Abstract
Background
Aerobic treadmill exercise (T-EX) therapy has been shown to benefit walking and cardiorespiratory fitness in stroke survivors with chronic gait impairment even long after their stroke. The response, however, varies between individuals.
Objective
The purpose of this post hoc analysis of 2 randomized controlled T-EX trials was to identify predictors for therapy response.
Methods
In all, 52 participants received T-EX for 3 (Germany) or 6 (United States) months. Improvements in overground walking velocity (10 m/6-min walk) and fitness (peak VO2) were indicators of therapy response. Lesion location and volume were measured on T1-weighted magnetic resonance scans.
Results
T-EX significantly improved gait and fitness, with gains in 10-m walk tests ranging between +113% and −25% and peak VO2 between −12% and 88%. Baseline walking impairments or fitness deficits were not predictive of therapy response; 10-m walk velocity improved more in those with subcortical rather than cortical lesions and in patients with smaller lesions. Improvements in 6-minute walk velocity were greater in those with more recent strokes and left-sided lesions. No variable other than training intensity, which was different between trials, predicted fitness gains.
Conclusions
Despite proving overall effectiveness, the response to T-EX varies markedly between individuals. Whereas intensity of aerobic training seems to be an important predictor of gains in cardiovascular fitness, lesion size and location as well as interval between stroke onset and therapy delivery likely affect therapy response. These findings may be used to guide the timing of training and identify subgroups of patients for whom training modalities could be optimized.
Keywords: stroke, lesion, gait, rehabilitation, aerobic treadmill exercise
Introduction
Impaired gait after hemiparetic stroke contributes strongly to overall disability. Aerobic treadmill exercise (T-EX) has been successfully used to retrain gait and improve cardiorespiratory fitness at the same time, thereby, reducing the disability related to immobility. Several randomized controlled trials have demonstrated benefits on various outcome parameters in patients with chronic gait impairments.1-4 Although group effects are significant, the individual response to T-EX is variable. The reasons for this variability are not known. Identifying predictors of therapy-related benefits will serve to select and adjust the intervention to the individual patient.
For other rehabilitative treatments, predictive parameters have been reported. Using functional magnetic resonance imaging,5,6 transcranial magnetic stimulation,7,8 or positron emission tomography,9 it was shown that different brain areas undergo lasting changes after stroke and after rehabilitative interventions. These changes in brain activation are associated with the degree to which motor function recovers. Cramer and coworkers10 suggested that lower baseline motor cortex activation predicts higher therapeutic benefit. Given that brain activation during hemiparetic movement depends on the location and size of the brain lesion,6 it is conceivable that lesion geometry has prognostic value. Lesion geometry indeed explains part of the variability in acute deficits after stroke and of functional outcomes at 3 months.11-15 However, some studies have failed to show such relationships.10,16
Age was also identified as a predictor of functional outcome in previous studies.17,18 This may be explained by higher frequency of comorbidity, stroke-related complications,14 and limited plasticity of the aging brain.19 The objective here was to investigate the value of clinical, demographic, and lesion-related variables to predict the benefit provided by T-EX in chronically disabled stroke survivors.
Materials and Methods
Participants
This post hoc analysis combines data from 2 randomized controlled trials that were conducted by the same collaborative group of researchers. In the first trial, they compared 6 months of aerobic T-EX with stretching exercises of equal duration in Baltimore, Maryland.2,20 In the second trial conducted in Stuttgart, Germany, they compared 3 months of T-EX to conventional care. Here, data for the 52 participants from the T-EX groups of both trials for whom structural imaging data were available are analyzed (Table 1).
