pooled plasma samples were used to evaluate recoveries at three concentrations (0.025 ng/mL, 0.250 ng/mL, and 2.5 ng/mL) spanning the calibration range of the method. RM, PHEN, CF, and CP recoveries were only determined at the latter two concentrations because of their higher LODs. The recoveries were measured by spiking six blank plasma samples (2mL) with the appropriate native standard spiking solution and preparing according to the described method. Six additional blank plasma samples (unspiked) were prepared concurrently. After the SPE step, the six unspiked plasma samples were then spiked with the appropriate native standard spiking solution to serve as control samples representative of 100% recovery. The extracts of all samples were then spiked with ISTD to correct for instrumental variation during analysis and resulted in a more accurate recovery calculation. The recoveries were determined as the area ratios (native: labeled) of spiked samples divided by the area ratios (native: labeled) of the control samples multiplied by 100.
2.6.2. Limits of detection
The LOD for each analyte within the method was calculated as 3s0, where s0 is the estimated standard deviation of measured concentration values as the concentration approaches zero. With this technique, s0 is an extrapolated value, and equivalent to the y- intercept of a regression line from the plot of the standard deviations of the measured concentrations versus their nominal concentration values. The LODs were verified by analyzing samples that were spiked at the LOD level.
2.6.3. Relative Recovery
Relative recovery is defined here as the degree of closeness of the determined mean values of samples to the nominal spiked values of those samples. The relative recovery for each analyte within the method was determined at two concentrations per analyte by comparing the mean concentration values of QC samples (n=36 for each concentration) with the nominal spiked concentrations (0.025 ng/mL and 0.250 ng/mL for DZN, CPFS, CY, c-PM, and t-PM and 0.250 ng/mL and 1 ng/mL for PPX, BNCB, FIP, PBUT, RM, TM, PHEN, CY, CP, FENV, and DM).
2.6.4. Precision
The precision of the method was determined by calculating the relative standard deviation (RSD) of repeat measurements (n=36 for each concentration) of the quality control materials at two concentrations (0.025 ng/mL and 0.250 ng/mL for DZN, CPFS, CY, c-PM, and t-PM and 0.250 ng/mL and 1 ng/mL for PPX, BNCB, FIP, PBUT, RM, TM, PHEN, CY, CP, FENV, and DM). Six samples at each concentration were analyzed on six different days, and the results were used to determine within-day, between-day, and total RSDs for each analyte.
2.6.5. Method Application
This method was used to measure pesticide concentrations in 273 maternal and umbilical cord plasma samples collected at delivery or immediately postpartum from a cohort of African American and Dominican women enrolled during 1999–2004 from the upper Manhattan and South Bronx area of New York City as a part of the Columbia Center for Children’s Environmental Health (CCCEH) [28, 29]. Plasma samples were collected into heparinized vacutainers. Within 12 hours, blood was transferred to centrifuge tubes and spun for 18 minutes at 1500 rpm. Plasma samples were stored at −70°C prior to shipment to the CDC for insecticide analysis.
3. Results
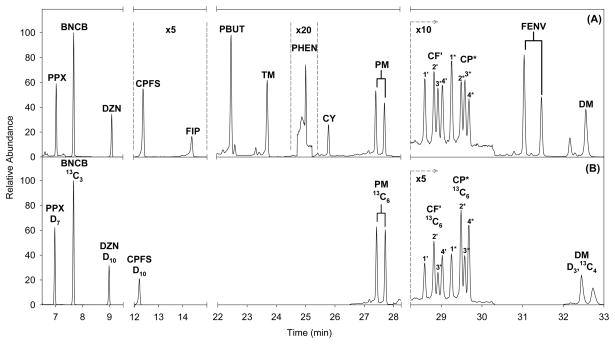
A typical mass chromatogram is shown in Figure 2. The total analysis time was 36 min with all analytes eluting between 7 and 33 min. Because PM, CF, CP and FENV were comprised of multiple isomers, either 2 or 4 peaks were monitored for these analytes.
Figure 2.
Mass chromatogram of an extracted plasma sample fortified with approximately 0.8 ng/mL of all insecticides. Only quantitation ions of unlabeled (A) and labeled (ISTD) (B) analytes are shown for clarity.
