ABSTRACT
Purpose: To investigate the extent to which different single-limb support (SLS) parameters predict mobility performance following traumatic brain injury (TBI).
Methods: Seventy-one people with mobility limitations following TBI were assessed for balance and mobility performance in a human movement laboratory. Participants performed a clinical test of static balance that involved balancing in SLS on each leg with eyes open and eyes closed. Mobility performance was measured by self-selected gait speed and performance on the High Level Mobility Scale (HiMAT). Dynamic stability during walking was measured by quantifying lateral centre of mass (COM) displacement, width of base of support, and proportion of double-support stance time.
Results: Total static balance scores were strongly correlated with HiMAT scores (r=0.57, p<0.001) and lateral COM displacement (r=−0.51, p<0.001). Despite these strong correlations, however, balance scores explained only 32% of the variance in advanced mobility skills (r2=0.32) and 26% of the variance in lateral COM displacement (r2=0.26).
Conclusions: Since mobility performance varied widely for people with similar levels of balance, SLS time was not able to predict dynamic stability during gait, self-selected gait speed, or advanced mobility skills in people with TBI.
Key Words: balance, gait, mobility, traumatic brain injury
RÉSUMÉ
Objectif : Étudier à quel point divers paramètres relatifs aux supports posturaux simples (SPS) peuvent influer de manière prévisible sur la mobilité à la suite d'une lésion cérébrale traumatique (LCT).
Méthode : Un échantillon de 71 personnes avec des limitations de mobilité résultant d'une LCT a été étudié ; on a évalué l'équilibre et la mobilité de chaque personne dans un laboratoire de motricité humaine. Les participants ont pris part à un test clinique de leur équilibre statique, pour lequel ils devaient se balancer d'une jambe à l'autre avec un SPS, les yeux ouverts, puis les yeux fermés. La mobilité des participants a été évaluée lors de la locomotion, suivant une vitesse et le rendement choisis par chacun, sur l'HiMAT (Level Mobility Scale). La stabilité dynamique au cours de la marche a été mesurée en quantifiant le déplacement latéral du centre de masse (CM), la largeur de la base d'appui et la proportion du temps passé en station debout avec double appui.
Résultats : Les pointages totaux d'équilibre statique étaient conformes aux pointages obtenus sur l'HiMAT (r=0,57, p<0,001) et pour le déplacement latéral du CM (r=−0,51, p<,001). Malgré ces fortes corrélations toutefois, les pointages touchant l'équilibre n'expliquaient que 32 % des variations des habiletés motrices avancées (r2=0,32) et 26 % des variations du déplacement latéral du CM (r2=0,26).
Conclusions : Puisque la mobilité varie grandement entre les gens ayant un même degré d'équilibre, le temps de SPS n'était pas en mesure d'influer sur la stabilité dynamique durant la locomotion, sur la vitesse de locomotion choisie ou sur les habiletés motrices avancées des personnes ayant subi une LCT.
Mots clés : démarche, équilibre, lésion cérébrale traumatique, mobilité
INTRODUCTION
Independent and safe mobility requires the complex interaction of many systems.1 Balance is one system that is important for functional mobility.1 Following traumatic brain injury (TBI), balance and mobility problems are common.2–4 In addition, associated difficulties with insight, impulsiveness, self-monitoring, and planning potentially place people with TBI at greater risk of falling. Clinicians working in rehabilitation are required to make judgements about when a patient may be safe to mobilize independently. A simple clinical test of balance that predicts safe walking ability would be valuable in rehabilitation, as such a test could assist clinical decision making with respect to when patients no longer require supervision and when they are able to ambulate safely indoors or outdoors, as well as helping to determine the extent to which they are at risk of falling. We are unaware of any simple clinical test of balance that is able to predict safe independent ambulation in persons with TBI.
