Abstract
Background
Physical activity is associated with reduced risks of chronic diseases and premature death. Whether physical activity is also associated with improved overall health among those who survive to older ages is unclear.
Methods
A total of 13,535 Nurses’ Health Study participants who were free of major chronic diseases at baseline in 1986 and had survived to age 70 years or older as of 1995–2001 comprised the study population. We defined successful survival as no history of 11 major chronic diseases and no cognitive impairment, physical impairment, or mental health limitations.
Results
After multivariate adjustment for covariates, higher physical activity levels at mid-life, as measured by metabolic equivalent tasks, were significantly associated with better odds of successful survival. Significant increases in successful survival were observed beginning at the third quintile of activity: Odds ratios (95% confidence interval) in the lowest to highest quintiles were 1.00 (reference), 0.98 (0.80, 1.20), 1.37 (1.13, 1.65), 1.34 (1.11, 1.61), and 1.99 (1.66, 2.38; P for trend < 0.0001). Increasing energy expenditure from walking was associated with a similar elevation in odds of successful survival: The odds ratios (95% confidence interval) of successful survival across quintiles of walking were 1.00 (reference), 0.99 (0.80, 1.21), 1.19 (0.97, 1.45), 1.50 (1.24, 1.82), and 1.47 (1.22, 1.79; P for trend < 0.0001).
Conclusion
These data provide evidence that higher levels of mid-life physical activity are associated with exceptional health status among women who survive to older ages, and corroborate the potential role of physical activity in improving overall health.
The past century has witnessed a dramatic increase in life expectancy in the United States, from 47.3 years in 1900 to 75.2 years for men and 80.4 years for women in 2005.1 Together with a decreased birth rate and the aging of baby boomers, it is projected that by 2030 one in every five Americans will be aged 65 years or older.2 Older adults are disproportionately affected by chronic diseases and functional disabilities, and the attendant medical and social costs are tremendous.2 However, development of chronic diseases and disabilities is not inevitable among aged populations. Several studies have demonstrated that as many as 10%–50% of those 65 years or older can maintain physical and cognitive integrity and remain free of major chronic illnesses.3–6 Indeed, limited epidemiologic studies conducted primarily among older male populations have identified several modifiable mid-life risk factors, such as smoking and obesity, associated with the probability of exceptional health among those who survive to older ages.3–5, 7
Physical activity is a well established approach to reducing risks of many chronic diseases,8–12 and potentially other aspects of health.13–19 However, findings from limited existing studies of the relation between mid-life physical activity and overall health and well-being at older ages have been inconsistent. For example, several studies among men and women found that physical activity increased healthy aging,3, 7 disability-free survival,20 or self-reported physical and overall health,21 while a more recent study of Japanese American men reported a null association with successful survival.5 Moreover, evidence specifically among women is lacking, despite the fact that women live longer than men and, thus, identifying risk factors for successful survival is particularly important among women. Finally, limited research has addressed the dose-response relationship and intensity of activities in relation to successful survival.
We, therefore, utilized the Nurses’ Health Study (NHS)22 data to further explore the relation between mid-life physical activity, including walking, and successful aging as measured by a full spectrum of health outcomes, including incidence of chronic diseases, cognitive and physical functioning, and mental status.23
METHODS
Study Population
The NHS is an ongoing prospective cohort study initially comprising 121,700 female registered nurses aged 30–55 years who responded to a baseline questionnaire in 1976. Follow-up questionnaires have been administered to the participants every two years since 1976 to collect and update the information on incidence of diseases and demographic and lifestyle risk factors. In 1986, we started collecting detailed information on physical activity. Through 2000, the close of follow-up for the majority of participants in the present analyses, the follow-up rate was greater than 95%.
Assessment of Physical Activity
In 1986, we inquired about the average time per week in the past year spent on leisure-time physical activities including walking or hiking outdoors, jogging (≥10 min/mile), running (<10 min/mile), bicycling, lap swimming, tennis, calisthenics/aerobics/aerobic dance/rowing machine, and squash or racquet ball. For each question, there were 10 possible response-categories, ranging from “zero” to “11+ hours/week”. Furthermore, we inquired about flights of stairs climbed each day, and, for walkers, the usual walking pace: easy or casual (< 2.0 mph), normal (2.0–2.9 mph), brisk (3–3.9 mph), and very brisk (4 mph or faster). Based on this information, we calculated energy expenditure in metabolic equivalent tasks (METs) measured in hours per week.24 Each MET-hour is the caloric need per kilogram of body weight per hour of activity, divided by the caloric need per kilogram of weight per hour at rest. According to this standard, we assigned a MET value of 12 to running; 8 to stair-climbing; 7 to jogging, bicycling, lap-swimming, and tennis/racquet sports; 6 to aerobics/calisthenics; and 2.5–4.5 to walking depending on the pace. In other words, for example, running for an hour will generate 12 METs’ energy expenditure, climbing stairs for an hour will generate 8 METs, and so on. The same amount of energy expenditure can be achieved by various physical activities. For example, to achieve 30 METs/week, a woman can run for 2.5 hours/week or swim for 4.3 hours/week. In analyses of activity intensity, we defined activity with a MET value larger than 6 or more as vigorous; walking was defined as a moderate-intensity activity due to the lower MET value.