Method validation results including LOD, linearity, extraction recoveries, precision, and accuracy are summarized in Tables 2 and 3. The LODs of the method ranged from 0.01 ng/mL to 0.158 ng/mL. Extraction recoveries of the SPE method ranged from 19% to 98%. Eight of the 16 analytes had relative recoveries within 15% of the expected concentration. Total method RSDs ranged from 3.9 % to 59% although most were below 15%. Similar RSDs were observed both within and between runs.
Table 2.
Limits of detection and extraction recoveries of each target analyte in spiked heparinized plasma. The numbers associated with cyfluthrin, cypermethrin, and fenvalerate denote the elution order of the isomeric structures for these analytes.
| Analyte | LOD (pg/mL) | Linear Range (ng/mL) | r2 | Extraction recoveries (%) | ||
|---|---|---|---|---|---|---|
| 25 pg/mL (SD) | 250 pg/mL (SD) | 1000 pg/mL (SD) | ||||
| n=7 | n=6 | n=6 | n=6 | |||
| Propoxur | 29 | 29–6400 | 0.9997 | 78 (4.7) | 85 (7.0) | 75 (2.7) |
| Bendiocarb | 10 | 10–6400 | 0.9987 | 95 (5.7) | 81 (12) | 81 (3.2) |
| Diazinon | 16 | 16–6400 | 0.9996 | 95 (4.3) | 94 (3.2) | 89 (3.4) |
| Chlorpyrifos | 21 | 21–6400 | 0.9974 | 80 (3.8) | 87 (4.4) | 73 (1.2) |
| Fipronil | 44 | 44–6400 | 0.9924 | 47 (8.8) | 73 (19) | 62 (8.1) |
| PBUT | 70 | 70–6400 | 0.9999 | 97 (6.0) | 93 (2.8) | 88 (5.2) |
| Resmethrin | 34 | 34–6400 | 0.9888 | NE | 53 (6.8) | 38 (3.4) |
| Tetramethrin | 27 | 27–6400 | 0.9952 | 98 (8.1) | 84 (4.6) | 84 (4.3) |
| Phenothrin | 93 | 93–6400 | 0.9964 | NE | 32 (7.2) | 24 (3.3) |
| Cyhalothrin | 17 | 17–6400 | 0.9846 | 30 (4.8) | 30 (9.0) | 22 (2.2) |
| cis-Permethrin | 31 | 31–6400 | 0.9924 | 35 (4.6) | 33 (4.0) | 22 (2.0) |
| trans-Permethrin | 20 | 20–6400 | 0.9934 | 37 (6.1) | 41 (4.9) | 26 (2.3) |
| Cyfluthrin-1 | 66 | 66–6400 | 0.9985 | NE | 42 (8.2) | 26 (3.3) |
| Cyfluthrin-2 | 23 | 23–6400 | 0.9932 | NE | 54 (23) | 26 (1.6) |
| Cyfluthrin-3 | 76 | 76–6400 | 0.9853 | NE | 38 (10) | 24 (3.4) |
| Cyfluthrin-4 | 92 | 92–6400 | 0.9921 | NE | 46 (8.6) | 27 (2.4) |
| Cypermethrin-1 | 158 | 158–6400 | 0.9984 | NE | 39 (3.9) | 23 (2.1) |
| Cypermethrin-2 | 56 | 56–6400 | 0.9986 | NE | 40 (7.3) | 26 (2.8) |
| Cypermethrin-3 | 118 | 118–6400 | 0.9987 | NE | 43 (14) | 25 (4.6) |
| Cypermethrin-4 | 96 | 96–6400 | 0.9952 | NE | 41 (7.6) | 26 (3.1) |
| Fenvalerate-1 | 31 | 31–6400 | 0.9964 | 23 (4.9) | 37 (11) | 19 (3.2) |
| Fenvalerate-2 | 26 | 26–6400 | 0.9999 | 30 (7.7) | 35 (13) | 19 (3.4) |
| Deltamethrin | 31 | 31–6400 | 0.9998 | 25 (6.1) | 37 (3.5) | 20 (3.5) |
LOD Limit of detection calculated as 3s0 where s0 is the estimated standard deviation at zero concentration; SD standard deviation; NE not evaluated.