A similar example (i.e., using a measure of impairment to predict mobility) has been developed for muscle strength. The Upright Motor Control (UMC) test, developed to measure the functional strength of hemiplegic limbs for walking following stroke,5 is able to differentiate household from community ambulators with a high level of accuracy.6 The UMC has high interrater reliability, is simple and quick to administer, and can be performed in any environment. An equivalent test of the ability to balance for safe mobility could assist clinical decision making, goal setting, and treatment planning.
Many different measures of balance have been developed. Some, such as single-limb support (SLS) or tandem stance, require the participant to maintain stability under varying conditions while the feet remain still; such balance tests are typically called “static.” “Dynamic” tests of balance, such as the Four Square Step Test (FSST)7 and Step Test,8 require the participant to maintain stability while moving about. More challenging dynamic tests of balance include the figure-of-8 task.9 Since dynamic balance tasks require mobility, it can become difficult to dissociate and attribute the causes of performance problems. That is, dynamic balance requires mobility, which is the activity that the clinician is attempting to predict. In this study, therefore, static measures of balance were used to predict mobility performance.
Lateral displacement of the centre of mass (COM)—that is, how much a person moves from side to side when walking—has been associated with dynamic stability during gait in TBI.2–4 This means that people with better dynamic stability have less lateral COM displacement, whereas people with reduced dynamic stability have greater lateral COM displacement. Reduced self-selected gait speed,10 increased width of base of support (BOS),11 and increased proportion of double-support stance time (%DS)11 have all been described as compensatory strategies for reduced dynamic stability following TBI. We are unaware of any studies that have used static tests of balance to predict dynamic stability during gait, but Williams and Goldie (2001)12 found static balance on the less affected leg to be a predictor of high-level mobility; that is, the ability to balance in SLS on the less affected leg accounted for some unique variance when predicting the ability to run. SLS was chosen as the static balance test for the present study because of its level of difficulty and its potential to discriminate the performance of more able participants.13 Less challenging measures of static balance, such as the Tinetti Balance Assessment14 and the Berg Balance Scale,15 are susceptible to ceiling effects in the TBI population.2,4,10,16 The main aim of the current investigation was to determine the extent to which different SLS-derived parameters predict mobility performance in individuals with TBI. It was hypothesized that because of the complex interactions of many systems required for mobility, SLS would predict only a small proportion of mobility performance.
METHODS
The research project was approved by Epworth Hospital's Human Research and Ethics Committee (study number 34006) and by the University of Melbourne (Ethics ID: 060496.1).
Participants
In this cross-sectional study, all participants with TBI currently attending physiotherapy for gait retraining at Epworth Hospital, Melbourne, Australia, were invited to participate in the project. Epworth Hospital is a large rehabilitation facility specializing in the treatment of traumatic brain injury. The majority of participants had sustained their TBI as a result of a motor-vehicle accident. Patients were eligible for inclusion if they (a) had sustained a TBI and (b) were able to walk independently over a distance of 20 m without the use of a gait aid (see Table 1). Patients were excluded if they (a) were unwilling or unable to provide informed consent, (b) presented with concurrent central nervous system disorders, or (c) had severe cognitive or behavioural problems that prevented assessment. All individuals who were invited to participate consented to do so.
Table 1.
Participant Characteristics
| Participant Characteristics | Value |
|---|---|
| Sex (male:female) | 66:15 |
| Age (mean±SD years) | 29.3±10.6 |
| Length of post-traumatic amnesia (mean±SD days) | 69.6±51.5 |
| Time to testing post-injury (median months) | 30.4 (IQR=4.7–103.4) |
IQR=inter-quartile range
Instrumentation and Procedures
Each participant was assessed for static balance and mobility performance. Since people with TBI may experience problems bilaterally, both legs were tested for each participant. Static balance was measured via SLS for each leg with eyes open (EO) and eyes closed (EC), up to a maximum of 30 seconds. Participants were instructed to stand in SLS with their hands on their hips (where possible) and not to let their legs touch. The trial ceased if the participant moved the stance foot, put the non-stance foot down, touched his or her legs together, or lost hand contact for more than a brief moment. The trial also ceased for the EC condition if participants opened their eyes. Participants performed three trials of each condition. The average time for the three trials for each condition was then calculated. Three methods for measuring static balance were collated to determine whether a single measure of static balance was sufficient to predict mobility performance. First, SLS with EO and SLS with EC on the more affected leg were recorded separately (maximum total score=30 s). Second, EO and EC scores were summed for the more and less affected legs to create a representative score for each leg (maximum total score=60 s). Third, the average times for each of the four conditions (left and right legs, EO and EC) were summed to create a total SLS score (maximum total score=120 s) to represent static balance performance for both legs.
