Abstract
The ingestion of excess dietary salt (defined as NaCl) is strongly correlated with cardiovascular disease, morbidity, mortality, and is regarded as a major contributing factor to the pathogenesis of hypertension. Although several mechanisms contribute to the adverse consequences of dietary salt intake, accumulating evidence suggest that dietary salt loading produces neurogenically-mediated increases in total peripheral resistance to raise arterial blood pressure (ABP). Evidence from clinical studies and experimental models clearly establish a hypertensive effect of dietary salt loading in a subset of individuals who are deemed “salt-sensitive”. However, we will discuss and present evidence to develop a novel hypothesis to suggest that while chronic increases in dietary salt intake do not elevate mean ABP in “non-salt-sensitive” animals, dietary salt intake does enhance several sympathetic reflexes thereby predisposing these animals and/or individuals to the development of salt-sensitive hypertension. Additional evidence raises an intriguing hypothesis that these enhanced sympathetic reflexes are largely attributed to the ability of excess dietary salt intake to selectively enhance the excitability of sympathetic-regulatory neurons in the rostral ventrolateral medulla. Insight into the cellular mechanisms by which dietary salt intake alters the responsiveness of RVLM circuits will likely provide a foundation for developing new therapeutic approaches to treat salt-sensitive hypertension.
Keywords: dietary salt, blood pressure, sympathetic, rostral ventrolateral medulla, salt-sensitivity, hypertension
INTRODUCTION
The ingestion of excess dietary salt (defined as NaCl) is strongly correlated with cardiovascular disease, morbidity, and mortality, and is regarded as a major contributing factor to the pathogenesis of hypertension [1, 2]. Several mechanisms contribute to the hypertensive effect of dietary salt including increased water and sodium retention with resultant blood volume expansion, vascular abnormalities, and/or neurogenically-mediated increases in peripheral resistance [2]. This review will briefly discuss the “neurogenic” mechanisms by which increased salt intake alters cardiovascular regulation and develop the hypothesis that dietary salt alters the excitability of central sympathetic-regulatory networks to predispose an individual to the development of hypertension.
Salt-Sensitivity of Arterial Blood Pressure (ABP)
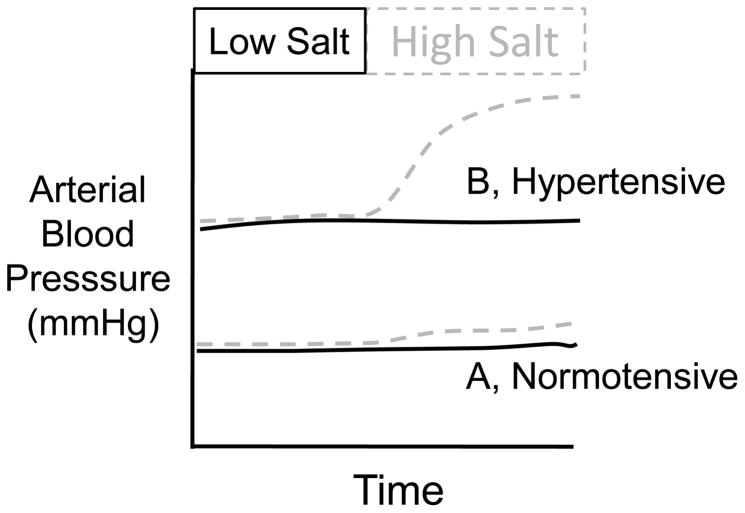
In humans, increases in dietary salt intake elevated ABP in a subset of individuals. Therefore, humans can be classified as “salt-sensitive” (SS) if ABP increases when fed a high salt diet versus “non salt-sensitive” (NSS) if ABP does not change (Figure 1). Although many studies have examined differences between SS and NSS individuals, there are no standard criteria applied uniformly to distinguish between SS and NSS individuals. That is, the duration of the salt load (hours to weeks) and magnitude of the blood pressure change (0–15 mmHg) has varied between studies. However, several consistent observations have been reported. First, approximately 30% of the normotensive population is SS – the majority are NSS [1, 2]. Second, the incidence of SS is significantly higher in the hypertensive population – the majority of hypertensive individuals are SS [1, 2]. Third, longitudinal studies indicate that normotensive SS individuals are more likely to develop hypertension than NSS counterparts [3]. Fourth, the incidence of SS is influenced by several factors including ethnic origin and age [2]. The distinction between SS and NSS likely represents a continuum ranging from the ability of dietary salt intake to produce a significant elevation in ABP to a negligible effect.
Figure 1.
Two common examples of the salt-sensitivity of ABP. (A) Dietary salt loading produces a small increase in ABP in approximately 30% of the normotensive population. (B) Elevated dietary salt intake causes a significant increase in ABP of hypertensive individuals – the incidence of salt-sensitivity is markedly higher in the hypertensive population.
