Abstract
Context
Because of a different degree of processing and nutrient contents, brown rice and white rice may have different effects on risk of type 2 diabetes.
Objective
To prospectively examine white rice and brown rice consumptions in relation to type 2 diabetes risk in US men and women aged 26–87 yr.
Design and Setting
The Health Professionals Follow-up Study (1986–2006) and the Nurses’ Health Study I (1984–2006) and II (1991–2005).
Participants
We prospectively ascertained diet, lifestyle practices, and disease status among 39,765 men and 157,463 women in these cohorts. All participants were free of diabetes, cardiovascular disease, and cancer at baseline. Intake of white rice, brown rice, other foods, and nutrients was assessed at baseline and updated every 2–4 years.
Results
During 3,318,196 person-years of follow-up, we documented 10,507 incident cases of type 2 diabetes. After multivariate adjustment for age and other lifestyle and dietary risk factors, higher intake of white rice was associated with a higher risk of type 2 diabetes. The pooled relative risk (95% confidence interval) of type 2 diabetes comparing ≥5 servings/week with <1 serving/month of white rice was 1.17 (1.02, 1.36). In contrast, high brown rice intake was associated with a lower risk of type 2 diabetes: The pooled multivariate relative risk (95% confidence interval) was 0.89 (0.81, 0.97) for ≥ 2 servings/week of brown rice as compared with <1 serving/month. We estimated that replacing 50 grams/day (cooked, equivalent to ⅓ serving/day) intake of white rice with the same amount of brown rice was associated with a 16% (95% confidence interval: 9%, 21%) lower risk of type 2 diabetes, whereas the same replacement with whole grains as a group was associated with a 36% (95% confidence interval: 30%, 42%) lower diabetes risk.
Conclusions
Substitution of whole grains, including brown rice, for white rice may lower risk of type 2 diabetes. These data support the recommendation that most carbohydrate intake should come from whole grains rather than refined grains to facilitate the prevention of type 2 diabetes.
Rice has been a staple food in Asian countries for centuries. By the twentieth century, the advance of grain-processing technology made it possible for large scale production of refined grains.1 Through refining processes, the outer bran and germ portions of intact rice grains (i.e., brown rice) is removed to produce white rice that primarily consists of starchy endosperm. Although not entirely consistent, consumption of white rice, in general, generates a stronger postprandial blood glucose response as measured by the glycemic index (GI) than the same amount of brown rice. A systematic review found that the mean GI was 64 ± 7 for white rice and 55 ± 5 for brown rice.2 Higher dietary GI has been consistently associated with elevated risk of type 2 diabetes (T2D) in prospective cohort studies.3–6 In addition, brown rice consumption may also impart beneficial effects on T2D risk by virtue of its high contents of multiple nutrients, such as fiber, vitamins, and minerals, the majority of which are lost during refining and milling processes.7 In line with these observations, high intake of white rice was associated with a monotonically elevated risk of developing T2D in a Chinese population, in which white rice consumption was the primary source of carbohydrate (74% of dietary glycemic load).6
Compared to Asian countries, rice consumption is much lower in the U.S., but is increasing rapidly. According to the U.S. Department of Agriculture 2009 food supply and disappearance data, rice consumption has increased more than 3 fold since the 1930s’, to reach 20.5 lbs per capita, and more than 70% of rice consumed is white rice.8 However, little is known whether rice intake is associated with diabetes risk in U.S. populations. We, therefore, evaluated the associations between intake of white rice and brown rice and risk of T2D in three large cohort studies with repeated prospective dietary assessments. We have previously observed an inverse association between whole grain consumption and risk of T2D in these cohorts.9, 10 In the present study, we extended the follow-up of these previously reported studies and evaluated whether substituting whole grains for white rice is associated with a lower risk of diabetes.
METHODS
Study Populations
We used data from three prospective cohort studies: the Health Professionals Follow-up Study (HPFS; age range, 32–87 yr) and the Nurses’ Health Study (NHS) I (age range, 37–65 yr) and II (age range, 26–45 yr). Detailed descriptions of these three cohorts were introduced elsewhere.11–13 In all three cohort studies, questionnaires were administered at baseline, as well as biennially after baseline, to collect and update information on lifestyle practice and occurrence of chronic diseases. The follow-up rates of the participants in these cohorts are all above 90%.
In the current analysis, we excluded men and women who had diagnoses of diabetes, cardiovascular disease, and cancer at baseline for the dietary analyses (1986 for HPFS, 1984 for NHS I, and 1991 for NHS II, when we first assessed white rice and brown rice consumption in these cohorts). In addition, we excluded HPFS participants who left more than 70 of the 131 food items blank on the baseline food frequency questionnaire (FFQ), or who reported unusual total energy intake levels (i.e., daily energy intake <800 or >4,200 kcal/day). For NHS I and II participants, we excluded those who left more than 10 (NHS I) or 9 (NHS II) items blank on baseline FFQs or whose total energy intake was <500 or >3,500 kcal/day. After exclusions, data from 39,765 (out of 51,530) HPFS participants, 69,120 (out of 81,755) NHS I participants, and 88,343 (out of 95,452) NHS II participants were available for the analysis. The study was approved by the Human Research Committee of Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard School of Public Health. The completion of the self-administered questionnaire was considered to imply informed consent.
Assessment of Rice Consumption
In 1984, a 116-item FFQ was administered among the NHS I participants to collect information on their usual intake of foods and beverages in the previous year. In 1986–2002, similar but expanded FFQs were sent to these participants to update their diet every 4 years. Using the expanded FFQ employed in the NHS I, dietary data were collected every four years during 1986–2002 among the HPFS participants, and during 1991–2003 among the NHS II participants. In all FFQs, we asked the participants how often, on average, they consumed each food of a standard portion size. In the current study, based on the distribution of responses to rice intake questions, we categorized participants into 5 categories (<1 serving/month, 1–3 servings/month, 1 serving/week, 2–4 servings/week, and ≥5 servings/week) of white rice intake and 3 categories (<1 serving/month, 1–4 servings/month, and ≥2 servings/week) of brown rice intake to warrant appropriate variation in rice consumption while preserving enough statistical power to make stable estimate for each category. The reproducibility and validity of these FFQs have been demonstrated in detail elsewhere.14–17 In a validation study conducted among a subsample of HPFS participants, assessments of white rice and brown rice intake were moderately correlated with diet record assessments. The corrected Pearson correlation coefficients between these two assessments were 0.53 for white rice and 0.41 for brown rice.14 Assessment of whole grain intake was described in detail elsewhere.9 We considered any intact or milled form of grain that consisted of the expected proportions of bran, germ, and endosperm as whole grains. By definition, brown rice is a whole grain.