Mobility performance was measured in several ways. First, dynamic stability during gait was assessed to evaluate mobility performance. To quantify this aspect of mobility performance, three-dimensional gait analysis (3DGA) was performed on all participants, using the Vicon 512 motion analysis system (Oxford Metrics, Oxford, UK), with eight cameras sampling at a rate of 120 Hz. The marker-placement protocol, calibration procedure, and data processing were conducted as previously described elsewhere.11 Data were collected for 10 trials, and the average performance for each variable was calculated. Lateral COM displacement was defined as the range between maximum and minimum values of the sacral marker, placed over S2. Width of BOS was defined as the perpendicular distance between the calcaneal markers, measured from the longitudinal axis of the laboratory. Self-selected gait speed was calculated from the toe marker during foot contact of consecutive steps (i.e., left/right stride length divided by time).
The second means of measuring mobility performance was the high-level mobility assessment tool (HiMAT). The HiMAT was selected as a clinical measure of mobility because of its ability to measure high-level mobility for people with TBI who can walk independently of gait aids.17,18 It measures walking under various conditions: stair use, running, skipping, hopping, and jumping. Higher scores indicate better performance (maximum score=54).
Data Analysis
Summary statistics (mean, standard deviation, and range) were generated for the measures of SLS (see Table 2) and mobility performance (see Table 3). All variables of interest were assessed for distribution normality. Pearson correlation coefficients, and their associated coefficients of determination, were calculated to determine how much of the variance in mobility performance (HiMAT, lateral COM displacement, self-selected gait speed, width of BOS, %DS) SLS could predict. SLS was recorded and analyzed in five ways:
EO on the affected leg
EC on the affected leg
Combined EO and EC scores on the affected leg
Combined EO and EC scores on the less affected leg
Total SLS balance score
Table 2.
Static Balance Performance of Persons with TBI (n=71)
| Mean (SD) seconds |
Range seconds |
|
|---|---|---|
| EO (affected limb) | 8.6 (9.7) | 0–30.0 |
| EC (affected limb) | 1.7 (2.2) | 0–12.8 |
| Combined EO+EC (affected limb) | 10.4 (11.3) | 0–37.4 |
| Combined EO+EC (less affected limb) | 20.0 (16.5) | 0–60.0 |
| Total static balance score | 30.4 (22.4) | 0–77.0 |
Table 3.
Mobility Performance of Persons with TBI (n=71)
| Mean (SD) | Range | |
|---|---|---|
| Self-selected gait speed (m/s) | 1.04 (0.33) | 0.30–1.84 |
| Lateral COM displacement (cm) | 8.7 (3.2) | 3.8–16.4 |
| Width of BOS (cm) | 24.2 (4.9) | 14.0–35.1 |
| %DS | 28.5 (7.5) | 19.3–61.6 |
| HiMAT score (maximum 54) | 21.6 (10.9) | 1–45 |
COM=centre of mass; BOS=base of support; %DS=proportion of double-support time; HiMAT=High-Level Mobility Assessment Tool
Self-selected gait speed was normalized for height in order to compare data across subjects prior to data analysis. Summary statistics involving self-selected gait speed are reported prior to normalization for ease of interpretation. Statistical tests involving self-selected gait speed were performed on the normalized data. Using Cohen's guidelines, a moderate relationship will be interpreted as r=0.30–0.50 and a strong relationship as r≥0.50.19
RESULTS
Table 1 outlines participant characteristics. The 71 participants with TBI were predominantly young and male; this is consistent with the broader TBI population. Of the 71 participants, 63 had a length of post-traumatic amnesia (PTA) in excess of 28 days, indicating that they had sustained an extremely severe brain injury.20 The TBI sample varied considerably with respect to time to testing post-injury.