The impact of changes in dietary salt intake on ABP has also been widely studied in experimental animals. Analogous to the human population, animals can show varying degrees of salt-sensitivity or resistance. This phenomenon has been extensively studied in rats. In general, standard laboratory rats would be considered NSS as even extremely large increases in dietary salt intake has minimal impact on ABP. Consequently, the majority of studies on cardiovascular regulation in rats (and other experimental animals) has used NSS animals. Several decades ago, Louis Dahl and colleagues bred substrains of Sprague-Dawley rat with high versus low sensitivity to salt-induced increases in ABP [4]. Through selective inbreeding over generations, substrains of rats were isolated that displayed substantial elevations in ABP when fed a high salt diet (Dahl Salt-Sensitive or DS rats) or were resistant to the impact of high salt diet on ABP (the Dahl Salt-Resistant or DR rats). This is probably the most widely used genetic model of salt-sensitive hypertension, although other forms of salt-sensitive hypertension also exist. On the other hand, there are several models of salt-sensitive hypertension using standard laboratory animals that rely on defined manipulations rather than a complex genetic background (ie, deoxycorticosterone plus dietary salt or chronic infusion of angiotensin II plus dietary salt [5–8]). Increased dietary salt intake, by itself, does not elevate ABP in these animals; however, the addition of these other manipulations (deoxycorticosterone or angiotensin II) consistently produces salt-sensitive hypertension. Altogether, the observation that these salt-sensitive models of hypertension in experimental animals require additional factors other than excess salt intake (e.g. specific genetic background, co-treatment with a mineralcorticoid or angiotensin II) to produce “salt-sensitivity” highlights the multifactorial complexity of salt-sensitive hypertension.
Neurogenic Mechanisms of Salt-Sensitive Hypertension
Salt-sensitive hypertension is likely a multi-system disorder that involves renal dysfunction (ie, water and salt retention), vascular abnormalities, and neurogenically-mediated increases in peripheral resistance. Support for a neurogenic component in salt sensitive hypertension arises from several lines of evidence in both humans and experimental models. First, salt-sensitive hypertension is associated with increases in peripheral resistance and activation of the sympathetic nervous system [7, 9–11]. Second, blockade of sympathetic outflow or transection of sympathetic nerves consistently lowers ABP in experimental models of salt-sensitive hypertension [12, 13]. Third, lesion of the anteroventral third ventricular region prevent or attenuate the development or severity of salt-sensitive hypertension [14–16]. Finally, interruption of neurotransmission in pivotal sympathetic-regulatory centers lowers and even normalizes sympathetic nerve activity and/or ABP in salt-sensitive models [17–19]. Altogether, these observations suggest that the central nervous system plays an important role in the development and maintenance of salt-sensitive hypertension.
Impact of Dietary Salt on Sympathetic Regulation
Experimental investigations in both humans and animals have suggested that chronic exposure to a high salt diet either reduces or does not affect sympathetic nerve activity [20–26]. However, the classification of SS versus NSS should be considered in these studies. A decrease in renal sympathetic nerve activity (at least in NSS subjects) may be consistent with the sodium-retaining actions of sympathetic input to the kidneys. On the other hand, a recent study by McBryde and colleagues using telemetry to chronically record sympathetic nerve activity in rabbits reported that 6 day salt loading did not alter ABP or renal sympathetic nerve activity [25]. These discrepancies likely reflect the various methodological approaches and factor that contribute to this relationship. First, sympathetic nerves innervating different targets are differentially regulated [7, 27, 28]. The profile of sympathetic nerve activity to various tissues during chronic salt loading is likely critical to the effects of cardiovascular regulation – clearly, the differential regulation of sympathetic nerve activity is a hallmark of other forms of hypertension and cardiovascular disease [27, 29, 30]. Second, it is not clear how information is coded within the pattern of sympathetic nerve discharge and how chronic salt loading will influence these dynamics. Third, studies must consider the background level of salt-sensitivity of the experimental subject or animal. The impact of dietary salt intake on sympathetic nerve activity may differ between SS and NSS individuals or animals. Clearly, further investigation is needed to clarify the effect of dietary salt on sympathetic outflow in context of these additional factors.