Table 1. Demographic and Baseline Characteristics.
| All (n = 52) | United States (n = 20) | Germany (n = 32) | PDifference (US vs German Trials) | |
|---|---|---|---|---|
| Age (years), mean (SEM) | 66.8 (1.1) | 64.0 (2.1) | 68.6 (1.1) | .055 |
| Gender, Female (%) | 18 (34.62) | 12 (60) | 6 (18.75) | .036a |
| Stroke therapy interval (months), mean (SEM) | 59.00 (9.28) | 60.06 (20.01) | 58.34 (8.77) | .93 |
| Stroke location, n (%) | ||||
| Brainstem | 8 (15.38) | 6 (30) | 2 (6.24) | |
| Cortex | 20 (38.46) | 5 (25) | 15 (46.88) | |
| Subcortical | 24 (46.15) | 9 (45) | 15 (46.88) | |
| Right-sided stroke, n (%) | 20 (38.46) | 8 (40) | 12 (37.5) | .86 |
| Lesion volume (mm3 SEM) | 37421.54 (8065.83) | 45775 (11484.75) | 24056 (9717.42) | .16 |
| NIHSS, mean (SEM) | 4.08 (0.35) | 3.67 (0.53) | 4.31 (0.47) | .39 |
Abbreviations: SEM, standard error of the mean; NIHSS, National Institutes of Health Stroke Scale.
Indicates significant differences between trials (P < .05).
Participants in both studies had suffered a first-ever ischemic stroke at least 6 months prior to enrollment. Exclusion criteria were heart failure, unstable angina, peripheral arterial occlusive disease, aphasia, dementia, untreated major depression, clinical and/or neuroimaging signs of stroke-independent neurological diseases (eg, Parkinsonian syndromes), patients already performing aerobic exercise training for >20 min/d and >1 d/wk, and other medical conditions precluding participation in exercise (for details see ACSM21). The trials were approved by the institutional review boards of the University of Maryland and the Johns Hopkins University (US trial) and the Ethics Committee of the University of Tübingen, Germany (German trial). All participants provided written informed consent.
Assessments of Gait Function and Cardiovascular Fitness
Participants were enrolled when capable of completing ≥3 consecutive minutes of treadmill walking at ≥0.1 m/s without personal or body weight support (use of hand rails was allowed) and without signs of myocardial ischemia or other contraindications to training. During a peak-effort T-EX test with open-circuit spiroergometry, cardiovascular fitness was determined by measuring VO2 in (mL/kg body weight)/min according to the standards of the American Heart Association21,22 under continuous monitoring of vital signs and ECG. For peak VO2 testing, a modified Balke protocol (increase of treadmill incline every 2 minutes with constant speed) was applied—a procedure to assess cardiovascular fitness in stroke patients with a reliability of repeated measurements of heart rate, systolic blood pressure, oxygen consumption (VO2 in L/min), VO2 (mL/kg/min), respiratory exchange ratio, rate pressure product, and oxygen pulse.23 Locomotor impairments were assessed by 2 widely used and well-characterized tests.24,25 The time required to walk 10 m at fastest and comfortable paces was used to assess the ability to walk short distances typical for the home environment. The distance walked during 6 minutes was added to evaluate sustained walking capacity. To render both tests comparable to each other and to published reference data, the mean velocity was calculated for both walking tests and was used in further analyses. Functional assessments were conducted before and after the training period.
Training
The T-EX training goal was three 40-minute exercise sessions per week at an aerobic intensity of 60% in the US trial and 80% of heart rate reserve (HRR) in the German trial. Duration and intensity started at low values (10-20 minutes, 40%-50% HRR) and increased by approximately 5 minutes and 5% HRR. To reach the training intensity target, treadmill velocity was increased by 0.05 m/s every 1 to 2 weeks as tolerated. In the US trial, training was conducted for 6 months and in the German trial, for 3 months.
MRI Data Acquisition
In the US trial, structural MRI data were collected using a 1.5 T Philips scanner (Philips, Eindhoven, Netherlands) within 2 weeks of the start and end of the training. In the German trial, MRI data were acquired from a 3T scanner (Vision, Siemens, Erlangen, Germany). T1-weighted images (3D-MPRAGE sequence, resolution 1 mm3) covering the entire brain were acquired to determine lesion location and size. Functional MRI data collected in the US trial are reported elsewhere.2
Image Analysis
Lesion location was first determined by visual inspection performed by 2 raters independently (ARL and BH for the US trial; JML and CG for the German trial). Lesions were stratified into cortical/subcortical white matter with or without basal ganglia involvement, referred to as cortical lesions and subcortical lesions. The latter were defined as lesions restricted to the region medial to the insula and inferior to the corpus callosum. Brainstem lesions were regarded as subcortical.