The TBI cohort also showed a great deal of variation in their physical performance. As Table 3 shows, self-selected gait speed ranged from very slow to very quick. The mean self-selected gait speed for the TBI cohort was slower than normative values that have been reported.11 Ability to balance in SLS also varied considerably; several participants (n=4, ≤0.5 seconds) were unable to balance with their eyes open on their more affected leg, despite being able to walk independently.
The total SLS score was the best predictor of mobility performance for the majority of the five measures (see Table 4). Despite several strong correlations between measures of SLS and mobility performance, however, the total SLS score was a poor predictor of dynamic stability. It was strongly negatively correlated with lateral COM displacement, indicating that higher balance scores were associated with lower lateral excursions of the COM, yet explained only 26% of the variance in lateral COM displacement (r2=0.26). Weaker correlations were obtained with width of BOS and proportion of double-support time (see Table 4), which accounted for only 19% (r2=0.19) and 5% (r2=0.05) of the variance in mobility performance respectively.
Table 4.
Relationships between Measures of Static Balance and Mobility Performance
| Measure of Mobility Performance |
Static Balance |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EO Affected | EC Affected | EO+EC Affected | EO+EC Less Affected | Total Static Balance Score | ||||||
| |
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
| Self-selected gait speed | 0.41 | <0.01 | 0.40 | <0.01 | 0.43 | <0.01 | 0.23 | 0.06 | 0.35 | <0.01 |
| Lateral COM displacement | −0.35 | <0.01 | −0.35 | <0.01 | −0.37 | <0.01 | −0.40 | <0.01 | −0.51 | <0.01 |
| Width of BOS | −0.29 | 0.02 | −0.25 | 0.04 | −0.30 | 0.01 | −0.33 | <0.01 | −0.44 | <0.01 |
| %DS | −0.15 | 0.23 | −0.28 | 0.02 | −0.18 | 0.14 | −0.19 | 0.12 | −0.22 | 0.07 |
| HiMAT score | 0.43 | <0.01 | 0.46 | <0.01 | 0.46 | <0.01 | 0.47 | <0.01 | 0.57 | <0.01 |
COM=centre of mass; BOS=base of support; %DS=proportion of double-support time; HiMAT=High-Level Mobility Assessment Tool
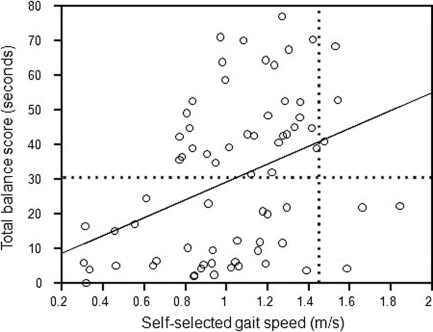
Although the total SLS score was moderately positively correlated with self-selected gait speed, indicating that higher SLS scores were associated with higher self-selected gait speeds (see Figure 1), it explained only 12% of the variance in self-selected gait speeds (r2=0.12). As Figure 1 shows, all participants with a total SLS score >30 seconds (horizontal reference line) were able to walk at ≥0.7 m/s. Post hoc analysis was performed on the sample, dichotomized at a total SLS score of 30 seconds, to determine whether higher scores were associated with greater predictive ability for mobility. For participants with a total SLS score >30 seconds, there was no significant relationship between total SLS scores and self-selected gait speed (r=0.26; p=0.13). Participants scoring above the horizontal reference line (Figure 1) varied from 0.77 to 1.54 m/s in their self-selected gait speed. Similarly, for participants with a total SLS score <30 seconds, there was no significant relationship between total SLS scores and self-selected gait speed (r=0.28; p=0.11). The vertical dotted line in Figure 1 represents the normal self-selected walking speed for young adults.21 For participants walking at a self-selected speed similar to that of healthy young adults, total balance scores varied from 3.6 to 70.4 seconds. This result suggests that SLS alone is a poor indicator of self-selected gait speed.