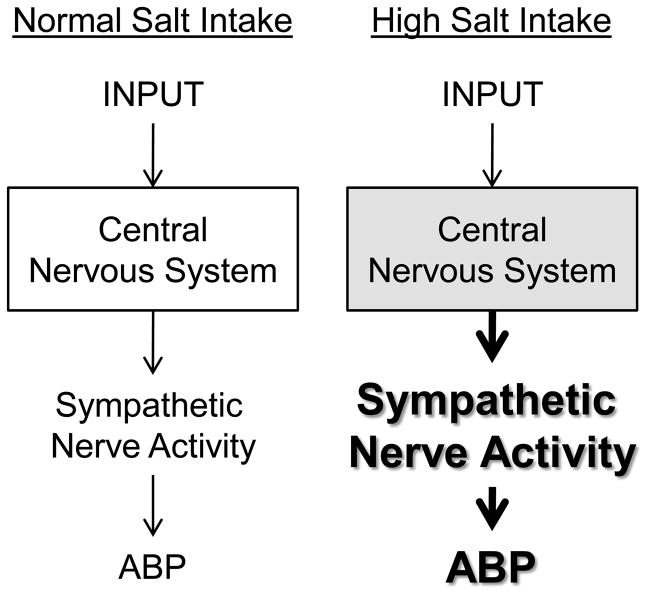
The ability of dietary salt with other factors (e.g., genetic background, mineralocorticoid, or angiotensin II) to produce hypertension and the dependence of the elevated ABP on the sympathetic nervous system suggests that dietary salt may influence the excitability of sympathetic-regulatory networks. In this context, we hypothesize that elevated dietary salt intake alters the excitability and increases the gain of central sympathetic neurons (Figure 2). Such a model would predict that elevated dietary salt intake would potentiate the sympathetic and ABP response evoked by a given input to the central nervous system, possibly even in NSS subjects. Support for this hypothesis arises from several lines of evidence that indicates elevated dietary salt intake potentiates the sympathetic and/or pressor responses to stress [31, 32], hyperinsulinemia [33], intracerebroventricular injection of angiotensin II [34], and activation of somatic afferents [24, 35] (Figure 3). Furthermore, several studies in humans and animals have suggested that elevated dietary salt intake enhances baroreflex gain [23, 36] and the depressor and bradycardic responses from stimulation of the aortic depressor nerve [26] or the site of baroreceptor input in the nucleus tractus solitarius [24, 37]. Future experiments are needed to identify which reflexes are altered by dietary salt intake in animal models and extend these findings to human subjects. Collectively, these observations indicate that excess dietary salt intake can sensitize both sympathoexcitatory and sympathoinhibitory responses to several physiological stimuli. This raises the possibility that the synergistic effect of dietary salt in salt-sensitive humans and animals stems from the ability of dietary salt to enhance the sympathoexcitatory effects of those inputs contributing to the elevated sympathetic outflow and ABP.
Figure 2.
Hypothetical model for the central actions of high salt intake on sympathetic-regulatory function. The central nervous system integrates a variety of inputs together with ongoing activity to generate a level of sympathetic nerve activity. During elevated dietary salt intake, we hypothesize that dietary salt alters the excitability of these central networks to amplify a given input to produce a much greater level of sympathetic nerve activity and ABP.
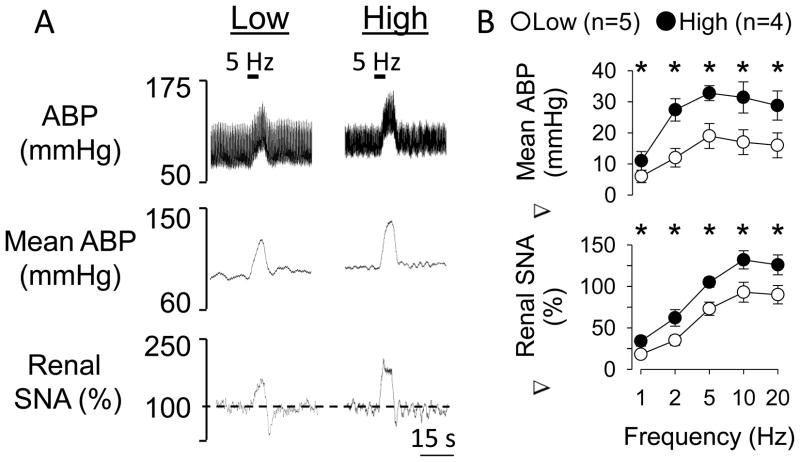
Figure 3.