Study Outcome
The study outcome was incident T2D that occurred between the return of the baseline FFQ and January 31, 2006 (HPFS), June 30, 2006 (NHS I), or June 30, 2005 (NHS II). In all three cohorts, men and women who reported a diagnosis of T2D in the biennial follow-up questionnaires were sent a supplementary questionnaire to confirm the diagnosis. In this supplementary questionnaire, information on symptoms, diagnostic tests, and treatment was collected. We utilized the criteria from the National Diabetes Data Group to confirm self-reported diagnosis of T2D.18 For cases of T2D identified after 1998, we applied the American Diabetes Association criteria.19 The validity of the supplementary questionnaire for the diagnosis of diabetes has been described previously.20, 21 Out of a random sample of 62 nurses reporting type 2 diabetes in the supplementary questionnaire, 61 (98%) of them were confirmed after their medical records were reviewed by an endocrinologist blinded to the supplementary questionnaire information.20 In another validation study conducted in HPFS participants, 97% (57/59) of self-reported type 2 diabetes cases were confirmed using medical record review.21 Deaths were identified by reports from next of kin, postal authorities, or by searching the National Death Index. At least 98% of deaths among the study participants were identified.22
Statistical Analysis
We counted each individual’s person-years of follow-up from the date of return of the baseline FFQ to the date of death, the date of diagnosis of T2D, or January 31, 2006 (HPFS), June 30, 2006 (NHS I), or June 30, 2005 (NHS II), whichever came first. The relative risks (RRs) were estimated using Cox proportional hazard regression,23 in which we stratified the analysis jointly by age in months at baseline and calendar year to control for confounding by these factors as finely as possible. In multivariate analysis, we further adjusted for ethnicity, body mass index (BMI), smoking status, alcohol intake, multivitamin use, physical activity, and family history of diabetes. Among nurses, we adjusted for oral contraceptive use (NHS II participants only), postmenopausal status, and hormone use. To minimize confounding by other dietary factors, we further adjusted for total energy intake and intake of red meat, fruits and vegetables, coffee, and whole grains. All of these covariates are established risk factors for type 2 diabetes and were correlated with white rice or brown rice consumption in these cohorts.
To address missing values of dietary variables in the follow-up FFQs, we replaced missing values with those from a previous FFQ without missing data. On average, 12.7% of NHS I, 11.3% of NHS II, and 23.1% of HPFS participants had missing data after baseline assessment. To better represent long-term diet and to minimize the within-person variation, we created cumulative averages of food and nutrient intake from baseline to the censoring events.24 We stopped updating diet when participants first reported having a diagnosis of hypertension, hypercholesterolemia, cardiovascular disease, or cancer. For these participants, we carried forward the cumulative averages of dietary intake prior to the occurrence of these diseases to represent diet for later follow-up. We have used this approach in our previous studies to avoid systematic errors in dietary assessment due to potential biased recall after occurrence of chronic diseases.24, 25 To estimate the association of substituting brown rice intake for the same amount of white rice, we included both white rice and brown rice intake as continuous variables (50 grams/day; equivalent to ⅓ serving/day of white rice) in the same multivariate model. We used the difference between regression coefficients for brown rice and white rice to derive the RR measuring this association of substitution. We used the same approach to examine such an association for whole grains treated as a single food item. This method has been used in our previous studies.26
Tests for trend were conducted by assigning the median value to each category and modeling this value as a continuous variable. To summarize the estimates of association across the three studies, we conducted a meta-analysis using fixed effects models. P values for heterogeneity of study results were calculated using the Cochran Q test.27 All P values were two-sided. Ninety-five percent confidence intervals (95% CI) were calculated for RRs. Data were analyzed with the Statistical Analysis Systems software package, version 9.1 (SAS Institute, Inc., Cary, North Carolina). The pooling analysis was conducted using STATA 10.0 (StataCorp, College Station, Texas).
Role of the Funding Source
The National Institutes of Health provided funding for this study. The funding source had no role in the collection, analysis, and interpretation of the data or in the decision to submit the manuscript for publication.
RESULTS
We documented 2,648 incident T2D cases during 20 years of follow-up in the HPFS, 5,500 cases during 22 years in the NHS I, and 2,359 cases during 14 years in the NHS II. TABLE 1 describes the distribution of baseline characteristics according to intake of white rice and brown rice. Men and women who had high white rice intake were less likely to have European ancestry or to smoke and more likely to have a family history of diabetes. In addition, high white rice intake was associated with high fruit and vegetable intake and low intake of whole grains, cereal fiber, and trans fat. In contrast, brown rice intake was not associated with ethnicity but with a more health-conscious lifestyle and dietary profile. For example, participants with higher brown rice intake were more physically active, leaner, less likely to smoke or have a family history of diabetes, and had higher intake of fruits, vegetables, and whole grains and lower intake of red meat and trans fat. Both white rice and brown rice intake were positively associated with a higher glycemic load in all three cohorts.
TABLE 1.
Baseline characteristics of study participants by levels of white rice and brown rice intake.