NOTE: LODs were calculated independently for each ion isomer.
Table 3.
Accuracy and precision of the method for each analyte spiked into heparinized plasma.
| Relative Recovery (%) |
Relative Standard Deviation (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Within-day |
Between-day |
Total |
|||||
| 25 pg/mL | 250pg/ mL | 25 pg/mL | 250pg/ mL | 25pg/m L | 250pg/ mL | 25pg/m L | 250pg/ mL | |
| n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | |
| Diazinon | 139 | 100 | 5.2 | 3.2 | 9.9 | 3.6 | 10 | 3.9 |
| Chlorpyrifos | 144 | 101 | 5.2 | 3.9 | 35 | 6.7 | 35 | 6.9 |
| Cyhalothrin | 132 | 83 | 50 | 13 | 42 | 14 | 47 | 15 |
| cis-Permethrin | 87 | 98 | 20 | 6.6 | 36 | 4.0 | 42 | 4.9 |
| trans-Permethrin | 104 | 100 | 21 | 9.2 | 59 | 4.9 | 59 | 6.2 |
| 250pg/ mL | 1000pg/ mL | 250pg/ mL | 1000pg/ mL | 250pg/ mL | 1000pg/ mL | 250pg/ mL | 1000pg/ mL | |
| n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | n=36 | |
| Propoxur | 102 | 108 | 11 | 9.2 | 4.2 | 2.4 | 6.2 | 9.5 |
| Bendiocarb | 126 | 118 | 11 | 9 | 6.6 | 1.2 | 13 | 9.1 |
| Fipronil | 107 | 113 | 11 | 11 | 11 | 12 | 15 | 16 |
| PBUT | 117 | 127 | 19 | 3.2 | 17 | 17 | 19 | 18 |
| Resmethrin | 100 | 118 | 11 | 14 | 11 | 15 | 12 | 21 |
| Tetramethri n | 112 | 122 | 9.1 | 11 | 8.9 | 5.9 | 9.6 | 13 |
| Phenothrin | 156 | 150 | 21 | 22 | 21 | 12 | 29 | 25 |
| Cyfluthrin-1 | 108 | 116 | 11 | 9.6 | 9.5 | 6.0 | 14 | 11 |
| Cyfluthrin-2 | 107 | 89 | 9.8 | 12 | 9.6 | 3.1 | 14 | 12 |
| Cyfluthrin-3 | 106 | 92 | 12 | 14 | 16 | 5.4 | 20 | 15 |
| Cyfluthrin-4 | 94 | 103 | 12 | 11 | 5.0 | 3.2 | 13 | 12 |
| Cypermethri n-1 | 97 | 106 | 15 | 11 | 2.8 | 11 | 15 | 16 |
| Cypermethri n-2 | 94 | 99 | 14 | 9.3 | 4.3 | 8.6 | 15 | 13 |
| Cypermethri n-3 | 112 | 78 | 17 | 10 | 17 | 8.9 | 24 | 13 |
| Cypermethri n-4 | 105 | 75 | 14 | 8.8 | 17 | 1.2 | 22 | 8.9 |
| Fenvalerate-1 | 111 | 120 | 11 | 13 | 15 | 18 | 19 | 22 |
| Fenvalerate-2 | 128 | 74 | 14 | 12 | 18 | 25 | 22 | 28 |
| Deltamethri n | 88 | 92 | 10 | 12 | 24 | 22 | 26 | 26 |
Accuracy average measured value compared to spiked concentration; RSD relative standard deviation; (%dev) percent of average deviation from spiked concentration.
None of the pesticides were detected with any significant frequency among the CCCEH maternal and cord samples. Cis- and trans- permethrin were detected in 4.5% and 6.5% of maternal plasma samples and in 9.2% and 12.6% of cord plasma samples, although these frequencies of detection include those detected at “trace” concentrations that were not quantifiable. The % > LOD and percentiles for cis- and trans-permethrin in maternal and cord plasma are presented in Table 4.