For the current analysis, we used 1986, when detailed physical activity information was first obtained, as the study baseline. Moreover, in all analyses, we only considered physical activity reported in 1986, since we wanted to minimize the possibility of reverse causation with aging (i.e., poor underlying health status caused decreased physical activity rather than the opposite). At baseline in 1986, the mean age was 60 for our study participants and, therefore, mid-life was defined as age 60 for the purposes of this report.
The physical activity questionnaire has been validated in a similar population (the NHS II).25 In a representative sample of 147 nurses, the physical activity scores based on this questionnaire administered 2 years apart were reasonably correlated, given some true changes in activity across two years; the test-retest correlation coefficient (r) was 0.59. The questionnaire estimate of physical activity levels was highly correlated with those reported in 1-week recalls (r = 0.79) and those logged in diaries during the year (r = 0.62).
Ascertainment of Chronic Diseases
A wide variety of major chronic diseases (i.e., cancer, diabetes, coronary heart disease, stroke, Parkinson’s disease, and multiple sclerosis) were reported by participants in 1976 and in biennial follow-up questionnaires. The self-reports were confirmed by study physicians through a variety of methods, such as medical record review, pathology report review, telephone interview, or supplementary questionnaire inquiries. The self-report of incidence of chronic diseases among these nurses has been previously demonstrated to be highly valid.26–29
Assessment of Physical Function and Mental Health
In 1992, 1996, and 2000, we added the Medical Outcomes SF-36 Health Status Survey to the follow-up questionnaires to assess the physical and mental status of the participants. The SF-36 is a 36-item questionnaire that measures eight health concepts including limitations of physical activities, usual role activities, social activities, mental health, bodily pain, vitality, and general health perceptions. The validity and reproducibility of the SF-36 have been extensively examined and reported elsewhere.30
Assessment of Cognitive Function
In 1995–2001, we invited all nurses who were free of stroke and reached age 70 years or older to a cognitive function study. Of 21,202 invited nurses, 19,415 (92%) agreed to participate and were administered the Telephone Interview for Cognitive Status (TICS),31 which is modeled on the Mini-Mental State Examination.32 Scores on the TICS have a range of 0 (worst) to 41 (perfect), with a score lower than 31 indicating cognitive impairment.31 The high test-retest reliability and validity of TICS compared to in-person cognitive testing have been demonstrated previously.32 Trained study nurses who were unaware of the study hypothesis and exposure status of the participants administered the TICS with high inter-interviewer reliability.31 The current analysis was conducted within these participants of the cognitive study, due to the availability of cognitive data in this group.
Definition and Ascertainment of Successful Aging
To evaluate the overall health status of the study participants, we employed the concept of successful aging raised by Rowe and Kahn, which takes into account both comorbidities and disabilities.23 The working definition of successful aging has been introduced in detail elsewhere.33 Briefly, our definition of successful aging addressed four domains: 1) no history of 11 major chronic diseases, including cancer (except nonmelanoma skin cancer), diabetes, myocardial infarction, coronary artery bypass graft surgery, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis; 2) no impairment in cognitive function (TICS score ≥ 31); 3) no physical disabilities (no limitations on moderate activities and no more than moderate limitations on more demanding physical performance measures); and 4) no mental health limitations (mental health score > 84, which is the median score in our study population). Any participant who survived to at least age 70 years and met all these criteria was defined as a “successful survivor”; the remaining participants who survived to at least aged 70 years but had a chronic disease history, cognitive impairment, physical or mental health limitations were defined as “usual survivors”. Since the cognitive function of the majority of study participants was assessed in 1999–2000 (87.5%), we used the year 2000 to define chronic disease status. Similarly, physical and mental health domains were primarily derived from the SF-36 administered in 2000.