To determine lesion volume, binary lesion masks were produced by manually segmenting the lesion area on all consecutive sections displaying the lesion. Lesion area was defined on T1 images as all voxels isointense to CSF plus hypodense voxels at the boundary of the lesion core. Manual segmentation was performed using MRIcro.26 All voxels defining the lesion (1 voxel = 1 mm3) were counted using a Matlab script.
Statistical Analysis
The changes in functional assessments (10-m walk test, 6-minute walk test, and peak VO2) were expressed as absolute change and change relative to baseline performance. Relative changes were analyzed because we expected patients with more impairment to show less absolute improvement as compared with patients with smaller deficits. General linear models were used to assess the effects of age, gender, stroke-onset to therapy-onset interval, and lesion volume, side, and location (cortical, subcortical) on the dependent variables. Dependent variables were either baseline performance or change of performance (absolute or relative to baseline) in fitness and walking tests. In the models investigating change variables, the baseline value of the respective change variable was added as a covariate. Independent variables were entered into the model in a stepwise fashion using a criterion of P < .25 and then removed if P > .05. After identifying significant predictors, 2-way interactions between them were first added to the model and then removed if their effect was insignificant (P < .05). The efficacy of T-EX to improve fitness and gait was tested using repeated-measures ANOVA models, one for each outcome parameter. All data are expressed as mean ± standard error of the mean.
Results
Baseline Functional Impairment
The patients enrolled in Germany walked faster at their self-selected pace at baseline and had better cardiovascular fitness (Table 2) compared with those in the US trial. Overground walking velocity as measured in the 6-minute walk test and velocity in the 10-m walk test was slower in women, in older participants, in those with larger lesions (for the 10-m walk test fastest pace), and at higher baseline NIHSS score (Table 3). For gait velocity derived from the 6-minute walk test, we found a higher negative correlation with NIHSS score among participants in the German trial than in the US trial. Low cardiorespiratory fitness was predicted by female gender, right-sided lesion, and high (indicating greater impairment) NIHSS score. No other interactions between trial and other independent variables were significant.
Table 2. Baseline and Absolute (absCh) and Relative Changes (relCh) in Gait Performance and Fitness.
| All (n = 52) | United States (n = 20) | Germany (n = 32) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | absCh | relCh | Baseline | absCh | relCh | Baseline | absCh | relCh | |
| 6-Minute walk velocity (m/s), mean (SEM) | 0.704 (0.054) | 0.127 (0.015) | 0.198 (0.023) | 0.585 (0.061) | 0.115 (0.024) | 0.206 (0.046) | 0.778 (0.077) | 0.135 (0.019) | 0.194 (0.024) |
| 6-Minute walk distance (m), mean (SEM) | 253.25 (19.38) | 45.90 (5.31) | 210.6 (21.82) | 41.35 (8.65) | 279.91 (27.61) | 48.75 (6.79) | |||
| 10-m Walk velocity (m/s), SSWS, mean (SEM) | 0.672 (0.048) | 0.059 (0.015) | 0.133 (0.035) | 0.558a (0.057) | 0.075 (0.030) | 0.217 (0.083) | 0.739a (0.067) | 0.0499 (0.017) | 0.083 (0.024) |
| 10-m Walk velocity (m/s), FCWS, mean (SEM) | 0.852 (0.063) | 0.100 (0.019) | 0.156 (0.035) | 0.748 (0.083) | 0.126 (0.036) | 0.25 (0.084) | 0.914 (0.087) | 0.084 (0.019) | 0.100 (0.022) |
| Peak VO2 (mL/kg/min), mean (SEM) | 17.856 (0.944) | 3.957 (0.527) | 0.232 (0.030) | 14.125a (1.214) | 2.089a (0.510) | 0.161 (0.039) | 20.188a (1.167) | 5.066a (0.720) | 0.274 (0.041) |
Abbreviations: SEM, standard error of the mean; SSWS, self-selected walking speed; FCWS, fast comfortable walking speed.
