Abstract
Objective
To determine whether Adaptive Physical Activity (APA-stroke), a community-based exercise program for participants with hemiparetic stroke, improves function in the community.
Methods
Nonrandomized controlled study in Tuscany, Italy, of participants with mild to moderate hemiparesis at least 9 months after stroke. Forty-nine participants in a geographic health authority (Empoli) were offered APA-stroke (40 completed the study). Forty-four control participants in neighboring health authorities (Florence and Pisa) received usual care (38 completed the study). The APA intervention was a community-based progressive group exercise regimen that included walking, strength, and balance training for 1 hour, thrice a week, in local gyms, supervised by gym instructors. No serious adverse clinical events occurred during the exercise intervention. Outcome measures included the following: 6-month change in gait velocity (6-Minute Timed Walk), Short Physical Performance Battery (SPPB), Berg Balance Scale, Stroke Impact Scale (SIS), Barthel Index, Hamilton Rating Scale for Depression, and Index of Caregivers Strain.
Results
After 6 months, the intervention group improved whereas controls declined in gait velocity, balance, SPPB, and SIS social participation domains. These between-group comparisons were statistically significant at P < .00015. Individuals with depressive symptoms at baseline improved whereas controls were unchanged (P < .003). Oral glucose tolerance tests were performed on a subset of participants in the intervention group. For these individuals, insulin secretion declined 29% after 6 months (P = .01).
Conclusion
APA-stroke appears to be safe, feasible, and efficacious in a community setting.
Keywords: Stroke, Exercise, Community, Rehabilitation
Stroke is one of the leading causes of death and long-term disability.1 The Framingham study found that at 6 months following a stroke (ie, after the period of natural recovery2) 50% of stroke survivors aged 65 years or older had some hemiparesis and 30% were unable to walk without assistance.3 Due in part to the sedentary lifestyle associated with these limitations, the stroke survivor is at increased risk of diabetes, glucose intolerance, heart disease, subsequent stroke death, and depression.4,5 There is substantial evidence supporting a protective role for exercise in the prevention of stroke.1 For stroke survivors, increasing evidence also links exercise to improved cardiovascular health, with decreased risk for cardiac events; improvements in indices of insulin sensitivity and glucose tolerance6; improved physical fitness, ambulatory function,7-12 and bone health13; as well as reduced depression and social isolation7 even years after the stroke.7-10 Despite the potential, there is limited experience translating this research into community-based exercise programs specifically adapted for the needs of stroke survivors.14-17 Most published research has been conducted in hospital or rehabilitation settings,14-18 and the optimal management strategy to prevent the progression of disability in chronic stroke survivors is unknown.19 Survivors 1 year after stroke report considerable levels of dissatisfaction with their quality of life, with the level of dissatisfaction correlated to the level of activity impairment.20 Whether the most severely impaired chronic patients can recover the ability to ambulate with intense rehabilitation is the subject of current investigations21; however, those with moderate disabilities may be more amenable to a community-based intervention.
In this context, we report the results of an evaluation of the Adaptive Physical Activity (APA) program for stroke (APA-stroke), conducted in the Tuscan region of Italy, under a memorandum of agreement between the US National Institutes of Health and its Italian counterpart, the Instituto Superiore di Sanità. The aim of this study was to investigate the safety, feasibility, and effectiveness of a community-based physical activity program for people with chronic stroke and moderate hemiparetic gait disorders.
Methods
APA-Stroke Program
This program evaluation was initiated as the first participants were enrolled in the APA-stroke program, beginning in October 2005, in communities served by the local health authority of Empoli, Italy. Both APA-stroke participants and controls were followed for 6 months after enrollment in the program. The APA-stroke program was designed to improve the long-term health and functioning of stroke survivors.22 Individuals who were medically cleared by their personal physician were eligible to participate in the exercise program that met for 1 hour, thrice a week, in classes of 9 to 13 people. Participants were also encouraged to do exercises at home. Both gymnasium and home APA-stroke exercises were aimed at improving muscle strength, joint flexibility, balance, and cardiorespiratory function.
APA-stroke provided walking, strength, and balance training exercises specifically designed for individuals with chronic stroke. Exercises were performed both standing and seated. Walking time and the intensity of exercises progressively increased during the first 3 months. The standing exercises were designed to improve lower extremity function, strength, and awareness and were performed as the participant held onto a bar for support and balance. The gait component (walking) was done at the beginning and at the end of each class. At the beginning of the class, each participant walked around the perimeter of the room for 6 minutes at a pace that he or she selected as being comfortable. At the end of the class, the participant walked around ropes placed on the floor as obstacles to step around for 6 minutes at a pace selected by each participant as comfortable. After the first month, both walking components were lengthened by 1 minute each week, until by week 13 walking time reached 15 minutes. Participants were able to sit and rest if the progression was too taxing for them, but this was infrequent. Rather, it appeared that participants adjusted their walking speed according to their individual capabilities. As walking time increased, time spent on other exercises was correspondingly reduced to maintain the 1-hour timeframe. Instructors had the discretion to reduce the minutes allocated to other exercises or alternate exercises through the week. Seated exercises included sit-to-stand, trunk twists, shoulder lifts, arm rolls, and neck stretches. Exercises were also performed at a bar, including weight shifting, mezzo squats, stretches, and leg/trunk flexion and extensions.