Table 4.
Cis - and trans-permethrin in maternal (n=154) and cord (n=119) plasma collected at or immediately postpartum.
| n >LOD (%)* | n>LOD (%)** | 95th Percentile*** | |
|---|---|---|---|
| cis-Permethrin LOD = 0.031 ng/mL | |||
| Maternal | 10 (6.5) | 1 (0.6%) | <LOD |
| Cord | 11 (9.2) | 5 (4.2% | <LOD |
| trans-Permethrin LOD = 0.02 ng/mL | |||
| Maternal | 7 (4.5) | Same | <LOD |
| Cord | 15 (12.6) | Same | 34.3 |
Limit of detection; note that methodology uses 2 mL of plasma. This calculation includes detectable but non-quantifiable concentrations below our LOD.
Only quantifiable concentrations.
uses quantifiable concentrations with values <LOD imputed as the LOD/sq rt 2.
4. Discussion
Our objective was to develop a sensitive and accurate GC-HRMS method capable of identifying and quantifying a battery contemporary insecticides in human serum. Because of recent evidence suggesting increased use and exposure to pyrethroid insecticides, the method was optimized to measure pyrethroid insecticides while including representative organophosphorus and carbamate insecticides. Because blood sampling is invasive and the amount of sample collected is typically small, it was appealing to develop not only a multi-analyte method, but one that encompassed more than one class of pesticide in order to achieve a greater exposure assessment with the limited sample volume available for analysis. However, optimizing the performance of each individual target analyte in a multi-class method is challenging when complex biological matrices are used and often the performance of some analytes must be somewhat sacrificed for the overall method performance. The diverse chemical and physical properties of the target analytes made method development and optimization challenging. Nonetheless, the method we report was the best compromise to achieve the most efficient overall extraction, cleanup and analysis of various classes of pesticides within the same 2-mL plasma sample.
Ions were selected based upon the relative abundance observed in EI spectra and the signal-to-noise ratio in the specified ion channel. For analytes that had a corresponding labeled ISTD, we also considered whether the fragment ion retained the label. Confirmation ions were selected based on relative abundance or if a naturally occurring isotope peak was present in the fragment.
Chromatographic separation was optimized to achieve separation of all analytes and to allow for analysis of individual analytes specified time segments so that optimum sensitivity could be obtained. PBUT and RM and CF and CP were the only analytes which were combined into shared time segments. For most analytes, we found no chromatographic interferences. However, small matrix peaks were present in the case of BNCB and FENV-2.
To obtain chemical behavior patterns of each analyte with a particular SPE sorbent, preliminary recovery experiments were performed by substituting the blood plasma matrix with dI-H2O. By doing so, we were able to collect information on the chemical interactions of each analyte on each SPE sorbent tested and eliminate any potential interferences or complications matrix components may present. Such information provided guidance into selecting an SPE sorbent for sample cleanup as well as the optimization of wash and elution steps for a given sorbent. Several reversed-phase sorbents (e.g. C18, C8, C2, phenyl, and CN) were evaluated for these behavioral patterns; however, we ultimately selected the Nexus cartridge for SPE because of its overall recovery efficiency and because it allowed for a greater organic wash of the sorbent which effectively minimized matrix co-extractants.
In an attempt to further minimize the presence of the blood plasma matrix in the final extract and to test the method robustness, plasma samples were prepared using the described procedure but with pH modifications of the dI-H2O and organic washes. Changes in pH might affect reversed-phase SPE retention of biological matrix components which possess various ionizable sites within their biochemical structures. Ionization of these components would thus disrupt the weak interactions between the SPE sorbent and that of the biological structure and would favor partitioning into the polar solvent and be washed away. We observed that the modifications to pH did not affect the analyte-sorbent interactions since all target analytes were non-ionizable or contained no significantly ionizable atoms at the pHs tested. No improvements or deleterious effects were observed in the analysis if at pHs of 3, 5, 7 and 9.