Elevated dietary salt intake enhances renal sympathetic nerve activity and ABP responses to electrical activation of sciatic nerve afferents. (A) Representative examples of ABP, mean ABP, and integrated and rectified renal sympathetic nerve activity in rats drinking water (low) or 0.9% NaCl (high) for 14 days during electrical stimulation of sciatic nerve afferents (500 uA, 1 ms pulse duration). (B) Mean ± SEM of the change in mean ABP and renal sympathetic nerve activity during electrical stimulation of sciatic nerve afferents of rats drinking water or 0.9% NaCl for 14 days. *Significant difference between low and high salt rats (P<0.05)
Dietary Salt Alters the Excitability of Sympathetic-Regulatory Circuits in the Rostral Ventrolateral Medulla (RVLM)
Recent evidence suggests that the ability of dietary salt to impact central neural control of the circulation depends upon sympathetic neurons in the rostral ventrolateral medulla (RVLM). The RVLM is the principle site responsible for basal sympathetic vasomotor tone and critical for several sympathetic reflexes [38, 39]. Inhibition of the RVLM in experimental animals decreases sympathetic nerve activity and ABP to similar levels observed after spinal cord transection. Conversely, chemical excitation of the RVLM produces a marked increase in sympathetic nerve activity and ABP. RVLM neurons are tonically-active, display discharge properties consistent with a role in sympathetic regulation, and directly innervate sympathetic preganglionic neurons in the thoracic and lumbar segments of the spinal cord. Interruption of neurotransmission in the RVLM decreases or normalizes ABP in salt-sensitive hypertension [17, 18, 34]. Interestingly, the sympathetic reflexes shown to be enhanced by dietary salt are mediated by the RVLM [39–42]. These observations suggest that dietary salt directly affects the excitability or responsiveness of RVLM neurons to enhance sympathetic reflexes.
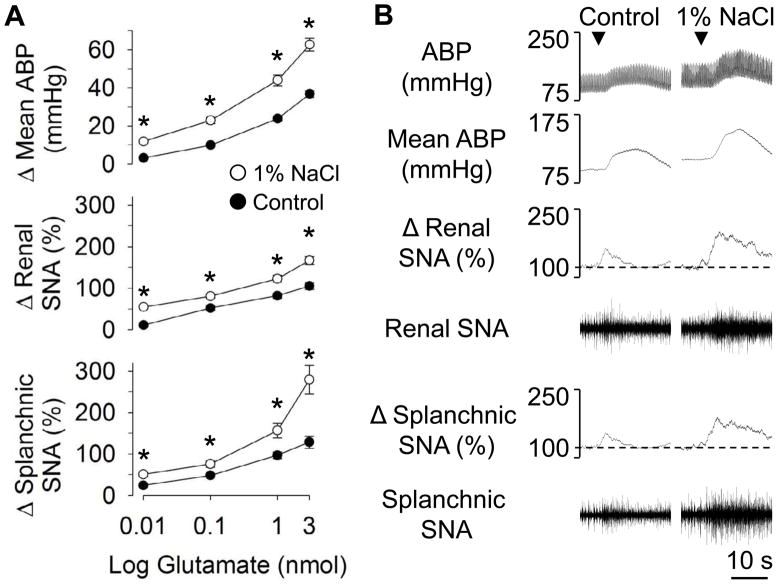
In a series of studies from different laboratories, elevated dietary salt intake has been reported to enhance the sympathetic and pressor responses to RVLM stimulation produced by local microinjection of glutamate [24, 26, 43] (Figure 4). This effect is not unique to glutamate-evoked stimulation, as increased dietary salt also enhances the sympathoexcitatory response to RVLM injection of other substances including the cholinergic agonist carbachol [24] and angiotensin II [20]. Interestingly, this effect was not restricted to excitatory neuroactive substances as the sympathoinhibitory and depressor responses produced by RVLM injection of GABA were enhanced by increased dietary salt intake [43, 44]. The mechanism for the enhanced sympathetic responses likely resides within the RVLM as vascular reactivity [24, 26, 45] and the sympathetic and pressor responses to stimulation of the dorsolateral funinculus [20, 26] are not altered by high dietary salt intake.
Figure 4.
(A) Peak changes in mean ABP, renal and splanchnic sympathetic nerve activity during microinjection of L-glutamate into the RVLM of rats fed standard chow and given access to water (control) or 1% NaCl solution for 14 days. (B) Individual examples of ABP, mean ABP, and renal and splachnic SNA. ▼ indicates microjection of L-glutamate. Results were reprinted with permission from Adams and colleagues [43].
The enhanced responses to RVLM stimulation or inhibition are not observed acutely following changes in dietary salt intake, but rather take several days to appear. For example, the enhanced responses to L-glutamate or GABA were not observed after 1 or 7 days but were present at 14 and 21 days of ingesting 1.0% NaCl versus water [43]. Interestingly, these enhanced responses persisted after the 1.0% NaCl was replaced by water for 1 day but had returned back to baseline by 7 days [43]. These observations suggest that the dietary salt-induced changes in RVLM function depend upon a slowly-developing and reversible form of neuronal plasticity within the RVLM.