Figure 1.
Relationship between total balance scores and self-selected gait speed. The solid line represents the line of best fit; the horizontal dotted line represents the mean total balance score for TBI; the vertical dotted line represents the normal self-selected walking speed for young adults.21
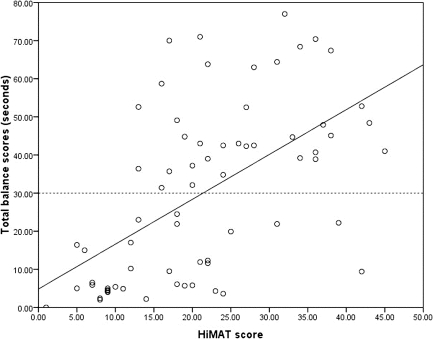
The total SLS score had a strong positive correlation with the HiMAT score (see Figure 2), yet explained only 32% of the variance in advanced mobility skills (r2=0.32). The horizontal dotted reference line in Figure 2, representing the mean total SLS score for TBI, demonstrates that despite a strong relationship between total SLS scores and HiMAT scores, HiMAT scores varied from 13 to 45 for total SLS scores >30 seconds.
Figure 2.
Relationship between total SLS scores and High-Level Mobility Assessment Tool scores. The solid line represents the line of best fit; the horizontal dotted line represents the mean total balance score for TBI.
DISCUSSION
Although several measures of mobility performance were investigated, the results suggest that SLS is a poor predictor of mobility performance. Of the four measures of gait performance, total SLS scores were most strongly correlated with lateral COM displacement; yet total SLS scores accounted for only 26% of the variance in lateral COM displacement, and much less of the variance in other indicators of dynamic stability during gait. Further, SLS scores accounted for only a small proportion of the variance in self-selected gait speed. Although SLS scores were most strongly correlated with the HiMAT, 68% of the variance in advanced mobility skills remained unaccounted for. The clinical implication of this result is that because of the complex interactions required for mobility, SLS alone cannot independently predict mobility performance for people with TBI. Following TBI, problems with muscle strength and tone, spasticity, vision, and vestibular impairment may all affect the ability to mobilize;2,22–24 clinicians are not able to predict with any certainty how stable and safe a person with TBI will be while walking based on his or her ability to stand on one leg. In the absence of a predictor for mobility performance, clinicians may need to assess mobility tasks directly in order to determine the combined impact of contributing impairments on stability and safety.
The results demonstrate a large range of participant abilities to balance and mobilize (see Tables 2 and 3 and Figure 1). Although all participants were able to walk unassisted, this sample ranged from limited household ambulators to higher-functioning individuals attempting to return to pre-morbid social, leisure, and sporting activities. This large range in participant performance optimized the opportunity to identify a relationship between SLS and mobility. Nevertheless, as Figure 1 demonstrates, there was considerable variance in self-selected gait speeds for any given total SLS score. Participants above the dotted horizontal reference line in Figure 1, representing the mean balance score, varied from 0.77 to 1.54 m/s in their self-selected gait speed. This result suggests that SLS alone is a poor indicator of self-selected gait speed.