We excluded nurses who had a history of any of the 11 aforementioned chronic diseases at baseline (n = 2,361), or who had missing physical activity data at baseline (n = 2,724). We further excluded those who skipped more than 2 items on the mental health scale at age 70 years or older, or more than 5 items on the physical function scale in the SF-36 (n = 795). After these participants were excluded, data from 13,535 nurses were available for analysis. All participants gave informed consent. The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital.
Statistical Methods
We grouped the study participants into quintiles of total METs. We used logistic regression to assess the odds ratios (OR) of successful survival versus usual survival associated with each quintile, with the lowest quintile as the reference level. In multivariate logistic regression models, we adjusted for variables defined in 1986, including age at baseline (yr), education (registered nurse, bachelor, master, or doctorate degree), husband’s education (less than high school, some high school, high school graduate, college graduate, or graduate school), marital status (unmarried, married, widow, separated, or divorced), postmenopausal hormone use (never used, past user, or current user), smoking status (never smoked, past smoker, current smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d), family history of heart disease/diabetes/cancer (yes, no), polyunsaturated to saturated fat ratio (in quintiles), intakes of trans fat, alcohol, and cereal fiber (all in quintiles), and intakes of fruits and vegetables and red meat (in tertiles). Since moderate-intensity physical activity, such as walking, was associated with lower risk of chronic diseases in our previous studies,8, 10 we further examined walking METs and pace in relation to successful survival in the current study. When examining the associations for walking MET quintiles, we further adjusted for vigorous activity METs to minimize potential confounding by vigorous physical activity. Similarly, when we examined the associations for walking pace, we further controlled for total METs.
Tests of linear trend across increasing MET quintiles were conducted by treating the quintiles as a continuous variable and assigning the median score for each quintile as its value. All P values were two-sided. Ninety-five percent confidence intervals (95% CI) were calculated for ORs. Data were analyzed with the Statistical Analysis Systemssoftware package, version 9.1 (SAS Institute, Inc., Cary, NC).
Sensitivity Analyses
We performed three secondary sensitivity analyses to examine the robustness of observed associations. First, although we excluded anyone with major chronic diseases at baseline, and imposed an average 14 year lag period between the assessment of activity levels and the assessment of successful survival, it is still possible that long-term physical disabilities at baseline may bias the current analysis. To address this issue, rather than comparing women with the least and the most activity, we repeated the analysis only within participants who reported having at least a minimum level of activity, which we defined as walking at least 1 hour per week or performing any vigorous activity at least 20 minutes per week at baseline. Second, to best address the independent effects of walking as exercise, we estimated the ORs associated with walking METs after excluding women who both walked and participated in vigorous activity. Finally, to examine the robustness of the current definition of successful aging, we repeated the analysis using an alternative definition that included the same criterion for chronic disease status, but used median score to define the cut-points for cognitive, physical, and mental health domains.33 We conducted this analysis because, while the domains we utilized for considering successful survival are widely accepted, the specific criteria for defining “successful” within each domain is less established.
RESULTS
Primary Analysis
Of the total of 13,535 participants, 1,456 (10.8%) met the criteria of successful survivor. Table 1 shows the characteristics of the participants in 1986. As expected, successful survivors were more active than usual survivors. The successful survivors were also leaner and less likely to smoke than usual survivors, and had a slightly lower prevalence of hypertension or high cholesterol.
Table 1.