| Characteristics | White rice intake | Brown rice intake | ||||
|---|---|---|---|---|---|---|
| <1/mo | 1/wk | ≥5/wk | <1/mo | 1/mo-1/wk | ≥2/wk | |
| HPFS | ||||||
| n | 9386 | 9968 | 795 | 21433 | 16134 | 2198 |
| Age (year) | 54.5 | 51.2 | 51.1 | 53.6 | 51.7 | 51.5 |
| Physical activity (MET-hr) | 22.0 | 22.0 | 20.8 | 19.3 | 23.4 | 28.4 |
| BMI (kg/m2) | 24.8 | 24.9 | 24.2 | 25.0 | 24.8 | 24.4 |
| White (%) | 96.7 | 96.2 | 49.0 | 94.5 | 96.4 | 93.0 |
| Current smoker (%) | 9.6 | 7.5 | 7.0 | 9.9 | 7.2 | 5.8 |
| Hypertension (%) | 19.0 | 18.0 | 21.9 | 20.0 | 17.5 | 17.0 |
| High cholesterol (%) | 9.7 | 10.4 | 11.5 | 9.7 | 10.5 | 12.9 |
| Family history of diabetes (%) | 12.3 | 13.5 | 14.6 | 13.2 | 12.4 | 13.6 |
| Total energy (kcal/day) | 1851 | 2099 | 2200 | 1943 | 2042 | 2229 |
| Red meat intake (servings/wk) | 7.6 | 8.3 | 8.3 | 8.6 | 7.6 | 5.7 |
| Fruit and vegetable intake (servings/day) | 5.0 | 5.8 | 5.8 | 4.8 | 5.8 | 7.6 |
| White rice (servings/wk) | 0 | 1.0 | 7.5 | 0.9 | 0.8 | 1.2 |
| Brown rice (servings/wk) | 0.5 | 0.5 | 0.6 | 0 | 0.7 | 3.6 |
| Whole grains (g/day) | 23.7 | 21.5 | 17.8 | 16.2 | 25.2 | 49.6 |
| P:S ratio | 0.57 | 0.58 | 0.62 | 0.54 | 0.59 | 0.71 |
| Glycemic load | 121 | 125 | 143 | 122 | 126 | 138 |
| trans fat (% of total energy) | 1.3 | 1.3 | 1.0 | 1.4 | 1.2 | 0.9 |
| Cereal fiber (g/day) | 5.9 | 5.9 | 5.7 | 5.4 | 6.2 | 7.8 |
| Magnesium (mg/day) | 357 | 351 | 359 | 335 | 364 | 426 |
| Alcohol (g/day) | 11.2 | 11.7 | 8.6 | 11.3 | 11.7 | 10.5 |
| Coffee (cups/day) | 1.9 | 1.9 | 1.7 | 2.0 | 1.9 | 1.6 |
| Multivitamin supplement user (%) | 44.3 | 40.6 | 44.8 | 39.1 | 43.7 | 51.6 |
| NHS I | ||||||
| n | 14690 | 17839 | 600 | 51673 | 16010 | 1437 |
| Age (year) | 50.9 | 49.2 | 49.8 | 50.0 | 50.2 | 50.9 |
| Physical activity (MET-hr) | 14.7 | 14.5 | 15.8 | 13.1 | 17.2 | 22.3 |
| BMI (kg/m2) | 24.8 | 24.8 | 24.1 | 24.9 | 24.4 | 24.0 |
| White (%) | 98.8 | 98.2 | 51.0 | 97.8 | 98.3 | 96.0 |
| Current smoker (%) | 25.1 | 23.8 | 16.1 | 25.4 | 20.8 | 15.0 |
| Postmenopausal (%) | 45.9 | 44.3 | 44.2 | 45.2 | 44.1 | 43.3 |
| Postmenopausal hormone use (%)* | 33.9 | 33.5 | 36.8 | 33.1 | 37.9 | 39.7 |
| Hypertension (%) | 19.1 | 20.1 | 19.9 | 19.7 | 18.8 | 17.4 |
| High cholesterol (%) | 7.3 | 7.6 | 8.4 | 7.2 | 7.8 | 10.2 |
| Family history of diabetes (%) | 24.6 | 24.6 | 27.6 | 25.4 | 23.8 | 22.1 |
| Total energy (kcal/day) | 1585 | 1852 | 1981 | 1724 | 1791 | 1958 |
| Red meat intake (servings/wk) | 8.1 | 9.3 | 9.6 | 9.2 | 8.2 | 6.3 |
| Fruit and vegetable intake (servings/day) | 4.4 | 4.8 | 5.4 | 4.4 | 5.2 | 6.5 |
| White rice (servings/wk) | 0 | 1.0 | 7.3 | 0.8 | 0.7 | 0.7 |
| Brown rice (servings/wk) | 0.3 | 0.2 | 0.3 | 0 | 0.6 | 3.5 |
| Whole grains (g/day) | 16.4 | 13.2 | 10.3 | 11.6 | 19.1 | 41.1 |
| P:S ratio | 0.56 | 0.56 | 0.61 | 0.54 | 0.57 | 0.68 |
| Glycemic load | 98 | 99 | 114 | 99 | 100 | 109 |
| trans fat (% of total energy) | 1.9 | 1.9 | 1.5 | 2.0 | 1.8 | 1.4 |
| Cereal fiber (g/day) | 4.3 | 4.1 | 3.7 | 4.0 | 4.5 | 6.0 |
| Magnesium (mg/day) | 296 | 288 | 293 | 278 | 312 | 368 |
| Alcohol (g/day) | 6.3 | 7.7 | 4.2 | 6.8 | 7.8 | 6.6 |
| Coffee (cups/day) | 2.4 | 2.5 | 2.2 | 2.5 | 2.4 | 2.0 |
| Multivitamin supplement user (%) | 39.6 | 36.2 | 37.8 | 34.6 | 43.4 | 51.7 |
| NHS II | ||||||
| n | 15753 | 25808 | 1901 | 51217 | 32382 | 4744 |
| Age (year) | 36.0 | 36.3 | 36.3 | 36.2 | 36.0 | 36.3 |
| Physical activity (MET-hr) | 22.2 | 20.7 | 21.8 | 18.5 | 23.5 | 30.4 |
| BMI (kg/m2) | 24.5 | 24.5 | 24.0 | 24.8 | 24.2 | 24.0 |
| White (%) | 94.5 | 94.2 | 51.6 | 92.1 | 94.4 | 92.1 |
| Current smoker (%) | 13.4 | 11.9 | 8.6 | 13.0 | 11.1 | 10.1 |
| Postmenopausal (%) | 4.1 | 3.3 | 3.0 | 3.7 | 3.4 | 3.2 |
| Postmenopausal hormone use (%)* | 81.9 | 82.4 | 72.4 | 82.3 | 82.2 | 80.9 |
| Hypertension (%) | 6.2 | 5.7 | 6.1 | 6.3 | 5.5 | 5.1 |
| High cholesterol (%) | 14.3 | 13.7 | 16.3 | 14.6 | 13.7 | 13.3 |
| Family history of diabetes (%) | 15.8 | 15.7 | 18.4 | 16.7 | 14.8 | 14.7 |
| Total energy (kcal/day) | 1633 | 1852 | 2083 | 1737 | 1829 | 2034 |
| Red meat intake (servings/wk) | 4.8 | 5.7 | 5.6 | 5.9 | 5.0 | 3.9 |
| Fruit and vegetable intake (servings/day) | 4.1 | 4.8 | 5.7 | 4.0 | 5.0 | 6.8 |
| White rice (servings/wk) | 0 | 1.0 | 7.8 | 1.1 | 0.9 | 1.2 |
| Brown rice (servings/wk) | 0.6 | 0.4 | 0.5 | 0 | 0.7 | 3.5 |
| Whole grains (g/day) | 23.3 | 20.1 | 16.5 | 15.5 | 24.4 | 46.4 |
| P:S ratio | 0.52 | 0.52 | 0.57 | 0.50 | 0.54 | 0.61 |
| Glycemic load | 121 | 121 | 137 | 120 | 122 | 131 |
| trans fat (% of total energy) | 1.7 | 1.6 | 1.3 | 1.7 | 1.5 | 1.2 |
| Cereal fiber (g/day) | 5.9 | 5.6 | 5.1 | 5.2 | 6.0 | 7.7 |
| Magnesium (mg/day) | 321 | 316 | 316 | 299 | 331 | 385 |
| Alcohol (g/day) | 2.8 | 3.3 | 2.4 | 2.8 | 3.6 | 3.8 |
| Coffee (cups/day) | 1.5 | 1.6 | 1.3 | 1.5 | 1.6 | 1.6 |
| Multivitamin supplement user (%) | 44.6 | 43.1 | 44.6 | 40.9 | 46.9 | 51.5 |
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; MET, metabolic equivalent task; BMI, body mass index; P:S, polyunsaturated to saturated fat ratio.