Chromatographic resolution of all 15 target analytes was achieved within a reasonable amount of time considering the number of compounds and the high boiling points of FENV and DM. A quantification ion and confirmation ion for each analyte and its respective ISTD was included in the measurement for increased specificity. The ions measured were selected based upon the abundance of the ion and/or the atomic composition of the fragment. In some instances, the most abundant fragment ion could not be used since the fragment ion of the ISTD was an unlabeled fragment. Use of an unlabeled fragment would result in a complete loss of specificity for that particular compound.
Most analytes were chromatographically separated and placed into individual time segments, which avoided any problems of sensitivity losses from a reduction of the accelerating voltage when the mass differences were too large. In instances when more than one analyte had to be placed in the same segment, their masses were relatively close to one another also avoiding significant reductions in the accelerating voltage. Separation allowed for increased scan times for each analyte for maximum sensitivity.
GC-injector and transfer line temperatures were set high (both at 300°C) in order to 1) vaporize the pyrethroids with high boiling points, and 2) to remove as much matrix material from the GC injector liner as possible. Still, large amounts of lipids were injected and the chromatography was negatively affected resulting in relatively large shifts in retention times over several injections. Retention shifts posed a problem for the separation of FIP and BIO making it difficult to place them into separate time segments. Separation of these two compounds was essential to maintain sensitivity for FIP. If combined into one time segment, the low masses for BIO (m/z = 123.1168 and 136.0883) and the large masses for FIP (m/z = 366.9429 and 368.9400) would result in a large reduction in the MS accelerating voltage which would ultimately result in a loss of sensitivity for FIP. The permanent presence of lipids on-column also resulted in a high background through the ion channels of BIO and PRAL which significantly increased their detection limits. Because of the varied performance of BIO and PRAL because of the lipid background, we decided to eliminate them from the quantitative method, thus their validation parameters are not reported here.
The fragmentation patterns of the pyrethroid pesticides limited the selection of ions which could be used for their analysis. The type I pyrethroids, BIO, PRAL, RM, TM, and PHEN (PM the one exception), produce extensive fragmentation of the parent molecule which typically results in a base peak at m/z = 123.1168. While the few available mass fragments proved useful as quantitation ions, the base peak ion seemed to be the only option for use as a confirmation ion. As previously mentioned, this ion channel was problematic due to the high levels of lipids present in the sample extract. Background levels were orders of magnitude higher at this mass throughout the GC run time.
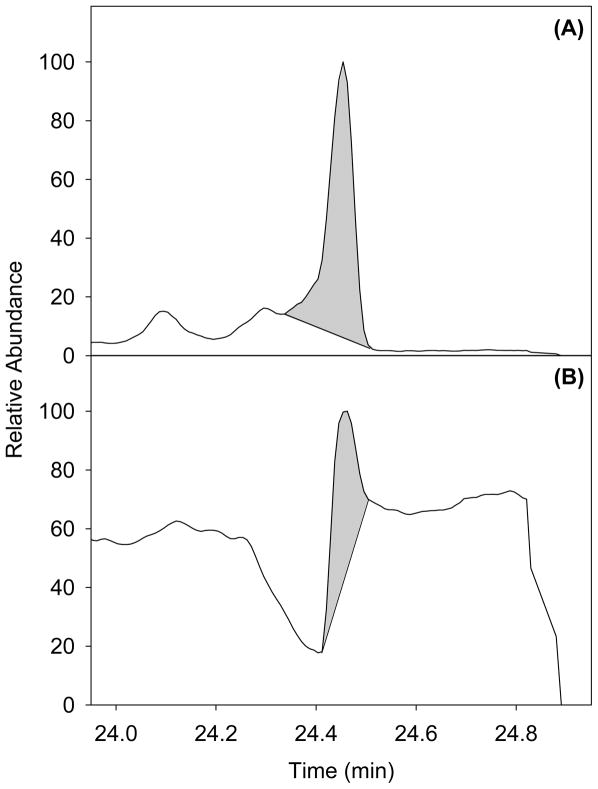
The limitations of selectable ions and the presence of high levels of matrix material injected greatly affected the analysis of PHEN. Figure 3 shows the problems presented with PHEN. A co-eluting matrix component interferes with PHEN at its quantification ion, and appears to suppress ionization of the confirmation ion. This effect caused the confirmation ion to be unusable, and quantification of PHEN difficult. This resulted in an increase in the limit of detection for PHEN.