A gait speed of ≥0.8 m/s has been suggested as a threshold for community mobility following stroke.25 In this cohort, every participant with a total SLS score ≥30 seconds walked at a speed ≥0.8 m/s (see Figure 1), which suggests that this score may also be a threshold for predicting community mobility following TBI. Further analysis showed that above the total SLS score threshold of 30 seconds, balance had no significant relationship with and no greater predictive ability for mobility performance. Although high-level mobility was measured in this study, community mobility was not directly assessed, so caution is required in interpreting this finding. The large variability in results further limits clinical interpretation. As Figure 1 shows, participants with TBI who walked at a self-selected gait speed comparable to that of normal healthy controls (vertical dotted line) varied considerably in their SLS performance.11 Although all were able to walk comfortably at 1.4 m/s, total SLS scores varied from several seconds to >70 seconds.
Large variability in results was also found when SLS scores and HiMAT scores were compared. HiMAT scores for participants who scored above the reference line in Figure 2 varied from 13 to 45; this result indicates that SLS alone is a poor predictor of ability to perform advanced mobility skills such as running, skipping, hopping, and jumping. Although statistical analyses suggest moderate to strong correlations between SLS and some measures of mobility, neither self-selected gait speed nor HiMAT scores can be predicted from the ability to balance in SLS.
The results support the findings of several prior studies that have identified a moderate to strong correlation between the ability to balance and the ability to walk.18,26 Despite this relationship, however, no previous study has demonstrated that gains achieved in balance retraining lead to subsequent gains in mobility.27–30 Although the results of the present study suggest that performance in balancing on one leg is unable to predict gait performance, it may be of some value in predicting falls. Despite its susceptibility to a ceiling effect,10,16 the Berg Balance Scale (BBS) is one of the most widely used measures of balance, particularly in patients following stroke;31 the individual items are primarily “static” in that the participant's feet are not required to move. The BBS score has been used to predict falls,32,33 and SLS has been found to be the most sensitive and specific of the individual BBS items for predicting falls.34 A simple test of static balance may be clinically useful for predicting gait performance; however, SLS appears to have limited predictive value. Therefore, clinicians may need to assess patients' stability while walking.
LIMITATIONS
This study focused on a single static measure of balance as a means to predict mobility performance. It is possible that other measures of static balance may be better predictors of mobility, but SLS was chosen because it is able to discriminate performance over a wide range of abilities and is less susceptible to a ceiling effect. It is also possible that other measures of dynamic balance may predict mobility performance better than measures of static balance can. One of the main benefits of using a dynamic measure of balance is that such measures require the ability to maintain stability during transitional movements. Transitional movements from leg to leg are required during gait, but successful performance on dynamic balance tasks also places greater demands on muscle strength, range of motion, and motor control. Therefore, the unique contribution of balance to dynamic measures of balance is uncertain.
A major benefit of identifying a predictor for safe gait is to reduce the risk of falling. Although the key gait parameters associated with stability were measured in this study, falls were not. Using this cross-sectional study design, we were able to investigate only mobility performance. A longitudinal study might be able to use a measure of balance to measure fall rates or to predict mobility outcomes.
CONCLUSION
The ability to balance in SLS is strongly correlated with some measures of mobility performance, such as dynamic stability in gait (lateral COM displacement) and high-level mobility (HiMAT scores). Despite these strong correlations, however, the ability to balance in SLS is only a weak predictor of mobility performance. Because of the wide range in mobility performance among people whose ability to balance is similar, SLS performance is not able to predict dynamic stability in gait, self-selected gait speed, or advanced mobility skills.
KEY MESSAGES
What Is Already Known on This Topic
Balance and mobility problems are common following TBI. Despite strong correlations between tests of static balance and mobility, effective strategies for retraining balance have had little impact on ability to mobilize.
What This Study Adds
Static balance does not predict mobility performance in TBI. Therefore, static balance cannot be used as a clinical decision-making tool for mobility.