Current and past use of hormone among postmenopausal women.
TABLE 2 shows the RRs of T2D according to white rice intake. In age-adjusted models, white rice intake was associated with an elevated risk of developing T2D across the three studies. After multivariate adjustment for lifestyle and dietary risk factors, these associations were attenuated, but a trend of increased risk associated with high white rice intake still remained. After summarizing the multivariate estimates across the three studies, in comparison to those in the lowest category of white rice intake, participants who ate white rice ≥5 servings/week had 17% (95% CI: 2%, 36%; P for trend < 0.0001) higher risk of developing T2D.
TABLE 2.
Relative risk (95% CI) of type 2 diabetes risk according to white rice intake in the HPFS, NHS I, and NHS II.
| White rice (servings/time) |
P for trend | |||||
|---|---|---|---|---|---|---|
| <1/mo | 1–3/mo | 1/wk | 2–4/wk | ≥5/wk | ||
| HPFS | ||||||
| Number of cases | 320 | 1150 | 537 | 553 | 88 | |
| Person-year | 101899 | 299985 | 161667 | 120197 | 19172 | |
| Model 1* | 1.0 | 1.12 (0.99, 1.27) | 1.06 (0.92, 1.22) | 1.35 (1.17, 1.55) | 1.37 (1.08, 1.74) | <0.0001 |
| Model 2† | 1.0 | 1.08 (0.95, 1.22) | 1.07 (0.93, 1.23) | 1.30 (1.12, 1.49) | 1.06 (0.81, 1.39) | 0.03 |
| Model 3‡ | 1.0 | 1.09 (0.96, 1.24) | 1.07 (0.93, 1.23) | 1.30 (1.12, 1.50) | 1.02 (0.77, 1.34) | 0.08 |
| NHS I | ||||||
| Number of cases | 415 | 2744 | 1183 | 1062 | 96 | |
| Person-year | 145718 | 691609 | 327215 | 221681 | 18150 | |
| Model 1* | 1.0 | 1.03 (0.93, 1.14) | 1.08 (0.96, 1.20) | 1.12 (1.00, 1.26) | 1.34 (1.07, 1.67) | 0.0005 |
| Model 2† | 1.0 | 1.02 (0.92, 1.14) | 1.13 (1.01, 1.26) | 1.16 (1.03, 1.30) | 1.22 (0.96, 1.56) | 0.0004 |
| Model 3‡ | 1.0 | 1.00 (0.90, 1.11) | 1.07 (0.96, 1.20) | 1.09 (0.97, 1.23) | 1.11 (0.87, 1.43) | 0.02 |
| NHS II | ||||||
| Number of cases | 248 | 924 | 585 | 500 | 102 | |
| Person-year | 134505 | 480405 | 324961 | 237765 | 33267 | |
| Model 1* | 1.0 | 0.82 (0.72, 0.95) | 0.83 (0.71, 0.96) | 0.82 (0.71, 0.96) | 1.27 (1.01, 1.60) | 0.03 |
| Model 2† | 1.0 | 0.93 (0.81, 1.07) | 0.95 (0.82, 1.11) | 0.96 (0.82, 1.12) | 1.43 (1.12, 1.83) | 0.005 |
| Model 3‡ | 1.0 | 0.93 (0.81, 1.07) | 0.94 (0.81, 1.10) | 0.95 (0.81, 1.11) | 1.40 (1.09, 1.80) | 0.01 |
| Pooled | ||||||
| Fixed effects model‡ | 1.0 | 1.01 (0.94, 1.08) | 1.04 (0.96, 1.12) | 1.11 (1.03, 1.20) | 1.17 (1.02, 1.36) | <0.0001 |
| Pheterogeneity | - | 0.26 | 0.38 | 0.02 | 0.20 | 0.87 |
Age-adjusted.
Age (yr), ethnicity (Caucasian, African American, Hispanic, and Asian), BMI (<21 kg/m2, 21–22.9 kg/m2, 23–24.9 kg/m2, 25–26.9 kg/m2, 27–29.9 kg/m2, 30–32.9 kg/m2, 33–34.9 kg/m2, or ≥35 kg/m2), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes/day, 15–24 cigarettes/day, or ≥25 cigarettes/day), alcohol intake (0 g/day, 0.1–4.9 g/day, 5.0–9.9 g/day, 10.0–14.9 g/day, 15.0–29.9 g/day, and ≥30 g/day for men; 0 g/day, 0.1–4.9 g/day, 5.0–9.9 g/day, 10.0–14.9 g/day, ≥15 g/day for women), multivitamin use (yes, no), physical activities (quintiles), family history of diabetes. For women, postmenopausal status and hormone use, and oral conceptive use were further adjusted for.
Based on model 2, model 3 was further adjusted for total energy (kcal/day) and intake of red meat, fruits and vegetables, whole grains, and coffee (all in quintiles).
In contrast to white rice, brown rice intake was associated with a lower risk of T2D in age-adjusted models (TABLE 3). After multivariate adjustment for covariates, these associations were attenuated but the statistical significance remained. When compared to the participants who ate less than one serving per month of brown rice, the pooled RR (95% CI) of T2D was 0.89 (0.81, 0.97) for intake of ≥2 servings/week with a P for trend of 0.005.
TABLE 3.