Indicates significant differences between trials (P < .05).
Table 3. Predictors of Baseline Walking Impairment and Fitness.
| Dependent Variable | Predictors | Mean | SEM | r | P |
|---|---|---|---|---|---|
| 10-m Walk velocity (m/s), comfortable | Trial (United States) | 0.56 | 0.06 | .009 | |
| Trial (Germany) | 0.74 | 0.07 | |||
| NIHSS | −0.54 | <.0001 | |||
| 10-m Walk velocity (m/s), fastest | Trial (United States) | 0.75 | 0.09 | .036 | |
| Trial (Germany) | 0.91 | 0.09 | |||
| Gender (female) | 0.69 | 0.07 | .032 | ||
| Gender (male) | 0.93 | 0.08 | |||
| Age | −0.1 | .012 | |||
| Lesion volume | −0.4 | .023 | |||
| NIHSS | −0.5 | .005 | |||
| 6-Minute walk velocity (m/s) | Gender (female) | 0.56 | 0.06 | .009 | |
| Gender (male) | 0.77 | 0.07 | |||
| NIHSS (United States) | −0.14 | <.0001 | |||
| NIHSS (Germany) | −0.69 | ||||
| Peak VO2 (mL/kg/min) | Trial (United States) | 14.1 | 1.21 | .013 | |
| Trial (Germany) | 20.2 | 1.17 | |||
| Gender (female) | 13.2 | 1.2 | .0004 | ||
| Gender (male) | 20.3 | 1.1 | |||
| Stroke side (left) | 19.7 | 1.2 | .009 | ||
| Stroke side (right) | 14.9 | 1.4 | |||
| NIHSS | −0.28 | .003 |
Abbreviations: SEM, standard error of the mean; NIHSS, National Institutes of Health Stroke Scale.
Exercise-Related Functional Gains
Treadmill training led to increased gait velocity as measured by the 10-m walk test (fastest pace 0.85 ± 0.06 to 0.96 ± 0.06 m/s, P < .0001; comfortable pace 0.67 ± 0.05 to 0.75 ± 0.05 m/s, P = .0006) and as measured during the 6-minute walk (0.70 ± 0.05 to 0.84 ± 0.06 m/s, P < .0001). T-EX also improved cardiorespiratory fitness (peak VO2 17.9 0.94 to 21.7 ± 1.18 mL/kg/min, P < .0001). There were no significant correlations between gains in fitness and velocity (P > .5 for all gait tests). Absolute and relative gains in these outcome parameters are presented in Table 2.
Predictors of Exercise-Related Functional Gains
Relative improvements in 10-m walk velocities were higher in participants with smaller lesions (Table 3). Relative gains in 6-minute walk velocity were higher in participants with more recent stroke events. Relative improvement in fitness (peak VO2) was higher in German than in US participants (Table 3).