Based on the above evidence, centrally-evoked sympathetic responses that are mediated by the RVLM should be enhanced. Indeed, elevated dietary salt intake enhances the sympathoinhibition evoked by excitation of NTS or CVLM neurons [24, 37] and the sympathoexcitation evoked by stimulation of the lateral parabrachial nucleus [44]. One notable exception is the observation of DiBona and Jones [46] that a high salt diet attenuates the renal sympathoexcitation produced by disinhibition of the hypothalamic paraventricular nucleus. This observation is surprising for two reaons: 1) the increase in renal SNA evoked by disinhibition of neurons within the PVH is partially dependent on the activation of angiotensin II type 1 (AT1) receptors within the RVLM [47], and 2) the increase in renal SNA evoked by RVLM microinjection of angiotensin II is enhanced by excess dietary salt intake [20]. The explanation for this apparent discrepancy is unknown. Since ~50% of the sympathoexcitatory response from disinhibition of the hypothalamic paraventricular nucleus is mediated by the RVLM [47], dietary salt may decrease the excitability of pathways independent of the RVLM thereby producing an attenuated response. Alternatively, the magnitude of the sympathetic and ABP responses of control animals in the study by DiBona and Jones [46] were considerably smaller than those reported by other investigators across a number of laboratories [47–50] thereby raising questions about this study. Clearly, additional studies are needed to clarify the effects of dietary salt on centrally-evoked sympathetic responses.
One interesting question that arises from these observations is whether dietary salt intake alters the excitability of all sympathetic-regulatory networks or only RVLM neurons. Although limited evidence is available, preliminary data indicate that dietary salt intake does not enhance the sympathetic and/or pressor responses evoked from the anterior hypothalamus [44] or medullo-cervical pressor area [35] – responses that are not dependent on neurotransmission within the RVLM [41, 51]. These observations suggest the dietary salt selectively alters RVLM sympathetic circuits.
The cellular mechanism(s) mediating the enhanced responsiveness of RVLM circuits during elevated dietary salt intake are unknown. However, it is noteworthy that both excitatory and inhibitory inputs to the RVLM are enhanced by increased dietary salt intake [20, 24, 26, 43]. One possible explanation is that dietary salt similarly alters the expression of multiple types of receptors on RVLM neurons, but this seems highly unlikely. Furthermore, elevated dietary salt intake enhanced sympathoexcitatory responses to RVLM injection of AngII despite the lack of an increase in AT1 or AT2 receptor mRNA [20]. A more likely explanation is that increased dietary salt intake alters the intrinsic excitability of RVLM circuits. This might include, for example, a general change in membrane conductance as this would permit both excitatory and inhibitory responses to be enhanced. Insight into the mechanism(s) of cellular plasticity responsible for these changes will require studies recording directly from RVLM neurons under conditions of elevated dietary salt intake.
Collectively, these observations indicate that dietary salt alters the responsiveness of sympathetic-regulatory neurons in the RVLM. Although limited data exists, experimental models of hypertension show exaggerated sympathetic and/or pressor responses to RVLM injection of L-glutamate [18, 52]. In fact, the Dahl-salt sensitive rat shows an exaggerated pressor response to L-glutamate on a low salt diet [18]. The significance of these observations to salt-sensitive hypertension in humans will be discussed in a later section of this review.
How Does Increased Dietary Salt Intake Alter the Responsiveness of RVLM Circuits?
How changes in dietary salt intake lead to changes in RVLM responsiveness is just speculation at this point. Large changes in dietary salt intake have been reported to change plasma or CSF sodium concentration in both rodents and humans [53–56]. However, there are some discrepancies. In carefully performed studies in rats consuming a diet containing a 4% versus 0.2% NaCl diet, plasma sodium concentrations increased 1–2% at night and remained elevated during the day [55]. Similar observations have been reported for rats fed normal chow (0.23% NaCl) and given access to 0.9% NaCl versus water [53]. Fang and colleagues [54] also reported a small increase plasma sodium concentrations of WKY and SHR rats fed 0.6% versus 8% NaCl diet, but the circadian rhythm was opposite. Regardless, similar changes in dietary salt intake have been reported to change plasma sodium concentrations in humans [56]. Importantly, mean ABP of SS subjects was found to vary directly with plasma sodium concentration [10]. Small increases in plasma sodium concentration or osmolality (1–2%) stimulate drinking behavior in mammals [57, 58] thereby suggesting that the reported changes in plasma sodium concentration during dietary salt loading could be sensed by the central nervous system. However, whether or not such small changes cause the enhanced responsiveness of RVLM neurons are presently unknown. Another relevant issue is the source of the dietary Na+. While it is often viewed that Na+ is the only important ion to consider, differences have been noted between NaCl and NaHCO3 in the impact on cardiovascular regulation [10]. Clearly, future studies are needed to provide a direct link between plasma sodium concentration and the responsiveness of RVLM neurons.