Relative risk (95% CI) of type 2 diabetes risk according to brown rice in the HPFS, NHS I, and NHS II.
| Brown rice intake (serving/time) |
P for trend | |||
|---|---|---|---|---|
| <1/mo | 1/mo-1/wk | ≥2/wk | ||
| HPFS | ||||
| Number of cases | 1142 | 1296 | 210 | |
| Person-year | 283230 | 358085 | 61606 | |
| Model 1* | 1.0 | 0.84 (0.77, 0.91) | 0.77 (0.67, 0.90) | 0.0002 |
| Model 2† | 1.0 | 0.95 (0.88, 1.03) | 0.96 (0.83, 1.12) | 0.53 |
| Model 3‡ | 1.0 | 0.96 (0.89, 1.04) | 0.96 (0.82, 1.12) | 0.51 |
| NHS I | ||||
| Number of cases | 3127 | 2167 | 206 | |
| Person-year | 785713 | 554634 | 64026 | |
| Model 1* | 1.0 | 0.78 (0.74, 0.83) | 0.63 (0.54, 0.72) | <0.0001 |
| Model 2† | 1.0 | 0.92 (0.87, 0.97) | 0.83 (0.72, 0.96) | 0.001 |
| Model 3‡ | 1.0 | 0.92 (0.87, 0.98) | 0.83 (0.72, 0.96) | 0.003 |
| NHS II | ||||
| Number of cases | 1271 | 941 | 147 | |
| Person-year | 580179 | 537974 | 92750 | |
| Model 1* | 1.0 | 0.71 (0.65, 0.78) | 0.62 (0.52, 0.73) | <0.0001 |
| Model 2† | 1.0 | 0.92 (0.85, 1.01) | 0.88 (0.74, 1.05) | 0.10 |
| Model 3‡ | 1.0 | 0.95 (0.87, 1.04) | 0.89 (0.75, 1.07) | 0.17 |
| Pooled | ||||
| Fixed effects model‡ | 1.0 | 0.94 (0.90, 0.98) | 0.89 (0.81, 0.97) | 0.005 |
| Pheterogeneity | - | 0.77 | 0.45 | 0.21 |
We observed a monotonically decreasing risk of diabetes associated with increasing consumption of whole grains, which include brown rice (TABLE 4). In comparison to the lowest quintile, the pooled RR (95% CI) for the highest quintile of whole grains was 0.73 (0.68, 0.78; P for trend < 0.0001). We further estimated the RRs of T2D associated with bran and germ intake (Table 4). Bran intake, but not germ intake, was associated with a lower risk of developing T2D. In comparison to those in the lowest quintile of bran intake, men and women in the highest quintile had a pooled RR (95% CI) of 0.76 (0.71, 0.82; P for trend < 0.0001). For germ intake, the corresponding RR was 0.95 (0.88, 1.03; P for trend = 0.40).
TABLE 4.
Relative risk (95% CI) of type 2 diabetes risk according to whole grain, as well as bran or germ intake in the HPFS, NHS I, and NHS II.
| Intake quintiles |
P for trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Whole grain | ||||||
| HPFS | ||||||
| Intake levels* | 5.1 (2.7–7.2) | 12.6 (10.4–14.9) | 20.4 (17.9–22.7) | 29.9 (27.0–32.9) | 47.1 (41.1–57.6) | |
| Number of cases | 667 | 574 | 559 | 475 | 373 | |
| Person-year | 132594 | 142608 | 143546 | 144486 | 139687 | |
| RR (95% CI)† | 1.0 | 0.82 (0.73, 0.92) | 0.86 (0.77, 0.97) | 0.78 (0.69, 0.88) | 0.72 (0.63, 0.83) | <0.0001 |
| NHS I | ||||||
| Intake levels* | 3.6 (2.2–5.0) | 8.2 (6.7–10.0) | 13.0 (11.2–15.1) | 19.4 (17.0–21.5) | 31.3 (27.0–38.3) | |
| Number of cases | 1346 | 1246 | 1158 | 971 | 779 | |
| Person-year | 271378 | 281807 | 283347 | 285978 | 281864 | |
| RR (95% CI)† | 1.0 | 0.89 (0.83, 0.97) | 0.85 (0.79, 0.92) | 0.76 (0.70, 0.83) | 0.70 (0.64, 0.77) | <0.0001 |
| NHS II | ||||||
| Intake levels* | 6.2 (4.0–8.0) | 12.6 (11.0–14.3) | 18.8 (16.9–20.4) | 26.2 (23.9–28.6) | 40.0 (35.1–48.4) | |
| Number of cases | 676 | 524 | 462 | 377 | 320 | |
| Person-year | 236653 | 241524 | 243592 | 244875 | 244259 | |
| RR (95% CI)† | 1.0 | 0.89 (0.80, 1.00) | 0.89 (0.79, 1.00) | 0.81 (0.71, 0.92) | 0.81 (0.70, 0.94) | 0.002 |
| Pooled RR (95% CI) | ||||||
| Fixed effects model† | 1.0 | 0.87 (0.83, 0.93) | 0.86 (0.81, 0.91) | 0.78 (0.73, 0.83) | 0.73 (0.68, 0.78) | <0.0001 |
| Pheterogeneity | - | 0.41 | 0.86 | 0.78 | 0.23 | 0.01 |
| Bran | ||||||
| HPFS | ||||||
| Intake levels* | 0.6 (0.3–1.0) | 2.1 (1.5–2.8) | 4.3 (3.3–5.3) | 7.5 (6.2–8.8) | 14.3 (11.8–18.6) | |
| Number of cases | 634 | 616 | 546 | 480 | 372 | |
| Person-year | 133254 | 144206 | 143049 | 142852 | 139558 | |
| RR (95% CI)† | 1.0 | 0.90 (0.79, 1.01) | 0.84 (0.74, 0.96) | 0.79 (0.68, 0.91) | 0.69 (0.60, 0.81) | <0.0001 |
| NHS I | ||||||
| Intake levels* | 0.6 (0.3–0.9) | 1.5 (1.1–2.1) | 2.8 (2.1–3.6) | 4.8 (3.9–5.8) | 9.5 (7.7–12.6) | |
| Number of cases | 1278 | 1262 | 1182 | 1009 | 769 | |
| Person-year | 270994 | 278327 | 284764 | 285877 | 284412 | |
| RR (95% CI)† | 1.0 | 0.96 (0.88, 1.04) | 0.92 (0.84, 1.01) | 0.85 (0.77, 0.94) | 0.77 (0.69, 0.86) | <0.0001 |
| NHS II | ||||||