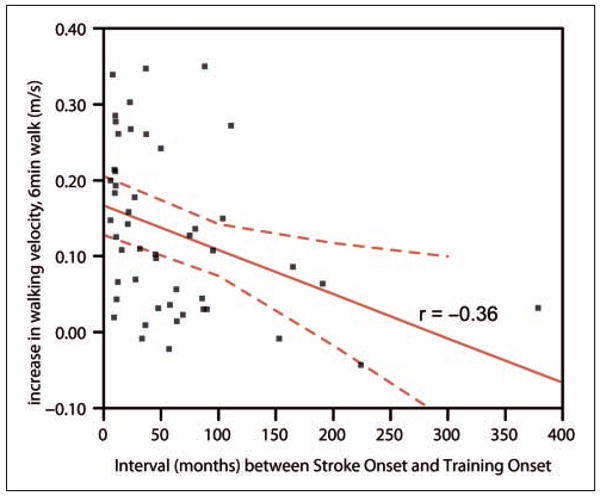
Absolute changes in walking or fitness were not predicted by baseline walking velocities or fitness. Absolute improvement in gait velocity measured during the 10-m walk test (fastest or comfortable pace) was greater in participants with subcortical than with cortical lesions (Table 4). Whereas improvements in patients with subcortical lesions were significant for both comfortable and fastest walking velocity (fastest pace: gain = 0.13 ± 0.02 m/s, P < .0001; comfortable pace: gain = 0.09 ± 0.02 m/s, P < .0001), gains in participants with cortical lesions failed to reach significance (fastest pace: gain = 0.05 ± 0.03 m/s, P = .08; comfortable pace: gain = 0.02 ± 0.02 m/s, P = .5). Participants with shorter stroke– therapy intervals and left-sided lesions showed greater improvement in 6-minute walk velocity (Table 4, Figure 1). Nevertheless, both left- and right-hemisphere-lesioned participants walked faster in the 6-minute walk test (left-hemisphere lesion: gain in velocity = 0.16 ± 0.02 m/s, P < .0001; right-hemisphere lesion: gain = 0.08 ± 0.02 m/s, P < .001). Predictive models explained between 10% and 33% of the variability in therapy response (r2 values Table 4).
Table 4. Predictors of Therapy Response.
| Dependent Variable | Predictors | Mean | SEM | r | P | r2overall |
|---|---|---|---|---|---|---|
| Absolute gain in 10-m walk velocity (m/s), comfortable | Location (cortical) | 0.015 | 0.021 | .014 | 0.12 | |
| Location (subcortical) | 0.091 | 0.020 | ||||
| Relative gain in 10-m walk velocity (m/s), comfortable | Lesion volume | −0.24 | .0023 | 0.32 | ||
| Absolute gain in 10-m walk velocity (m/s), fastest | Location (cortical) | 0.047 | 0.025 | .018 | 0.11 | |
| Location (subcortical) | 0.134 | 0.023 | ||||
| Relative gain in 10-m walk velocity (m/s), fastest | Lesion volume | −0.24 | .0035 | 0.27 | ||
| Absolute gain in 6-minute walk velocity (m/s) | Stroke side (left) | 0.16 | 0.02 | .035 | 0.33 | |
| Stroke side (right) | 0.08 | 0.02 | ||||
| Stroke–Therapy interval | −0.36 | .0088 | ||||
| Relative gain in 6-minute walk velocity (m/s) | Stroke–Therapy interval | −0.31 | .017 | 0.1 | ||
| Absolute gain in peak VO2 (mL/kg/min) | Trial (United States) | 2.09 | 0.51 | .005 | 0.15 | |
| Trial (Germany) | 5.07 | 0.72 | ||||
| Relative gain in peak VO2 (mL/kg/min) | Trial (United States) | 0.16 | 0.04 | .0312 | 0.15 | |
| Trial (Germany) | 0.27 | 0.04 |
Abbreviations: SEM, standard error of the mean.
Figure 1. The absolute improvement in 6-minute walk velocity after treadmill exercise is greater in participants who were trained earlier after the stroke.
Discussion
This post hoc analysis of 2 trials on aerobic T-EX demonstrates that despite overall significant benefits, the response to T-EX varies between individuals. Predictors of greater benefit in walking parameters were subcortical and left-sided lesion location, smaller lesions, and shorter interval time between stroke onset and onset of treadmill training.