Baseline characteristics of successful survivors and usual survivors in the Nurses’ Health Study
| Characteristicsa | Successful survivor (n = 1,456) | Usual survivors (n = 12,079) | P valueb |
|---|---|---|---|
| Age at baseline (year) | 60.1±2.5 | 60.6±2.5 | <0.0001 |
| Age at cognitive function assessment (year) | 73.7±2.1 | 74.2±2.3 | <0.0001 |
| Physical activity (METs, hr/wk) | 19.1±22.0 | 14.1±19.7 | <0.0001 |
| Walking activity (METs, hr/wk) | 9.5±11.5 | 7.2±9.7 | <0.0001 |
| BMI (kg/m2) | 23.8±3.3 | 25.5±4.4 | <0.0001 |
| Waist circumference (cm) | 76.2±8.2 | 80.6±10.3 | <0.0001 |
| Waist-to-hip ratio | 0.78±0.08 | 0.79±0.07 | <0.0001 |
| Saturated fat (g/d) | 12.5±2.5 | 12.9±2.4 | <0.0001 |
| Polyunsaturated fat (g/d) | 5.8±1.3 | 5.9±1.3 | 0.003 |
| Polyunsaturated to saturated fat ratio | 0.50±0.15 | 0.50±0.14 | 0.07 |
| Trans fat (g/d) | 1.8±0.6 | 1.9±0.5 | <0.0001 |
| Alcohol intake (g/d) | 7.1±9.9 | 6.7±9.9 | 0.15 |
| Cereal fiber (g/d) | 4.2±2.1 | 4.0±2.0 | 0.004 |
| Red meat (serving/d) | 1.0±0.5 | 1.1±0.5 | <0.0001 |
| Fruits and vegetables (serving/d) | 5.4±2.0 | 5.2±2.0 | <0.0001 |
| Smoking status(%)c | <0.0001 | ||
| Never smoked | 53.5 | 46.4 | |
| Past smoker | 34.8 | 36.2 | |
| Current smoke 1–14 cigarettes/d | 5.7 | 6.3 | |
| Current smoke 15–24 cigarettes/d | 4.3 | 6.9 | |
| Current smoke ≥25 cigarettes/d | 1.7 | 4.2 | |
| Education (%) | <0.0001 | ||
| Registered nurse | 74.0 | 79.0 | |
| Bachelor | 17.3 | 14.9 | |
| Master | 8.0 | 5.8 | |
| Doctorate | 0.7 | 0.4 | |
| Husband’s education (%)c | 0.009 | ||
| Less than high school | 2.2 | 2.6 | |
| Some high school | 5.4 | 6.4 | |
| High school graduate | 39.4 | 42.9 | |
| College graduate | 28.7 | 27.6 | |
| Graduate school | 24.2 | 20.5 | |
| Marriage status (%) | 0.47 | ||
| Married | 63.2 | 62.2 | |
| Widowed | 33.2 | 34.6 | |
| Separated/divorced/never married | 3.6 | 3.2 | |
| Hormone status (%)c | 0.01 | ||
| PMH never use | 34.1 | 31.0 | |
| PMH current use | 36.9 | 36.7 | |
| PMH past use | 29.0 | 32.4 | |
| Family history (%) | |||
| Heart disease | 15.0 | 17.7 | 0.01 |
| Diabetes | 26.7 | 29.7 | 0.01 |
| Cancer | 16.7 | 18.3 | 0.14 |
| History of hypertension (%) | 21.5 | 33.5 | <0.0001 |
| History of high cholesterol (%) | 12.2 | 17.8 | <0.0001 |
Abbreviations: BMI, body mass index, calculated as weight (kilogram) divided by height (meter squared); PMH, postmenopausal hormone use.
SI conversion factors: To convert values from kilogram to pound, multiply by 2.2046; to convert values from centimeter to inch, multiply by 0.3937.
Values are mean (standard deviation) for continuous variables or n (percentage) for categorical variables.
P values are based on Student t test for continuous variables or χ2 test for categorical variables.
Proportions are based on nonmissing values.
Table 2 displays the age- and multivariate-adjusted ORs of successful survival associated with quintiles of total physical activity METs and walking METs. After adjustment for multiple covariates, the ORs for successful survival across quintiles were 1.00, 0.98, 1.37, 1.34, and 1.99 (P for trend < 0.0001) for total METs. We also found associations of similar strength between walking METs and the odds of successful aging. After multivariate adjustment of covariates, ORs for successful survival across walking METs quintiles were 1.00, 0.99, 1.17, 1.47, and 1.45 (P for trend < 0.0001). Further adjustment for possible intermediate variables, such as BMI, history of hypertension, and history of hypercholesterolemia, did not change these associations materially (data not shown).
Table 2.
Odds ratios (95% CI) of successful survival among women surviving to age 70 years or older, according to physical activity level at mid-life in the Nurses’ Health Study.