| Intake levels* | 1.0 (0.7–1.4) | 2.6 (2.2–3.0) | 4.3 (3.8–4.8) | 6.7 (5.9–7.5) | 12.1 (10.0–15.9) | |
| Number of cases | 667 | 512 | 446 | 393 | 341 | |
| Person-year | 234309 | 244811 | 242569 | 245347 | 243867 | |
| RR (95% CI)† | 1.0 | 0.87 (0.77, 0.98) | 0.84 (0.74, 0.96) | 0.85 (0.74, 0.98) | 0.83 (0.71, 0.97) | 0.07 |
| Pooled RR (95% CI) | ||||||
| Fixed effects model† | 1.0 | 0.92 (0.87, 0.98) | 0.88 (0.82, 0.94) | 0.84 (0.78, 0.90) | 0.76 (0.71, 0.82) | <0.0001 |
| Pheterogeneity | - | 0.39 | 0.43 | 0.68 | 0.28 | 0.17 |
| Germ | ||||||
| HPFS | ||||||
| Intake levels* | 0.2 (0.1–0.3) | 0.5 (0.4–0.6) | 0.8 (0.7, 0.9) | 1.2 (1.1–1.4) | 2.3 (1.9–3.4) | |
| Number of cases | 577 | 592 | 563 | 506 | 410 | |
| Person-year | 129975 | 147061 | 141461 | 145060 | 139362 | |
| RR (95% CI)† | 1.0 | 1.00 (0.88, 1.13) | 1.06 (0.93, 1.21) | 1.04 (0.90, 1.20) | 1.04 (0.89, 1.21) | 0.65 |
| NHS I | ||||||
| Intake levels* | 0.2 (0.1–0.2) | 0.3 (0.3–0.4) | 0.6 (0.5–0.6) | 0.8 (0.7–0.9) | 1.5 (1.3–2.1) | |
| Number of cases | 1224 | 1163 | 1237 | 1038 | 838 | |
| Person-year | 256119 | 295315 | 287868 | 288588 | 276483 | |
| RR (95% CI)† | 1.0 | 0.93 (0.85, 1.02) | 0.99 (0.90, 1.09) | 0.92 (0.83, 1.02) | 0.88 (0.79, 0.97) | 0.02 |
| NHS II | ||||||
| Intake levels* | 0.3 (0.2–0.4) | 0.6 (0.5–0.7) | 0.9 (0.8–1.0) | 1.2 (1.1–1.4) | 2.0 (1.7–2.4) | |
| Number of cases | 617 | 464 | 484 | 391 | 403 | |
| Person-year | 234430 | 245739 | 241509 | 245747 | 243479 | |
| RR (95% CI)† | 1.0 | 0.93 (0.82, 1.05) | 1.04 (0.91, 1.19) | 0.90 (0.78, 1.04) | 1.04 (0.90, 1.21) | 0.56 |
| Pooled RR (95% CI) | ||||||
| Fixed effects model† | 1.0 | 0.95 (0.89, 1.01) | 1.02 (0.95, 1.09) | 0.94 (0.88, 1.01) | 0.95 (0.88, 1.03) | 0.40 |
| Pheterogeneity | - | 0.65 | 0.67 | 0.29 | 0.09 | 0.06 |
Median (interquartile range)
Adjusted for the same set of covariates for model 3 in Table 3. Bran and germ intake were mutually adjusted in the analysis.
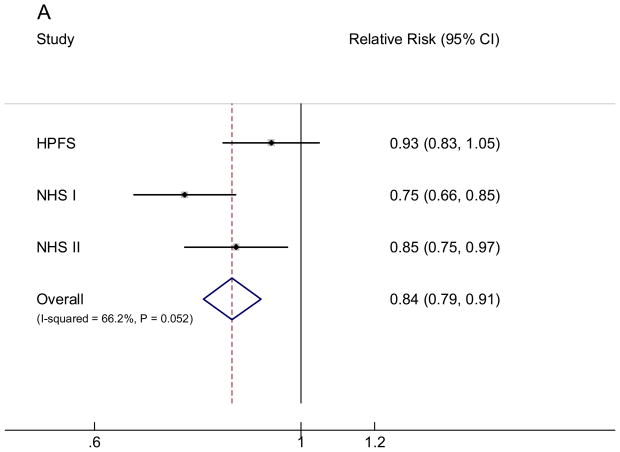
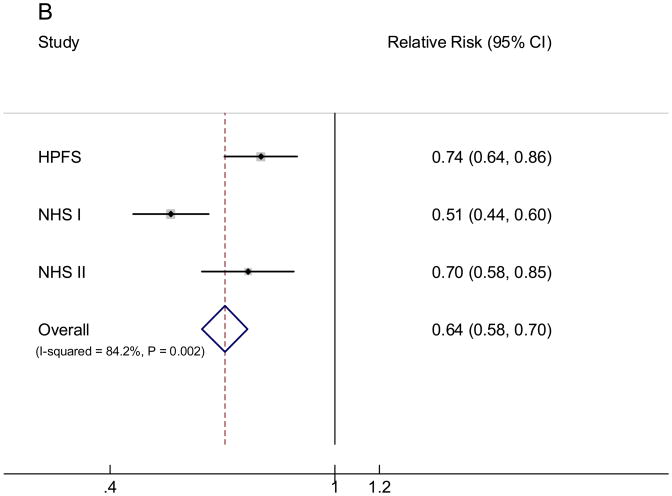
We subsequently examined the RR associated with the replacement of 50 grams/day (⅓ serving/day) of white rice with the same amount of brown rice intake. In all three cohorts, substituting brown rice for white rice was consistently associated with a lower risk of T2D (FIGURE). In the pooled analysis, each 50 grams/day intake of brown rice replacing white rice was associated with an RR (95% CI) of 0.84 (0.79, 0.91). We further examined the RR associated with replacing 50 grams/day of white rice intake with the same amount of whole grains: the RR (95% CI) was 0.64 (0.58, 0.70).
FIGURE.
Pooled fixed effects relative risk (95% CI) of type 2 diabetes of substituting 50 grams/day brown rice (A) or whole grains (B) intake for the same amount of white rice intake. Bars indicate 95% CIs and P values are P for heterogeneity. Individual associations were controlled for the same set of covariates for model 3 in Table 3.
Since ethnicity was associated with both white rice consumption and diabetes risk,28 the observed associations can be a consequence of confounding by ethnicity. However, in secondary analyses, when we repeated these associations among white participants only, we found similar results. For example, after excluding non-white participants (African American, Hispanic, and Asian), the pooled RRs (95% CI) of T2D associated with white rice intake were 1.00 (0.93, 1.07) for 1–3 servings/month, 1.04 (0.96, 1.13) for 1 serving/week, 1.10 (1.01, 1.19) for 2–4 servings/week, and 1.19 (1.00, 1.41; P for trend < 0.0001) for ≥5 servings/week. The pooled RRs (95% CI) for brown rice intake levels were 0.94 (0.90, 0.99) for 1–4 servings/month and 0.87 (0.79, 0.96; P for trend = 0.003) for ≥ 2 servings/week. Among white participants, the pooled RRs (95% CI) of T2D were 0.84 (0.78, 0.92; P < 0.0001) for replacing 50 grams/day of white rice intake with the same amount of brown rice, and 0.64 (0.58, 0.71; P < 0.0001) for substituting 50 grams/day of whole grains for the same amount of white rice. When we restricted our analysis within minority groups only, although we observed largely similar results, most associations became non-significant due to the dramatically diminished power (we identified 624 T2D among 9,644 non-white participants).