Williams GP, Morris ME. Tests of static balance do not predict mobility performance following traumatic brain injury. Physiother Can. 2011;preprint. doi:10.3138/ptc.2009-53
REFERENCES
- 1.Patla AE, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Activity. 1999;7:7–19. [Google Scholar]
- 2.Basford JR, Chou LS, Kaufman KR, Brey RH, Walker A, Malec JF, et al. An assessment of gait and balance deficits after traumatic brain injury. Arch Phys Med Rehabil. 2003;84:343–9. doi: 10.1053/apmr.2003.50034. doi: 10.1053/apmr.2003.50034. [DOI] [PubMed] [Google Scholar]
- 3.Chou LS, Kaufman KR, Walker-Rabatin AE, Brey RH, Basford JR. Dynamic instability during obstacle crossing following traumatic brain injury. Gait Posture. 2004;20:245–54. doi: 10.1016/j.gaitpost.2003.09.007. doi: 10.1016/j.gaitpost.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman KR, Brey RH, Chou L-S, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med Eng Phys. 2006;28:234–9. doi: 10.1016/j.medengphy.2005.05.005. doi: 10.1016/j.medengphy.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Keenan MA, Perry J, Jordan C. Factors affecting balance and ambulation following stroke. Clin Orthop Relat R. 1984;182:165–71. doi: 10.1097/00003086-198401000-00021. [PubMed] [Google Scholar]
- 6.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 7.Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil. 2002;83:1566–71. doi: 10.1053/apmr.2002.35469. doi: 10.1053/apmr.2002.35469. [DOI] [PubMed] [Google Scholar]
- 8.Hill KD, Bernhardt J, McGann AM, Maltese D, Berkovits D. A new test of dynamic standing balance for stroke patients: reliability, validity and comparison with healthy elderly. Physiother Can. 1996;48:257–62. doi: 10.3138/ptc.48.4.257. [Google Scholar]
- 9.Johansson G, Jarnlo GB. Balance training in 70 year old women. Physiother Theory Pract. 1991;7:121–5. doi: 10.3109/09593989109106962. [Google Scholar]
- 10.McFadyen BJ, Swaine B, Dumas D, Durand A. Residual effects of a traumatic brain injury on locomotor capacity—a first study of spatiotemporal patterns during unobstructed and obstructed walking. J Head Trauma Rehabil. 2003;18:512–25. doi: 10.1097/00001199-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Williams G, Morris ME, Schache A, McCrory PR. Incidence of gait abnormalities after traumatic brain injury. Arch Phys Med Rehabil. 2009;90:587–93. doi: 10.1016/j.apmr.2008.10.013. doi: 10.1016/j.apmr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Williams G, Goldie P. Validity of motor tasks for predicting running ability in acquired brain injury. Brain Inj. 2001;15:831–41. doi: 10.1080/02699050110048546. [DOI] [PubMed] [Google Scholar]
- 13.Rinne MB, Pasanen ME, Vartiainen MV, Lehto TM, Sarajuuri JM, Alaranta HT. Motor performance in physically well-recovered men with traumatic brain injury. J Rehabil Med. 2006;38:224–9. doi: 10.1080/16501970600582989. doi: 10.1080/16501970600582989. [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, Speechley M, Ginter SF. Risk factors for fall among elderly persons living in the community. N Engl J Med. 1988;319:1071–7. doi: 10.1056/NEJM198812293192604. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 15.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–311. doi: 10.3138/ptc.41.6.304. [Google Scholar]
- 16.Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Arch Phys Med Rehabil. 2001;82:174–82. doi: 10.1053/apmr.2001.19744. doi: 10.1053/apmr.2001.19744. [DOI] [PubMed] [Google Scholar]