| Total Physical Activity, Quintiles |
P for trenda | |||||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 | 4 | 5 (Highest) | ||
| METs, hr/wk | ||||||
| Median | 0.9 | 3.6 | 7.9 | 16.2 | 37.1 | - |
| Range | 0.2–2.3 | 2.4–5.1 | 5.2–11.4 | 11.5–22.8 | ≥22.9 | - |
| Usual/successful survivor | 2603/213 | 2349/195 | 2466/307 | 2382/303 | 2279/438 | |
| Age-adjusted | 1.0 | 1.01 (0.83, 1.24) | 1.53 (1.28, 1.84) | 1.57 (1.31, 1.89) | 2.39 (2.01, 2.85) | <0.0001 |
| Multivariate model 1b | 1.0 | 0.98 (0.80, 1.20) | 1.37 (1.13, 1.65) | 1.34 (1.11, 1.61) | 1.99 (1.66, 2.38) | <0.0001 |
| Multivariate model 2c | 1.0 | 0.96 (0.78, 1.18) | 1.30 (1.08, 1.57) | 1.25 (1.03, 1.51) | 1.76 (1.47, 2.12) | <0.0001 |
| Walking, Quintiles | ||||||
| 1 (Lowest) | 2 | 3 | 4 | 5 (Highest) | ||
| METs, hr/wk | ||||||
| Median | 0 | 2.0 | 3.0 | 7.5 | 20.0 | - |
| Range | 0–0.5 | 0.6–2.5 | 2.7–4.5 | 5.0–11.2 | ≥12.5 | - |
| Usual/successful survivor | 2231/195 | 2536/230 | 2553/295 | 2423/379 | 2336/357 | |
| Age-adjusted | 1.0 | 1.04 (0.86, 1.28) | 1.32 (1.09, 1.60) | 1.82 (1.52, 2.18) | 1.80 (1.50, 2.17) | <0.0001 |
| Multivariate model 1b | 1.0 | 0.99 (0.80, 1.21) | 1.19 (0.97, 1.45) | 1.50 (1.24, 1.82) | 1.47 (1.22, 1.79) | <0.0001 |
| Multivariate model 2c | 1.0 | 0.99 (0.80, 1.22) | 1.15 (0.94, 1.40) | 1.42 (1.17, 1.72) | 1.37 (1.10, 1.67) | 0.0003 |
Abbreviations: BMI, body mass index; CI, confidence interval.
Estimates of P value for linear trend are based on linear scores derived from the medians of each physical activity category.
Multivariate model is adjusted for age at baseline (yr), education (registered nurse, bachelor, master, or doctorate degree), husband’s education (less than high school, some high school, high school graduate, college graduate, or graduate school), marital status (unmarried, married, widow, separated or divorced), postmenopausal hormone use (never used, past user, or current user), smoking status (never smoked, past smoker, current smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d), family history of heart disease (yes, no), family history of diabetes (yes, no), family history of cancer (yes, no), polyunsaturated to saturated fat ratio (in quintiles), intakes of trans fat, alcohol, and cereal fiber (all in quintiles), and intakes of fruits and vegetables and red meat (in tertiles). For walking METs, vigorous physical activity METs were further adjusted.
Further adjusted for BMI, (<18.5 kg/m2, 18.5–22.9 kg/m2, 23–24.9 kg/m2, or ≥25 kg/m2), history of hypertension (yes, no), and history of hypercholesterolemia (yes, no).
Independent of the total physical activity levels, increasing walking pace was also strongly associated with a significant increase in odds of successful aging (Table 3). Compared with women whose walking pace was easy, women with moderate walking pace had a 90% increase in the odds of successful aging; women whose walking pace was brisk or very brisk had 2.68 fold increased odds. To help disentangle the effects of the amount walked on the association with walking pace, we stratified the analysis by lower or higher levels of walking METs. Walking pace was similarly associated with increased odds of successful aging for both groups.
Table 3.
Odds ratios (95% CI)a of successful survival among women surviving to age 70 years or older, according to walking pace at mid-life in the Nurses’ Health Study
| Walking Pace |
P for trend | |||
|---|---|---|---|---|
| Easy (<2 mph) | Moderate (2–2.9 mph) | Brisk/Very Brisk (≥3 mph) | ||
| Overall | ||||
| Usual/successful survivor | 1972/98 | 6244/672 | 3738/678 | |
| Age-adjusted | 1.0 | 2.16 (1.74, 2.68) | 3.60 (2.89, 4.48) | <0.0001 |
| Multivariate model 1 | 1.0 | 1.90 (1.52, 2.38) | 2.68 (2.13, 3.37) | <0.0001 |
| Multivariate model 2 | 1.0 | 1.75 (1.40, 2.19) | 2.30 (1.82, 2.91) | <0.0001 |
| Walking METs < 3.1 | ||||
| Usual/successful survivor | 1515/78 | 3474/322 | 1063/159 | |
| Age-adjusted | 1.0 | 1.77 (1.37, 2.28) | 2.80 (2.11, 3.72) | <0.0001 |
| Multivariate model 1 | 1.0 | 1.69 (1.30, 2.20) | 2.50 (1.86, 3.35) | <0.0001 |
| Multivariate model 2 | 1.0 | 1.55 (1.19, 2.01) | 2.12 (1.58, 2.85) | <0.0001 |
| Walking METs ≥ 3.1 | ||||
| Usual/successful survivor | 457/20 | 2770/350 | 2675/519 | |
| Age-adjusted | 1.0 | 2.91 (1.83, 4.61) | 4.38 (2.77, 6.92) | <0.0001 |
| Multivariate model 1 | 1.0 | 2.58 (1.62, 4.11) | 3.46 (2.17, 5.50) | <0.0001 |
| Multivariate model 2 | 1.0 | 2.42 (1.51, 3.86) | 3.04 (1.91, 4.85) | <0.0001 |
Abbreviations: CI, confidence interval.