When we used more recent intake of white rice or brown rice instead of the cumulative average in the analyses, the results did not substantially change. For example, the pooled RR (95% CI) was 1.25 (1.08, 1.45; P for trend = 0.001) for ≥2 servings/week vs. <1 serving/month of white rice intake, 0.90 (0.85, 0.95; P < 0.0001) for replacing 50 grams/day of white rice intake with the same amount of brown rice, and 0.76 (0.71, 0.82; P < 0.0001) for substituting 50 grams/day of whole grains for the same amount of white rice. Because rice consumption for most of our study participants was relatively stable over time (data not shown), these results indicated that the cumulative averages could better represent long-term rice consumption because of reduced random within-person measurement errors.24 Lastly, we did not find any interactions between rice consumption and other diabetes risk factors, including age, BMI, and various co-morbidities.
COMMENT
In these three prospective cohort studies of U.S. men and women, we found that regular consumption of white rice was associated with higher risk of T2D, whereas brown rice intake was associated with lower risk. In addition, our data suggest that replacing white rice intake with the same amount of brown rice or whole grains was associated with a lower risk. These associations were independent of lifestyle and dietary risk factors for T2D, as well as ethnicity.
In Asian populations in whom rice is a staple food, higher white rice consumption has been associated with elevated risk of diabetes or metabolic syndrome.6, 29, 30 For example, white rice consumption was prospectively associated with developing type 2 diabetes in Chinese women living in Shanghai.6 In addition, in Asian Indians and Japanese, higher intake of refined grain including white rice was associated with metabolic risks in cross-sectional analyses.29, 30 In comparison to Asian populations, white rice intake in Western populations was much lower. White rice consumption contributed, on average, less than 2% of total energy intake in our study populations. In contrast, in the aforementioned Chinese female population, white rice consumption accounted for 53.7% of total energy intake (Xiao-Ou Shu, personal communication).6 Likewise, according to the Japanese National Nutrition Survey, white rice accounted for 29% of daily total energy intake in Japanese.31 Consumption of rice or food groups consisting of rice in relation to risk of type 2 diabetes was also evaluated in Western populations, but mixed results were observed.32–34 However, brown rice was not separated from white rice or other refined grains in these studies 32–34. To our knowledge, the current studies are the first prospective investigations conducted among Western populations that have specifically evaluated white rice and brown rice intake in relation to T2D risk. In our cohorts only 0.9% (NHS I) to 2.2% (NHS II) of total participants reported having five or more servings per week (≥107 grams/day) of white rice, which was within the lowest reference level (<200 grams/day) in the prospective study of Chinese women.6 However, by pooling data from the three studies, we detected a significant association for white rice intake. Our data are consistent with the Chinese study, in which white rice intake of 300 grams/day or more (equivalent to 2 servings/day in our analysis) was associated with a 78% increased risk of T2D in comparison to intake levels of less than 200 grams/day.6
We observed a moderate, inverse association for brown rice intake. Because brown consumption levels were rather low in our participants, we could not determine whether brown rice intake at much higher levels is associated with a further reduction of diabetes risk. Nonetheless, we found that substitution of brown rice for white rice was associated with a significantly lower risk of developing diabetes. Consistent with our previous analyses,9, 10 we found a significant inverse association between whole grain consumption and diabetes risk. Substitution of whole grains for white rice was more strongly associated with diabetes risk than the substitution of brown rice. This observation may result from the more reliable estimates of the association with diabetes for whole grains than those for brown rice, because of the low overall consumption of brown rice. In addition, whole grains included multiple grains with various nutrient compositions and, thus, possibly various effects on glucose response. For example, whole wheat and barley generate lower glucose response than brown rice: the GI values were 41 ± 3 for whole wheat, 25 ± 1 for barley, and 55 ± 5 for brown rice.2 As a consequence, in comparison to whole wheat and barley, the same amount of brown rice likely bears a higher glycemic load, which is an established risk factor for T2D.35
Depending on the botanical structure, amylase contents, and processing methods, both white rice and brown rice demonstrated a wide variety of GI values,2, 36–38 which made it difficult to directly compare white rice with brown rice for effects on postprandial glucose response.2 Despite of this inconsistency inherent to rice GI values, in general, white rice consumption generates a relatively stronger postprandial glucose response than the same amount of brown rice2 This notion was corroborated by the observation that isocaloric replacement of white rice with whole grains (66.6%; primarily composed of brown rice and barley) and legume powder (22.2%) significantly decreased postprandial glucose and insulin levels in a randomized clinical trial.39 The high GI of white rice consumption is likely the consequence of disrupting the physical and botanical structure of rice grains during the refining process, in which almost all of the bran and some of the germ are removed.40 The other consequence of the refining process includes loss of fiber, vitamins, magnesium and other minerals, lignans, phytoestrogens, and phytic acid,7 many of which may be protective factors for diabetes risk. Intact rice grains contain nearly exclusively insoluble fiber.7 In both observational and experimental studies, insoluble fiber intake was consistently associated with improved insulin sensitivity and decreased risk of developing T2D.4, 5, 41, 42 In addition, higher magnesium intake has been consistently associated with reduced risk of T2D in cohort studies or improved glucose metabolism in clinical trials.43–45 The combination of these mechanisms may explain the beneficial effects of replacing white rice with brown rice or other whole grains.
The strengths of the current study include a large sample size, high rates of follow-up, and repeated assessments of dietary and lifestyle information. The consistency of the results across all three cohorts indicates that our findings are unlikely due to chance. The current study was subject to a few limitations as well. First, our study populations primarily consisted of working health professionals with European ancestry. Although the homogeneity of socioeconomic status helps reduce confounding, the generalizability of the observed associations may be limited to similar populations. However, the biological mechanisms underlying the positive associations observed in both our study populations and the Chinese study6 are likely to be the same in other populations. Second, because diet was assessed by FFQs, some measurement error of rice intake assessment is inevitable. However, the FFQs used in these studies were validated against multiple diet records, and reasonable correlation coefficients between these assessments of rice intake were observed.14 Since we employed a prospective study design, any measurement errors of rice intake are independent of study outcome ascertainment, and, therefore, are likely to attenuate the associations towards the null. Moreover, we calculated cumulative averages of rice intake to minimize the random measurement errors caused by within-person variation. To minimize the possibility of systemic measurement error incurred by recall bias, we not only excluded participants with a history of major chronic diseases at baseline but also stopped updating dietary intake after participants reported having diagnoses of diseases that might influence their subsequent report of diet. Third, we did not perform oral glucose tolerance tests to confirm diabetes diagnoses because this is infeasible in large cohort studies. However, the supplementary questionnaire that we used for the confirmation of self-reported diabetes diagnoses has been demonstrated to be highly accurate.20, 21 Lastly, although we adjusted for established and potential risk factors for T2D, residual confounding is still possible.