Previous studies have demonstrated a relationship between lesion location and size and stroke-related deficits or benefits of conventional rehabilitation.11,12,14,15,27,28 Whereas in laboratory animals lesion volume predicts functional deficits,27,29,30 findings are heterogeneous in humans. Saunders and colleagues15 reported that for middle cerebral artery (MCA) territory infarctions, lesion volume is a prognostic outcome indicator. Other studies failed to show this relationship.16,31 Chen et al12 reported critical lesion sizes for different brain areas: motor impairment was high when lesions were larger than 75 cm3 for the cortex, 4 cm3 for the corona radiata, 0.75 cm3 for the internal capsule, 22 cm3 for the putamen, and 12 cm3 for the thalamus. This indicates that functional outcome depends not only on lesion size but also on a combination of lesion size and location. Dawes and coworkers31 reported a trend for a correlation between corticospinal tract lesion volume and walking performance after a partial body weight support treadmill training. Beloosesky and coworkers11 reported a correlation between lesion size and rehabilitation success for cortical infarcts. In our data set, lesion volume was an independent predictor in relative gains in 10-m walk gait velocity (independent of baseline deficit). For absolute improvement, lesion location (subcortical vs cortical) was an independent predictor, representing the same association as the association between lesion volume and relative gain because cortical strokes were substantially larger then subcortical strokes. Whereas patients with subcortical strokes showed significant improvements in the 10-m walk, patients with cortical strokes failed to achieve significant effects. We also found an association between improvement in gait velocity during the 6-minute walk (absolute gain) and lesioned hemisphere. Participants with left-sided lesions improved twice as much in gait velocity as those with right-sided ones; however, both subgroups benefited significantly. Although it has been shown that overall stroke outcomes at 3 months poststroke (modified Rankin scale) were similar for those with left- and right-sided lesions,32 locomotion was reported to recover better in patients with recent (mean 52 days) left-hemisphere lesions using conventional rehabilitation techniques.33 This difference may be related to the fact that visuospatial or attention deficits are more prominent in participants with right-hemisphere infarction, and this could interfere with locomotion because these cognitive functions are required for locomotion.34 It is plausible that 6-minute walks have higher cognitive demands, for example, higher demands for navigation in space, than 10-m walks and might, therefore, be more sensitive to right-hemisphere damage.
Age has been reported to predict poor response to constraint-induced movement therapy,35 but age was unrelated to the benefits conveyed by treadmill therapy here. Similarly, Luk and coworkers36 found in 878 stroke survivors that, if corrected for disability before the stroke, age per se does not predict functional independence at the time of discharge from the rehabilitation hospital. In the healthy elderly population, King and coworkers37 report younger age and better health and physical function at baseline to be predictors of exercise benefits. The reason for not observing predictive effects of age here, especially on fitness gains, may be the smaller age span and younger mean age in our participant sample as compared with those in the study by King et al.
A longer time interval between stroke onset and beginning of treadmill therapy were associated with less improvement in gait velocity measured during the 6-minute walk (absolute and relative). It is noteworthy that this relationship does not reflect differences in the efficacy of interventions delivered in the acute versus the chronic period after stroke because both trials recruited chronic participants at more than 6 months after their stroke. Although it does not qualify the finding that training on a treadmill can improve walking even long after stroke, this observation stresses the need for continued rehabilitation beyond the commonly prescribed 3 to 6 weeks.
It has been reported that apart from lesion-related parameters, more severe neurological deficits predicted less improvement after therapy for the recovery of arm function.38 A similar finding was reported for constraint-induced movement therapy.20 Here, we did not find an association between baseline deficits and therapy response—that is, there was no effect of a baseline functional measure on its absolute change after therapy. However, certain predictors of response also predicted baseline function—that is, lesion volume for the 10-m walk velocity. Their predictive value may therefore be explained via their effect on baseline function. Participants in the German trial had higher fitness levels at baseline than US participants and showed greater improvements, but baseline fitness itself did not predict gains in fitness in the combined study sample. Thus, the effects on fitness gains are not likely to be explained by the differences in baseline values between trials, particularly as one would expect even greater benefits in an unfit patient. The effects could, however, be explained by higher training intensity in the German trial (mean HRR at the end of training was 76% for Germany and 58% for the United States). Apart from that, none of the investigated independent variables (age, gender, baseline walking, stroke–therapy interval, and lesion volume, side, and location [cortical, subcortical]) seemed to predict gains in cardiovascular fitness in the combined sample.