Aside from changes in plasma sodium concentration, elevated dietary salt intake causes widespread changes in neurohumoral profiles including suppression of the renin-angiotensin-aldosterone system [59], increased atrial naturetic peptide [60], and alter inflammatory markers [61, 62]. Any one of these factors may signal the brain and potentially impact the excitability of RVLM neurons. However, the list of potential intermediaries should not be limited as other hormonal and even neural signals potentially respond to changes in dietary salt intake, providing an extensive array of possible signaling mechanisms. For example, increased dietary salt intake also alters potassium dynamics (increased potassium excretion). Interestingly, potassium supplementation during dietary salt loading has been reported to prevent salt-sensitive hypertension [63, 64]. Thus, it would be interesting to determine whether potassium supplementation impacts the increased RVLM responsiveness caused by increased dietary salt intake.
Forebrain lesion studies may help suggest the potential mechanisms by which changes in dietary salt intake impact RVLM responsiveness. Destruction of structures along the ventral lamina terminalis, a lesion that interferes with sodium-dependent and angiotensin-dependent models of hypertension in experimental animals [14–16, 65], also interferes with the increased RVLM responsiveness associated with increased dietary salt intake [53]. This is consistent with a recent report that intracarotid infusion of hypotonic saline decreased sympathetic nerve activity and ABP in DOCA-salt hypertensive animals [6]. While this might seem to suggest a mechanism involving sensation of sodium or angiotensin II at these forebrain structures, these findings might also be the result of altering inputs to RVLM that are critical for the response.
Consideration of the potential mechanism underlying the salt induced increased RVLM responsiveness must also take into account the time course of the increased responsiveness. Reported changes in plasma sodium and angiotensin II levels occur rapidly in response to changes in dietary salt intake, while the change in RVLM responsiveness takes many days to develop. While this might reflect a signal that develops slowly in response to changes in dietary salt intake, it may also reflect the temporal dynamics of whatever intervening plastic changes are required for the changes in RVLM function. Clearly, the physiological responses to changes in dietary salt intake are plentiful and complex. Unraveling how the brain senses and responds to changes in dietary salt intake is critical for understanding neural control of the cardiovascular system and salt-sensitive hypertension.
Relevance to Salt-Sensitive Hypertension in Humans
The findings in experimental animals discussed above raise new hypotheses regarding the effect of dietary salt on sympathetic and ABP regulation in “salt-resistant” and “salt-sensitive” individuals. First, these experiments were conducted in male laboratory rats that would be classified NSS as dietary salt loading does not raise ABP yet enhanced the responsiveness of RVLM neurons and exaggerated sympathetic reflexes. Similarly, rats selectively bred for salt-resistance (Dahl-salt-resistant rat) show exaggerated pressor responses evoked from the RVLM after dietary salt loading for 3 weeks [18]. These findings would predict that chronic salt loading may not increase ABP in salt-resistant individuals but promote exaggerated sympathetic responses to a variety of stimuli. Unfortunately, there is no readily available data in humans to test this hypothesis. This seems to reflect, in part, a bias in the literature that salt-resistant individuals are resistant to any adverse cardiovascular effects of dietary salt loading. However, the findings noted above in experimental animals suggest that important changes in central cardiovascular regulation do occur. Second, salt-sensitive rats (Dahl-salt-sensitive) do show exaggerated responses to RVLM injection of L-glutamate or intraventricular Na+ even on a normal salt diet when they are normotensive [18, 66]. In an analogous manner, multiple studies have reported that salt-sensitive individuals consuming a normal salt diet are normotensive but show greater cardiovascular reactivity to stress [67–70]. Over time, these greater increases in ABP would be expected to increase the probability of developing hypertension. One question that remains unanswered is whether dietary salt loading causes a similar change in the properties of RVLM neurons from salt-resistant humans (or animals) to those already present in salt-sensitive humans (or animals).
Extrapolating the experimental findings in rats discussed above to human hypertension might suggest that one aspect of apparent salt-sensitivity could be an increased excitatory drive of the RVLM, which is exaggerated by increased dietary salt intake. From this perspective, salt sensitivity has less to do with the impact of salt and how it is handled by salt-sensitive individuals and more with the background neural activity. Thus, one contributor would be the underlying central neural circuits involved in cardiovascular regulation. For example, an overactive excitatory input to the RVLM, which itself might have minimal impact on sympathetic vasomotor tone and ABP, does produce a much larger increase in ABP when potentiated by increased dietary salt intake.
Summary and Conclusions
Studies in experimental animals show that increased dietary salt intake alters cardiovascular regulation, and this effect is observed in both SS and NSS animals. In NSS animals such as the standard laboratory rat, increased dietary salt intake appears to sensitize the RVLM to a variety of inputs without overt changes in ABP. This enhanced sensitivity of RVLM function with increased dietary salt intake results in enhanced sympathetic and ABP responses to a variety of stimuli. How this interacts with salt sensitivity to promote hypertension is unclear at present, but understanding the cellular mechanisms by which dietary salt alters RVLM responsiveness will likely provide a foundation for developing new therapeutic approaches to treat salt-sensitive hypertension.