Figure 3.
Mass chromatogram of phenothrin (PHEN) showing a co-eluting matrix component interfering at the quantification ion channel (A) (m/z =183.0804), and apparently suppressing ionization at the confirmation ion channel (m/z=123.1168) (B).
Many of the pyrethroid insecticides exhibited extraction recoveries that were lower than desired. This was attributed to the strong retention of these fairly non-polar compounds to the sorbent of the Nexus SPE cartridge. Recoveries were not significantly improved when using lower polarity solvents. Extraction recoveries with toluene resulted in similar recoveries as other non-polar solvents as well as increased recoveries for all other analytes. Recoveries could have also been increased with the use of another reverse-phase SPE cartridge. These low recoveries were acceptable considering the limits of detection achieved for these compounds were in the low pg/mL range. In addition, the extraction recoveries were corrected for during quantification by the use of isotopically labeled internal standards.
The LODs we report were determined statistically using the precision of repeat measurements at multiple concentrations. Therefore, the reported LODs are average estimates and do not necessarily reflect the lowest level measureable for a given analyte during a given run. In some instances, we could clearly discern a peak with a signal-to-noise ratio greater than 3 at concentrations well below the calculated LOD. However, the calculated LODs are conservative and are appropriate for average LODs over a given time period.
The RSDs and relative recoveries for each analyte were acceptable with some exceptions. The precision of the method was evaluated as within-day, between-day, and total RSDs. In general, our method presented RSD values (for all three evaluated) less than, or just above, 15%, especially for analytes possessing labeled analogues. Analytes without labeled analogues also yielded RSD values less than, or just above 15%. For analytes with higher RSDs (e.g., RM, PHEN, FENV, DM at 1000 pg/mL and CPFS, CY, c-PM, t-PM at 25 pg/mL), we found that a lack of ISTD (for FENV and RM or approaching lower concentrations near the LOD (for CPFS, CY, c-PM and t-PM), resulted in increased imprecision for those analytes. Acquisition of additional ISTDs may allow us to improve our precision for some of these analytes.
Few methods exist in the literature that have the capability of measuring the low levels of pyrethroid and OP insecticides in plasma [24, 30–31]. We previously published a method to measure a large grouping of pesticides in serum and plasma with similar LODs but only one pyrethroid insecticide was included. Methods with higher LODs allowing detection of OP pesticides following acute poisonings has also been reported [30]. This method is highly selective but lacks sufficient LODs to be useful for general population samples. Ramesh and Ravil [31] reported a method that measured a suite of OP pesticides in 5 mL of blood at sub pg/mL concentrations. Although their method possessed superior characteristics, our method is more selective, uses less blood, and includes OP insecticides and synergists that are also of interest.
Application of our method to archived plasma samples from a New York City cohort demonstrated that our method possessed enough sensitivity to detect cis- and trans-permethrin in a small percentage of the samples tested. Given a more acute exposure scenario, we should be able to measure low levels of these chemicals in plasma samples collected from those exposed. Notably, these samples had been stored in freezers at −80 C for up to 6 years. Because some pyrethroid insecticides have been shown to be unstable in plasma over long periods of time, we may have detected them in fewer samples because of storage biodegradation [32].
5. Conclusions
We have developed a method for the measurement of a variety of pesticides in human blood plasma. The method focused primarily on measuring synthetic pyrethroid insecticides and piperonyl butoxide, the pyrethroid synergist and included representative organophosphorus, and carbamate pesticides. The method employs a simple SPE extraction and cleanup followed by analysis using isotope-dilution GC-HRMS. Our method can be used for measuring exposure levels of specific pesticides at low levels, in some instances, in the general population.
Acknowledgments
We thank Samuel Baker for his assistance in the understanding and proper validation of the described method. The opinions expressed in this manuscript are solely the opinions of the authors and are not necessarily the opinions of the CDC.
References
- 1.Kiely T, Donaldson D, Grube A. U.S. EPA; Washington, DC: 2004. [Google Scholar]
- 2.Donaldson D, Kiely T, Grube A. 1998 and 1999 market estimates. Pesticides industry sales and usage report. U.S. Environmental Protection Agency; Washington, DC: 2002. [Google Scholar]
- 3.U.S.EPA, 2002.