Despite identifying significant predictors here, predictive models explained at most 33% (for 6-minute walk velocity) of the variability in therapy effects. Other parameters, such as the degree of microvascular encephalopathy, brain atrophy, or mental factors such as motivation and ambition to achieve the training goals, were not evaluated here but may be important for predicting the response to T-EX.
The limitation of this study is the combination of 2 trials that were conducted in different populations and used different durations and intensities of T-EX. Because it is difficult to recruit large numbers of chronically disabled stroke survivors for prolonged training within a research study, we decided to pool the data despite these design differences. Trial (the United States, Germany) was a covariate in all analyses, and interaction terms were tested to identify differences between the data sets. As discussed above, the effect of trial was significant only in models predicting fitness gains. This may have been a confounder precluding an identification of predictors of fitness gains.
Conclusion
In summary, the present study provides further support for the efficacy of aerobic treadmill training in chronic stroke survivors. Walking benefits might be related to lesion characteristics, with participants with large and right-sided lesions improving the least. Additionally, earlier intervention after the stroke may optimize treatment effects. These findings might be important to consider when prescribing exercise interventions after stroke but require further confirmation by randomized controlled trials.
Acknowledgments
Funding: This research was supported by the Hertie Foundation and the Deutsche Forschungsgemeinschaft (Lu 748/5-1), National Institutes of Health, NIA (P60AG 12583) University of Maryland Claude D. Pepper Older Americans Independence Center, the Department of Veterans Affairs and Baltimore Veterans Affairs Medical Center Geriatrics Research, Education and Clinical Center (GRECC), Rehabilitation Research & Development Exercise and Robotics Center of Excellence, Johns Hopkins GCRC (NCRR #MO1-00052).
Footnotes
Declaration of Conflicting Interests: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 2.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaney BM, Boyd LA, McDowd J, He J, Mayo M, Macko R. Exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 5.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luft AR, Forrester L, Macko RF, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage. 2005;26:184–194. doi: 10.1016/j.neuroimage.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 8.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 9.Calautti C, Leroy F, Guincestre JY, Marie RM, Baron JC. Sequential activation brain mapping after subcortical stroke: changes in hemispheric balance and recovery. Neuroreport. 2001;12:3883–3886. doi: 10.1097/00001756-200112210-00005. [DOI] [PubMed] [Google Scholar]
- 10.Cramer SC, Parrish TB, Levy RM, et al. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 11.Beloosesky Y, Streifler JY, Burstin A, Grinblat J. The importance of brain infarct size and location in predicting outcome after stroke. Age Ageing. 1995;24:515–518. doi: 10.1093/ageing/24.6.515. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Tang FT, Chen HC, Chung CY, Wong MK. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil. 2000;81:447–452. doi: 10.1053/mr.2000.3837. [DOI] [PubMed] [Google Scholar]
- 13.Pineiro R, Pendlebury ST, Smith S, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke. 2000;31:672–679. doi: 10.1161/01.str.31.3.672. [DOI] [PubMed] [Google Scholar]
- 14.Pan SL, Wu SC, Wu TH, Lee TK, Chen TH. Location and size of infarct on functional outcome of noncardioembolic ischemic stroke. Disabil Rehabil. 2006;28:977–983. doi: 10.1080/09638280500404438. [DOI] [PubMed] [Google Scholar]
- 15.Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke. 1995;26:2272–2276. doi: 10.1161/01.str.26.12.2272. [DOI] [PubMed] [Google Scholar]
- 16.Dromerick AW, Reding MJ. Functional outcome for patients with hemiparesis, hemihypesthesia, and hemianopsia: does lesion location matter? Stroke. 1995;26:2023–2026. doi: 10.1161/01.str.26.11.2023. [DOI] [PubMed] [Google Scholar]