Regression models were adjusted for the same sets of covariates as in Table 2, plus total physical activity (METs, hr/wk; in quintiles) in multivariate models.
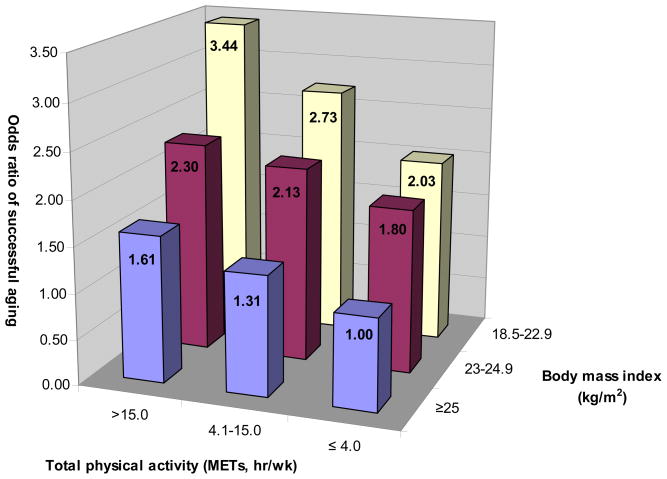
Since BMI and physical activity are intertwined, we also examined the joint associations of BMI in 1986 and total physical activity with successful survival (Figure). The positive associations between physical activity and successful aging persisted within each category of BMI. Nonetheless, women who were both lean (BMI = 18.5–22.9 kg/m2) and active (highest tertile of total METs) had the highest odds of successful survival in comparison with women who were overweight (BMI ≥ 25 kg/m2) and sedentary (bottom tertile of total METs): The OR was 3.44 (95% CI: 2.74, 4.31).
Figure.
BMI and physical activity at baseline in relation with the odds of successful survival in the Nurses’ Health Study. The odds ratios were adjusted for the same covariates in the multivariate model 1 in Table 2.
We also considered specific types of vigorous activities. After controlling for moderate-intensity activity METs, several individual vigorous activities were each associated with significant or borderline significant elevated odds of successful aging. The multivariate ORs (95% CI) comparing any vs. none were 1.66 (1.30, 2.14) for jogging, 1.87 (1.33, 2.61) for running, 1.12 (0.96, 1.31) for swimming, 1.34 (1.03, 1.74) for tennis, and 1.23 (1.09, 1.39) for aerobics/calisthenics.
Secondary Analysis
We observed similar associations for total METs when we restrict our analysis within women who were capable of performing at least low- to moderate-intensity activities at baseline: The ORs (95% CI) across total METs quintiles were 1.00, 1.53 (1.20, 1.95), 1.38 (1.08, 1.77), 1.83 (1.44, 2.32), and 2.04 (1.61, 2.58; P for trend < 0.001) in this group.
Likewise, associations for walking METs were largely unchanged when we repeated the analysis among women who did not engage in any vigorous activity: The OR (95% CI ) were 1.32 (1.03, 1.69) for women in the middle tertile and 1.64 (1.32, 2.04) for women in the highest tertile of walking METs.
Lastly, of 13,535 participants, 1,252 (9.3%) met the criteria of the alternate successful survival definition. In analyses of physical activity and this alternate definition, we found similar associations. For example, the odds ratios (95% CI) for total activity METs quintiles were 1.0 (reference), 1.27 (1.02, 1.57), 1.49 (1.22, 1.83), 1.63 (1.33, 2.00), and 1.93 (1.58, 2.36; P for trend < 0.0001), indicating that our results were robust to different definitions of successful survival.
COMMENT
In this large study of women, we documented a strong, positive association between mid-life leisure-time physical activity and the odds of successful survival, or exceptional overall health in later life. This included a positive relation between moderate-intensity activity, such as walking, and odds of maintaining overall health status among aging women.