Acknowledgments
The research was supported by American Heart Association Scientist Development Grants (S.D.S. and C.J.M.) and NIH Heart, Lung, and Blood Institute Grants HL090826 (S.D.S.), HL55687 (A.F.S.), HL076312 (A.F.S.) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK082558 (C.J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8:166–70. doi: 10.1007/s11906-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 2002;4:274–6. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–63. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 5.O’Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension. 2006;47:680–5. doi: 10.1161/01.HYP.0000214362.18612.6e. [DOI] [PubMed] [Google Scholar]
- 6.O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48:658–63. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- 7.Osborn JW, Fink GD. Region specific changes in sympathetic nerve activity in AngII-salt hypertension. Exp Physiol. 2009 doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn JW, et al. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–35. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 9.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep. 2002;4:129–35. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- 10.Schmidlin O, et al. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50:1085–92. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King AJ, et al. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1262–7. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 12.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 13.Jacob F, et al. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–29. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 14.Berecek KH, et al. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension. 1982;4:131–7. [PubMed] [Google Scholar]
- 15.Goto A, et al. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol. 1982;243:H614–8. doi: 10.1152/ajpheart.1982.243.4.H614. [DOI] [PubMed] [Google Scholar]
- 16.Sanders BJ, Johnson AK. Lesions of the anteroventral third ventricle prevent salt-induced hypertension in the borderline hypertensive rat. Hypertension. 1989;14:619–22. doi: 10.1161/01.hyp.14.6.619. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, et al. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–50. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- 18.Ito S, et al. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- 19.Nakata T, et al. Paraventricular nucleus lesions attenuate the development of hypertension in DOCA/salt-treated rats. Am J Hypertens. 1989;2:625–30. doi: 10.1093/ajh/2.8.625. [DOI] [PubMed] [Google Scholar]
- 20.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52:932–7. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson EA, et al. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–83. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 22.Friberg P, et al. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension. 1990;16:121–30. doi: 10.1161/01.hyp.16.2.121. [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, et al. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106:1957–61. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 24.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276:R1600–7. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 25.McBryde FD, et al. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R396–402. doi: 10.1152/ajpregu.90741.2008. [DOI] [PubMed] [Google Scholar]
- 26.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22:929–33. doi: 10.1161/01.hyp.22.6.929. [DOI] [PubMed] [Google Scholar]
- 27.Esler M, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–96. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–98. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 29.Lambert E, et al. Single-unit sympathetic discharge pattern in pathological conditions associated with elevated cardiovascular risk. Clin Exp Pharmacol Physiol. 2008;35:503–7. doi: 10.1111/j.1440-1681.2008.04905.x. [DOI] [PubMed] [Google Scholar]
- 30.Lambert E, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–8. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 31.Scrogin KE, Hatton DC, McCarron DA. The interactive effects of dietary sodium chloride and calcium on cardiovascular stress responses. Am J Physiol. 1991;261:R945–9. doi: 10.1152/ajpregu.1991.261.4.R945. [DOI] [PubMed] [Google Scholar]
- 32.Scrogin KE, Hatton DC, McCarron DA. Effects of dietary sodium and calcium on blood pressure reactivity in the SHR and WKY. Clin Exp Hypertens A. 1991;13:699–707. doi: 10.3109/10641969109042073. [DOI] [PubMed] [Google Scholar]
- 33.Muntzel MS, et al. Dietary salt loading exacerbates the increase in sympathetic nerve activity caused by intravenous insulin infusion in rats. Metabolism. 2007;56:373–379. doi: 10.1016/j.metabol.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Mann JF, et al. Central actions and brain receptor binding of angiotensin II: Influence of sodium intake. Hypertension. 1980;2:437–43. doi: 10.1161/01.hyp.2.4.437. [DOI] [PubMed] [Google Scholar]
- 35.Stocker SD, Madden CJ. Excess dietary salt intake selectively enhances the excitability of sympathetic neurons in the rostral ventrolateral medulla. FASEB J. 2009;23 Abstract # 958.9. [Google Scholar]
- 36.Huang BS, Leenen FH. Dietary Na and baroreflex modulation of blood pressure and RSNA in normotensive vs. spontaneously hypertensive rats. Am J Physiol. 1994;266:H496–502. doi: 10.1152/ajpheart.1994.266.2.H496. [DOI] [PubMed] [Google Scholar]