- 17.Williams G, Robertson V, Greenwood K, Goldie P, Morris ME. The concurrent validity and responsiveness of the high-level mobility assessment tool for measuring the mobility limitations of people with traumatic brain injury. Arch Phys Med Rehabil. 2006;87:437–42. doi: 10.1016/j.apmr.2005.10.028. doi: 10.1016/j.apmr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Williams GP, Robertson V, Greenwood KM, Goldie PA, Morris ME. The high-level mobility assessment tool (HiMAT) for traumatic brain injury. Part 2: content validity and discriminability. Brain Inj. 2005;19:833–43. doi: 10.1080/02699050500058711. doi: 10.1080/02699050500058711. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1977. [Google Scholar]
- 20.Shores EA, Marosszeky JE, Sandanam J, Batchelor J. Preliminary validation of a clinical scale for measuring the duration of post-traumatic amnesia. Med J Australia. 1986;144:569–72. doi: 10.5694/j.1326-5377.1986.tb112311.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams G, Morris ME, Schache AG, McCrory P. People preferentially increase hip joint power generation to walk faster following traumatic brain injury. Neurorehabil Neural Repair. 2010;24:550–8. doi: 10.1177/1545968309357925. doi: 10.1177/1545968309357925. [DOI] [PubMed] [Google Scholar]
- 22.Marshall S, Teasell R, Bayona N, Lippert C, Chundamala J, Villamere J, et al. Motor impairment rehabilitation post acquired brain injury. Brain Inj. 2007;21:133–60. doi: 10.1080/02699050701201383. doi: 10.1080/02699050701201383. [DOI] [PubMed] [Google Scholar]
- 23.Esquenazi A. Evaluation and management of spastic gait in patients with traumatic brain injury. J Head Trauma Rehabil. 2004;19:109–18. doi: 10.1097/00001199-200403000-00004. doi: 10.1097/00001199-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kerrigan DC, Bang M, Burke DT. An algorithm to assess stiff-legged gait in traumatic brain injury. J Head Trauma Rehabil. 1999;14:136–45. doi: 10.1097/00001199-199904000-00004. doi: 10.1097/00001199-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 26.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–66. doi: 10.2522/ptj.20070205. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 27.Au-Yeung SSY, Hui-Chan CWY, Tang JCS. Short-form Tai Chi improves standing balance of people with chronic stroke. Neurorehabil Neural Repair. 2009;23:515–22. doi: 10.1177/1545968308326425. doi: 10.1177/1545968308326425. [DOI] [PubMed] [Google Scholar]
- 28.Eser F, Yavuzer G, Karakus D, Karaoglan B. The effect of balance training on motor recovery and ambulation after stroke: a randomized controlled trial. Eur J Phys Rehabil Med. 2008;44(1):19–25. [PubMed] [Google Scholar]
- 29.Winstein CJ, Gardner ER, McNeal DR, Barto PS, Nicholson DE. Standing balance training: effect on balance and locomotion in hemiparetic adults. Arch Phys Med Rehabil. 1989;70:755–62. [PubMed] [Google Scholar]
- 30.Yavuzer G, Eser F, Karakus D, Karaoglan B, Stam HJ. The effects of balance training on gait late after stroke: a randomized controlled trial. Clin Rehabil. 2006;20:960–9. doi: 10.1177/0269215506070315. doi: 10.1177/0269215506070315. [DOI] [PubMed] [Google Scholar]
- 31.Korner-Bitensky N, Wood-Dauphinee S, Teasell R, Desrosiers J, Malouin F, Thomas A, et al. Best versus actual practices in stroke rehabilitation: results of the Canadian National Survey. Stroke. 2006;37(2):631. [Google Scholar]
- 32.Maeda N, Kato J, Shimada T. Predicting the probability for fall incidence in stroke patients using the Berg Balance Scale. J Int Med Res. 2009;37:697–704. doi: 10.1177/147323000903700313. [DOI] [PubMed] [Google Scholar]
- 33.Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age Ageing. 2008;37:270–6. doi: 10.1093/ageing/afn066. doi: 10.1093/ageing/afn066. [DOI] [PubMed] [Google Scholar]
- 34.Alzayer L, Beninato M, Portney LG. The accuracy of individual Berg Balance Scale items compared with the total Berg score for classifying people with chronic stroke according to fall history. J Neurol Phys Ther. 2009;33:136–43. doi: 10.1097/NPT.0b013e3181b51307. [DOI] [PubMed] [Google Scholar]