- 17.Masiero S, Avesani R, Armani M, Verena P, Ermani M. Predictive factors for ambulation in stroke patients in the rehabilitation setting: a multivariate analysis. Clin Neurol Neurosurg. 2007;109:763–769. doi: 10.1016/j.clineuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Paolucci S, Grasso MG, Antonucci G, et al. Mobility status after inpatient stroke rehabilitation: 1-year follow-up and prognostic factors. Arch Phys Med Rehabil. 2001;82:2–8. doi: 10.1053/apmr.2001.18585. [DOI] [PubMed] [Google Scholar]
- 19.Weimar C, Ziegler A, Konig IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol. 2002;249:888–895. doi: 10.1007/s00415-002-0755-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin KC, Huang YH, Hsieh YW, Wu CY. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23:336–342. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- 21.ACSM. American College of Sports Medicine's (ACSM) Guidelines for Exercise Testing and Prescription. 6th. Baltimore, MD: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 22.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 23.Dobrovolny CL, Ivey FM, Rogers MA, Sorkin JD, Macko RF. Reliability of treadmill exercise testing in older patients with chronic hemiparetic stroke. Arch Phys Med Rehabil. 2003;84:1308–1312. doi: 10.1016/s0003-9993(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 24.Friedman PJ. Gait recovery after hemiplegic stroke. Int Disabil Stud. 1990;12:119–122. doi: 10.3109/03790799009166265. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Drutz C, Kumar R, et al. Use of the six-minute walk test poststroke: is there a practice effect? Arch Phys Med Rehabil. 2008;89:1686–1692. doi: 10.1016/j.apmr.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 27.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. discussion 2066. [DOI] [PubMed] [Google Scholar]
- 28.Menezes NM, Ay H, Wang Zhu M, et al. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke. 2007;38:194–197. doi: 10.1161/01.STR.0000251792.76080.45. [DOI] [PubMed] [Google Scholar]
- 29.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 30.Grabowski M, Nordborg C, Johansson BB. Sensorimotor performance and rotation correlate to lesion size in right but not left hemisphere brain infarcts in the spontaneously hypertensive rat. Brain Res. 1991;547:249–257. doi: 10.1016/0006-8993(91)90968-2. [DOI] [PubMed] [Google Scholar]
- 31.Dawes H, Enzinger C, Johansen-Berg H, et al. Walking performance and its recovery in chronic stroke in relation to extent of lesion overlap with the descending motor tract. Exp Brain Res. 2008;186:325–333. doi: 10.1007/s00221-007-1237-0. [DOI] [PubMed] [Google Scholar]
- 32.Fink JN, Frampton CM, Lyden P, Lees KR. Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke?: an analysis of placebo-treated patients from prospective acute stroke trials. Stroke. 2008;39:3335–3340. doi: 10.1161/STROKEAHA.108.523365. [DOI] [PubMed] [Google Scholar]
- 33.Goto A, Okuda S, Ito S, et al. Locomotion outcome in hemiplegic patients with middle cerebral artery infarction: the difference between right- and left-sided lesions. J Stroke Cerebrovasc Dis. 2009;18:60–67. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz SL, Light KE, Clifford SN, Patterson TS, Behrman AL, Davis SB. Descriptive characteristics as potential predictors of outcomes following constraint-induced movement therapy for people after stroke. Phys Ther. 2006;86:825–832. [PubMed] [Google Scholar]
- 36.Luk JK, Cheung RT, Ho SL, Li L. Does age predict outcome in stroke rehabilitation? A study of 878 Chinese subjects. Cerebrovasc Dis. 2006;21:229–234. doi: 10.1159/000091219. [DOI] [PubMed] [Google Scholar]
- 37.King MB, Whipple RH, Gruman CA, Judge JO, Schmidt JA, Wolfson LI. The Performance Enhancement Project: improving physical performance in older persons. Arch Phys Med Rehabil. 2002;83:1060–1069. doi: 10.1053/apmr.2002.33653. [DOI] [PubMed] [Google Scholar]
- 38.Chen SY, Winstein CJ. A systematic review of voluntary arm recovery in hemiparetic stroke: critical predictors for meaningful outcomes using the international classification of functioning, disability, and health. J Neurol Phys Ther. 2009;33:2–13. doi: 10.1097/NPT.0b013e318198a010. [DOI] [PubMed] [Google Scholar]