There is persuasive evidence supporting an inverse association between physical activity and many individual aspects of health, including multiple chronic diseases, cognitive function, physical function, and mental health.9–19, 34 However, fewer epidemiological studies have examined the association of physical activity with overall health status as evaluated by multiple domains among those who have survived to older ages. In addition, existing data are primarily for men,3, 5, 7 despite the fact that women live, on average, longer than men. Among Cardiovascular Health Study participants and male Harvard college alumni, mid-life physical activity was associated with an improved overall health status at older ages.3, 7 In contrast, among male Japanese Americans, mid-life physical activity was not associated with the probability of exceptional overall health at older ages.5 In the latter study, adjustment of risk factors that can mediate the effects of physical activity on human health, such as plasma glucose, plasma triacylglycerol, hypertension, and BMI, is likely one explanation for the null association. Despite this, it is difficult to directly compare our findings to these studies, since our cohort only included women, for whom physical activity patterns tend be different from those among men. Nonetheless, similar to the Cardiovascular Health Study and the Harvard alumni study, we observed a strong, positive association between physical activity and exceptional survival at ages 70 years and older in women. Our observations are also compatible with previous studies that used disability-free survival or self-rated overall health as a surrogate measure of successful survival.20, 21
In the previous studies of successful survival, walking was not distinguished from more vigorous activities. While approximately 85% of Americans do not participate in any regular vigorous physical activities, 44% walk for exercise.35 Consistent with the literature on walking in relation to chronic diseases and other specific, adverse health outcomes,36–38 our results suggested that energy expenditure from walking at moderate to brisk pace could also increase the likelihood of exceptional survival. Our observations provide initial support for the consideration of walking in broad public health recommendations, since walking is sustainable and can often be easily incorporated into people’s daily schedule.
Importantly, in the current study, being physically active was associated with increased odds of successful survival for both lean and overweight women. This observation was consistent with our previous findings that physical activity was related to a substantial reduction in risk of chronic diseases and premature death among participants with various body weights.10, 34, 39 Together, our data strongly support the notion that, regardless of body weight, engaging in physical activity may increase the probability of preserving an optimal health status. Meanwhile, our study also demonstrated that maintaining a healthy body weight and high physical activity levels simultaneously at mid-life likely convey the highest odds of successful survival.
The strengths of the current study include a comprehensive measurement of overall health of aging women, large sample size, high follow-up rate, accurate self-reported incidence of chronic diseases, and validated methods to quantify physical and mental disabilities and cognitive function. Further unique aspects of our study are the focus on women, who live longer than men on average, and thus merit particular attention in considering risk factors for successful survival, as well as the examination of walking, one of the more common types of activity in women. An additional strength is the multiple analyses conducted to consider possible reverse causation. For example, we excluded anyone with existing chronic diseases at baseline, and also imposed an average 14 year lag period between exposure and outcome assessments – to both address reverse causation as well as the biologic likelihood that health and chronic conditions at older ages are influenced by lifestyle factors adopted at younger ages.
Our study also has several limitations. First, the generalizability of the current study may be limited to women who had primarily European ancestry and were largely healthy at mid-life. Further research should be conducted in minority populations and populations with various specific health issues at earlier life. In addition, we considered successful survival as of age 70 years. Whether the observed associations can be generalized to populations at much older ages is unknown. Second, although our questionnaire to measure physical activity has been validated in a similar population and showed reasonable accuracy, the self-reported physical activity levels were inevitably subject to measurement error. However, since these data were collected before any of the study outcomes occurred, the measurement errors would most likely be non-differential and bias true associations to the null. Third, as in any observational study, residual confounding is also an alternative explanation of our observations. However, the strength and the dose-response gradient of the multivariate associations support a causal relationship between physical activity and successful aging. In addition, the homogeneity of our study population with respect to demographic characteristics and access to health care further reduce possibilities for confounding. Fourth, we did not assess physical and mental health status at baseline. Long-term physical impairment or mental limitations may, thus, bias our observations. However, when we restricted our analysis to women with sufficient function to engage in at least low to moderate physical activity levels at baseline, we observed similar associations. Lastly, approximately 16% of eligible women were excluded from the current analysis because of missing physical activity data at baseline. These participants had slightly higher BMI and worse physical, cognitive, and mental status at older ages, and were less likely to be active at baseline than women who provided data on their physical activity. This combination could lead to bias towards the null.
In summary, the current study provides new evidence that mid-life physical activity, including walking, is associated with increased odds of exceptional health among women who are initially healthy at mid-life and survive to older ages. Since the American population is aging rapidly2 and nearly a quarter of Americans do not engage in any leisure-time activity,40 our findings appear to support federal guidelines regarding physical activity to promote health among older people and further emphasize the potential of activity to enhance overall health and well-being with aging. The notion that physical activity can promote successful survival rather than simply extend the lifespan, may provide particularly strong motivation for initiating activity.