- 4.ATSDR. Toxicological Profile for Pyrethrins and Pyrethroids. Agency for Toxic Substances and Disease Registry; Atlanta: 2003. [PubMed] [Google Scholar]
- 5.U.S.EPA, 2003.
- 6.E. Spitzer, Attorney General of New York State, (2002).
- 7.Bekarian N, Payne-Sturges D, Edmondson S, Chism B, Woodruff TJ. Environ Health. 2006;5:15. doi: 10.1186/1476-069X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. Environ Health Perspect. 2003;111:79. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Toxicology. 2002;171:3. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 10.Heudorf U, Angerer J, Drexler H. Int Arch Occup Environ Health. 2004;77:67. doi: 10.1007/s00420-003-0470-5. [DOI] [PubMed] [Google Scholar]
- 11.Anand SS, Bruckner JV, Haines WT, Muralidhara S, Fisher JW, Padilla S. Toxicol Appl Pharmacol. 2006;212:156. doi: 10.1016/j.taap.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Amweg EL, Weston DP, Ureda NM. Environ Toxicol Chem. 2005;24:966. doi: 10.1897/04-146r1.1. [DOI] [PubMed] [Google Scholar]
- 13.Sudakin DL. Clin Toxicol (Phila) 2006;44:31. doi: 10.1080/15563650500394647. [DOI] [PubMed] [Google Scholar]
- 14.Bradman MA, Harnly ME, Draper W, Seidel S, Teran S, Wakeham D, Neutra R. J Expo Anal Environ Epidemiol. 1997;7:217. [PubMed] [Google Scholar]
- 15.Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Environ Health Perspect. 1995;103:1126. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP. Environ Health Perspect. 2003;111:749. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.USDA, Agricultural Chemical Usage, 2001 Field Crops Summary, National Agricultural Statistics Service, 2002.
- 18.Battaglin WA, Furlong ET, Burkhardt MR, Peter CJ. Sci Total Environ. 2000;248:123. doi: 10.1016/s0048-9697(99)00536-7. [DOI] [PubMed] [Google Scholar]
- 19.Scribner EA, Battaglin WA, Goolsby DA, Thurman EM. Sci Total Environ. 2000;248:255. doi: 10.1016/s0048-9697(99)00547-1. [DOI] [PubMed] [Google Scholar]
- 20.Riederer AM, Bartell SM, Barr DB, Ryan PB. Environ Health Perspect. 2008;116:1015. doi: 10.1289/ehp.11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Environ Health Perspect. 2004;112:186. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano MA, Nguyen JV, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD, Jr, Schober SE, Needham LL. 2004 doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM. Environ Health Perspect. 2008;116:1681. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr D, Barr J, Maggio V, Whitehead R, Sadowski M, Whyatt R, Needham L. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:99. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- 25.Barr DB, Barr JR, Driskell WJ, Hill RH, Jr, Ashley DL, Needham LL, Head SL, Sampson EJ. Toxicol Ind Health. 1999;15:168. doi: 10.1191/074823399678846556. [DOI] [PubMed] [Google Scholar]
- 26.Barr D, Needham L. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:5. doi: 10.1016/s1570-0232(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 27.Westgard JO. Basic QC Practices: Training in Statistical Quality Control for Health Care Laboratories. Westgard QC, Inc; Madison, WI: 2002. [Google Scholar]
- 28.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Environ Health Perspect. 2004;112:1125. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP. Environ Health Perspect. 2003;111:749. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salm P, Taylor PJ, Roberts D, de Silva J. J Chrom B. 2009;877:568. doi: 10.1016/j.jchromb.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh A, Ravi PE. J Anal Toxicol. 2004;28:660. doi: 10.1093/jat/28.8.660. [DOI] [PubMed] [Google Scholar]
- 32.Leng G, Kuhn KH, Idel H. Sci Total Environ. 1997;199:173. doi: 10.1016/s0048-9697(97)05493-4. [DOI] [PubMed] [Google Scholar]