- 37.Isogai O, et al. High salt diet enhances cardiovascular responses from the nucleus tractus solitarius and ventrolateral medulla of Sprague-Dawley rats. Clin Exp Hypertens. 2005;27:33–44. doi: 10.1081/ceh-200044252. [DOI] [PubMed] [Google Scholar]
- 38.Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 39.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 40.Blessing WW. Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla. Am J Physiol. 1988;254:H686–92. doi: 10.1152/ajpheart.1988.254.4.H686. [DOI] [PubMed] [Google Scholar]
- 41.Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol. 1994;267:H1549–56. doi: 10.1152/ajpheart.1994.267.4.H1549. [DOI] [PubMed] [Google Scholar]
- 42.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic Receptor Activation in the Rostral Ventrolateral Medulla Mediates the Sympathoexcitatory Response to Hyperinsulinemia. Hypertension. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams JM, et al. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 44.Madden CJ, et al. Dietary salt selectively alters cardiovascular responses mediated by the rostral ventrolateral medulla (RVLM) [abstract] FASEB J. 2000;14:A626. [Google Scholar]
- 45.Kaufman LJ, Vollmer RR. Low sodium diet augments plasma and tissue catecholamine levels in pithed rats. Clin Exp Hypertens A. 1984;6:1543–58. doi: 10.3109/10641968409044068. [DOI] [PubMed] [Google Scholar]
- 46.DiBona GF, Jones SY. Effect of dietary sodium intake on the responses to bicuculline in the paraventricular nucleus of rats. Hypertension. 2001;38:192–7. doi: 10.1161/01.hyp.38.2.192. [DOI] [PubMed] [Google Scholar]
- 47.Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–7. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 48.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–31. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- 49.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1231–9. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- 50.Kenney MJ, et al. Paraventricular nucleus bicuculline alters frequency components of sympathetic nerve discharge bursts. Am J Physiol Heart Circ Physiol. 2001;281:H1233–41. doi: 10.1152/ajpheart.2001.281.3.H1233. [DOI] [PubMed] [Google Scholar]
- 51.Seyedabadi M, et al. A novel pressor area at the medullo-cervical junction that is not dependent on the RVLM: efferent pathways and chemical mediators. J Neurosci. 2006;26:5420–7. doi: 10.1523/JNEUROSCI.1190-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergamaschi C, et al. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–20. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- 53.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–14. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang Z, et al. Circadian rhythm of plasma sodium is disrupted in spontaneously hypertensive rats fed a high-NaCl diet. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1490–5. doi: 10.1152/ajpregu.2000.278.6.R1490. [DOI] [PubMed] [Google Scholar]
- 55.Habecker BA, et al. Ganglionic tyrosine hydroxylase and norepinephrine transporter are decreased by increased sodium chloride in vivo and in vitro. Auton Neurosci. 2003;107:85–98. doi: 10.1016/S1566-0702(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 56.He FJ, et al. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 57.Fitzsimons JT. The effects of slow infusions of hypertonic solutions on drinking and drinking thresholds in rats. J Physiol. 1963;167:344–54. doi: 10.1113/jphysiol.1963.sp007154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf AV. Osmometric analysis of thirst in man and dog. Am J Physiol. 1950;161:75–86. doi: 10.1152/ajplegacy.1950.161.1.75. [DOI] [PubMed] [Google Scholar]
- 59.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 60.Melo LG, et al. ANP in regulation of arterial pressure and fluid-electrolyte balance: lessons from genetic mouse models. Physiol Genomics. 2000;3:45–58. doi: 10.1152/physiolgenomics.2000.3.1.45. [DOI] [PubMed] [Google Scholar]
- 61.Larrousse M, et al. Increased levels of atherosclerosis markers in salt-sensitive hypertension. Am J Hypertens. 2006;19:87–93. doi: 10.1016/j.amjhyper.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–43. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris RC, Jr, et al. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 64.Whelton PK, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–32. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 65.Buggy J, et al. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res. 1977;40:I110–7. [PubMed] [Google Scholar]
- 66.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol. 2001;281:H1881–9. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 67.Weber CS, et al. Salt-sensitive men show reduced heart rate variability, lower norepinephrine and enhanced cortisol during mental stress. J Hum Hypertens. 2008;22:423–31. doi: 10.1038/jhh.2008.11. [DOI] [PubMed] [Google Scholar]
- 68.Buchholz K, et al. Enhanced affective startle modulation in salt-sensitive subjects. Hypertension. 2001;38:1325–9. doi: 10.1161/hy1101.096055. [DOI] [PubMed] [Google Scholar]
- 69.Deter HC, et al. Salt-sensitivity and other predictors of stress-related cardiovascular reactivity in healthy young males. Clin Exp Hypertens. 2001;23:213–25. doi: 10.1081/ceh-100102661. [DOI] [PubMed] [Google Scholar]
- 70.Deter HC, et al. Psychophysiological reactivity of salt-sensitive normotensive subjects. J Hypertens. 1997;15:839–44. doi: 10.1097/00004872-199715080-00006. [DOI] [PubMed] [Google Scholar]