Acknowledgments
Supported by research grants AG13482, AG15424, and CA40356 from the National Institutes of Health and the Pilot and Feasibility program sponsored by the Boston Obesity Nutrition Research Center (DK46200). Dr. Sun is supported by a Postdoctoral Fellowship from the Unilever Corporate Research. Dr. Townsend is supported by the Yerby Postdoctoral Fellowship Program. Dr. Hu is a recipient of American Heart Association Established Investigator Award. We are grateful to Dr. Frans van der Ouderaa for his insightful comments.
ROLE OF THE FUNDING SOURCE:
The funding source had no role in the collection, analysis, and interpretation of the data or in thedecision to submit the manuscript for publication.
Footnotes
AUTHOR CONTRIBUTION
Dr. Grodstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Hu and Grodstein. Acquisition of data: Grodstein. Analysis and interpretation of data: Sun, Townsend, Okereke, Hu, and Grodstein. Drafting of the manuscript: Sun. Critical revision of the manuscript for important intellectual content: Townsend, Okereke, Franco, Hu, and Grodstein. Statistical analysis: Sun and Townsend. Obtained funding: Hu and Grodstein. Administrative, technical, and material support: Sun, Townsend, and Okereke. Study supervision: Hu and Grodstein.
References
- 1.National Center for Health Statistics. Health, United States, 2007 with Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2007. [PubMed] [Google Scholar]
- 2.He W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. Washington DC: U.S. Census Bureau; 2005. [Google Scholar]
- 3.Newman AB, Arnold AM, Naydeck BL, et al. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–22. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 4.Reed DM, Foley DJ, White LR, Heimovitz H, Burchfiel CM, Masaki K. Predictors of healthy aging in men with high life expectancies. Am J Public Health. 1998;88:1463–8. doi: 10.2105/ajph.88.10.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. Jama. 2006;296:2343–50. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 6.von Faber M, Bootsma-van der Wiel A, van Exel E, et al. Successful aging in the oldest old: Who can be characterized as successfully aged? Arch Intern Med. 2001;161:2694–700. doi: 10.1001/archinte.161.22.2694. [DOI] [PubMed] [Google Scholar]
- 7.Vaillant GE, Mukamal K. Successful aging. Am J Psychiatry. 2001;158:839–47. doi: 10.1176/appi.ajp.158.6.839. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. Jama. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. Jama. 2000;283:2961–7. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–8. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 11.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 12.Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33:S530–50. doi: 10.1097/00005768-200106001-00025. discussion S609–10. [DOI] [PubMed] [Google Scholar]
- 13.Almeida OP, Norman P, Hankey G, Jamrozik K, Flicker L. Successful mental health aging: results from a longitudinal study of older Australian men. Am J Geriatr Psychiatry. 2006;14:27–35. doi: 10.1097/01.JGP.0000192486.20308.42. [DOI] [PubMed] [Google Scholar]
- 14.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 15.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 16.Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156:328–34. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- 17.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338:1035–41. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 19.Yates LB, Djousse L, Kurth T, Buring JE, Gaziano JM. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168:284–90. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 20.Leveille SG, Guralnik JM, Ferrucci L, Langlois JA. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999;149:654–64. doi: 10.1093/oxfordjournals.aje.a009866. [DOI] [PubMed] [Google Scholar]
- 21.He XZ, Baker DW. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. Am J Public Health. 2004;94:1567–73. doi: 10.2105/ajph.94.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 23.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–40. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 26.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Alonso A, Hernan MA, Ascherio A. Allergy, family history of autoimmune diseases, and the risk of multiple sclerosis. Acta Neurol Scand. 2008;117:15–20. doi: 10.1111/j.1600-0404.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 28.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155:965–71. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Ma J, Campos H, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007;115:1858–65. doi: 10.1161/CIRCULATIONAHA.106.679985. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 31.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–53. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- 32.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 33.Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women: prospective cohort study. Bmj. 2009;339:b3796. doi: 10.1136/bmj.b3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in overweight white men aged 40–64 years. Am J Epidemiol. 1999;149:491–503. doi: 10.1093/oxfordjournals.aje.a009843. [DOI] [PubMed] [Google Scholar]
- 35.Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta: National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 36.Hu FB, Manson JE. Walking: the best medicine for diabetes? Arch Intern Med. 2003;163:1397–8. doi: 10.1001/archinte.163.12.1397. [DOI] [PubMed] [Google Scholar]
- 37.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 38.Hakim AA, Curb JD, Petrovitch H, et al. Effects of walking on coronary heart disease in elderly men: the Honolulu Heart Program. Circulation. 1999;100:9–13. doi: 10.1161/01.cir.100.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 40.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]