Our data suggest that regular consumption of white rice is associated with an increased risk of T2D, whereas replacement of white rice by brown rice or other whole grains is associated with a lower risk. The current Dietary Guidelines for Americans identifies grains, including rice, as one of primary sources for carbohydrate intake and recommends at least half of carbohydrate intake should come from whole grains.46 Rice consumption in the U.S. population is increasing.8 However, most rice consumption is refined white rice,8 as seen in our studies. From a public health point of view, replacing refined grains such as white rice by whole grains, including brown rice, should be recommended to facilitate the prevention of type 2 diabetes.
Acknowledgments
Supported by research grants CA87969, CA055075, CA050385, and DK58845 from the National Institutes of Health. Dr. Sun is supported by a Postdoctoral Fellowship from the Unilever Corporate Research. Dr. Frank B. Hu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure:
None of the authors had any financial or personal conflict of interest to disclose.
References
- 1.Miller G, Prakash A, Decker E. Whole-grain foods in health and disease. St. Paul, MN: American Association of Cereal Chemists; 2002. [Google Scholar]
- 2.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002 Jul;76(1):5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004 Aug;80(2):348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 4.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997 Apr;20(4):545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 5.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. Jama. 1997 Feb 12;277(6):472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 6.Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Shu XO. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007 Nov 26;167(21):2310–2316. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- 7.Slavin JL, Martini MC, Jacobs DR, Jr, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. 1999 Sep;70(3 Suppl):459S–463S. doi: 10.1093/ajcn/70.3.459s. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed 16 June 2009.];Economic Research Service, United States Department of Agriculture. Available: http://www.ers.usda.gov/Data/
- 9.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007 Aug;4(8):e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002 Sep;76(3):535–540. doi: 10.1093/ajcn/76.3.535. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994 Oct;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 12.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. Am J Nurs. 1978 Jun;78(6):1039–1040. [PubMed] [Google Scholar]
- 13.Rimm EB, Stampfer MJ, Colditz GA, Giovannucci E, Willett WC. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol. 1990 Jun;131(6):1068–1071. doi: 10.1093/oxfordjournals.aje.a115598. [DOI] [PubMed] [Google Scholar]
- 14.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993 Jul;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989 Dec;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979 Dec;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 19.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991 Sep 28;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001 Jun 25;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 22.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984 May;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR, Oakes D. Analysis of survival data. London: Chapman and Hall; 1984. [Google Scholar]
- 24.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999 Mar 15;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009 Apr;32(4):629–634. doi: 10.2337/dc08-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006 Feb;83(2):284–290. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006 Jul;29(7):1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 29.Murakami K, Sasaki S, Takahashi Y, Okubo H, Hosoi Y, Horiguchi H, Oguma E, Kayama F. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr. 2006 May;83(5):1161–1169. doi: 10.1093/ajcn/83.5.1161. [DOI] [PubMed] [Google Scholar]
- 30.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57) Metabolism. 2009 May;58(5):675–681. doi: 10.1016/j.metabol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama M, Tang AC, Wakaki Y, Koyama W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr. 2003 Jun;57(6):743–752. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 32.Williams DE, Wareham NJ, Cox BD, Byrne CD, Hales CN, Day NE. Frequent salad vegetable consumption is associated with a reduction in the risk of diabetes mellitus. J Clin Epidemiol. 1999 Apr;52(4):329–335. doi: 10.1016/s0895-4356(99)00006-2. [DOI] [PubMed] [Google Scholar]
- 33.Hodge AM, English DR, O’Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004 Nov;27(11):2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Manson JE, Stampfer MJ, Hu FB, Giovannucci E, Colditz GA, Hennekens CH, Willett WC. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. 2000 Sep;90(9):1409–1415. doi: 10.2105/ajph.90.9.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008 Mar;87(3):627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 36.Larsen HN, Christensen C, Rasmussen OW, Tetens IH, Choudhury NH, Thilsted SH, Hermansen K. Influence of parboiling and physico-chemical characteristics of rice on the glycaemic index in non-insulin-dependent diabetic subjects. Eur J Clin Nutr. 1996 Jan;50(1):22–27. [PubMed] [Google Scholar]
- 37.Larsen HN, Rasmussen OW, Rasmussen PH, Alstrup KK, Biswas SK, Tetens I, Thilsted SH, Hermansen K. Glycaemic index of parboiled rice depends on the severity of processing: study in type 2 diabetic subjects. Eur J Clin Nutr. 2000 May;54(5):380–385. doi: 10.1038/sj.ejcn.1600969. [DOI] [PubMed] [Google Scholar]
- 38.Miller JB, Pang E, Bramall L. Rice: a high or low glycemic index food? Am J Clin Nutr. 1992 Dec;56(6):1034–1036. doi: 10.1093/ajcn/56.6.1034. [DOI] [PubMed] [Google Scholar]
- 39.Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol. 2001 Dec;21(12):2065–2071. doi: 10.1161/hq1201.100258. [DOI] [PubMed] [Google Scholar]
- 40.Bjorck I, Granfeldt Y, Liljeberg H, Tovar J, Asp NG. Food properties affecting the digestion and absorption of carbohydrates. Am J Clin Nutr. 1994 Mar;59(3 Suppl):699S–705S. doi: 10.1093/ajcn/59.3.699S. [DOI] [PubMed] [Google Scholar]
- 41.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000 Apr;71(4):921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 42.Weickert MO, Mohlig M, Schofl C, Arafat AM, Otto B, Viehoff H, Koebnick C, Kohl A, Spranger J, Pfeiffer AF. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006 Apr;29(4):775–780. doi: 10.2337/diacare.29.04.06.dc05-2374. [DOI] [PubMed] [Google Scholar]
- 43.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care. 2006 Oct;29(10):2238–2243. doi: 10.2337/dc06-1014. [DOI] [PubMed] [Google Scholar]
- 44.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007 May 14;167(9):956–965. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 45.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006 Oct;23(10):1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 46.U.S. Department of Health and Human Services and U.S. Department of Agriculture. [Accessed 16 June 2009.];Dietary guidlines for Americans. (6). 2005 Available: http://www.health.gov/dietaryguidelines/dga2005/document/