To keep the price low and promote geographic access to the APA-stroke classes, the local health authority negotiated agreements with local gymnasiums throughout the region served. Participants paid €1.9 (approximately $3.00) per class and provided for their own transportation. Classes were typically offered at “off-hours” on weekday mornings. To enroll in APA, prospective participants called the rehabilitation service of the local health authority. New classes were started as waiting lists developed.
To maintain safety and quality standards, physical therapists working for the rehabilitation service recruited and trained the instructors for APA classes. The instructors were generally recruited from among those already working for the gym where the classes would be offered. They were not required to be physical therapists, but they were selected for their experience and motivational qualities. The physical therapists also observed the exercise classes at random intervals to ensure that the exercise protocol was being followed. The rehabilitation service conducted telephone satisfaction surveys of APA participants. If participants reported deterioration in their functional abilities as a reason for stopping exercise, they were immediately scheduled for a physician's visit. Gym instructors contacted the rehabilitation service to report any concern regarding participant health or functioning of their students. All participant complaints or incident reports from the gyms were promptly followed-up by physical therapists who work for the local health authority.
Throughout the APA-stroke evaluation, adverse clinical events (ACEs) that occurred to APA participants either in the APA classes or outside were reported to the study team. Clinical events were classified for gravity, type, and place. An ACE was classified as serious if a subject presented to the emergency room or was admitted to hospital for any reason. Attendance was taken at each class. The study team contacted participants who failed to attend 3 consecutive classes to determine the cause for absence.
Prior to initiation of the community-based APA-stroke program, a pilot safety study of APA-stroke was conducted in an outpatient setting in Empoli, Italy. In the pilot study, 20 subjects with chronic hemiparesis completed a 2-month group exercise class that included home exercise, and they showed significant improvements as measured by the Berg Balance, 6-Minute Timed Walk (6MTW), and Short Physical Performance Battery (SPPB).23 There were no serious ACEs during the pilot.
Subjects
Study subjects included 93 stroke survivors with chronic mild-to-moderate hemiparetic gait deficits who resided in 1 of 3 neighboring health authorities in Tuscany—Empoli, Pisa, and Florence. Forty-nine subjects recruited from Empoli were enrolled in the APA-stroke program. Forty-four stroke survivors recruited from Florence and Pisa served as nonexercising controls and received usual care. Subjects were recruited for the APA program through flyers, word-of-mouth, and notification of physicians by the local health authority. Random assignment of subjects to APA-stroke versus control groups was considered, but deemed politically unacceptable by the local health authorities. Usual care consisted of medical care as needed, but no additional exercise program. The study was powered based on the data obtained in the pilot program and the results of glucose tolerance in stroke survivors after treadmill training.4,6
The Local Ethical Committee of the Health Authority of Empoli as well as the University of Maryland Internal Review Board reviewed and approved the study. All participants gave written informed consent.
To be eligible for participation, subjects had to be in the chronic phase of stroke recovery (ie, at least 9 months after the acute stroke) and have a mild-to-moderate hemiparetic gait. Nine months poststroke was chosen to ensure that any functional improvement observed was not due to the natural trajectory of recovery.24 Mild-to-moderate hemiparetic gait disorder was defined as the ability to walk independently for 6 minutes at a velocity ≥30 to 90 cm/s, either with or without an assistive device.
Patients were referred to the APA-stroke study by their general practitioner and were further screened by the study team to determine that the subjects met study participation criteria. To be included in the study, chronic stroke survivors had to be at least 40 years of age with onset more than 9 months prior to enrolling in the study and have no comorbid conditions that were contraindications to participation, such as dementia, aphasia with inability to follow 2-step commands, symptomatic heart failure, unstable angina, and hypertension (diastolic BP ≥95 mm Hg; systolic BP ≥160 mm Hg), which would preclude participation in exercise. Stroke survivors who were too deconditioned to meet study criteria were offered an outpatient preparatory course under the supervision of a physical therapist and subsequently reevaluated for inclusion in APA-stroke.
Measurements and Statistical Methods
Gait velocity, measured during the 6MTW, was the primary outcome measure for the study. The course in each location was 10 meters long, and subjects were required to make 180° turns. Evaluators received a 2-day training session prior to the initiation of the study to ensure that the same procedures for encouraging patients were used in all locations. Evaluators were observed periodically by an external review team to determine whether these procedures were being followed. Subjects were allowed to stop and rest; however, the rest time was included in their total time and computation of gait velocity. Evaluators were not blinded to subject assignment. The evaluation team at each location included a neurologist and a physical therapist. The physical therapists at Empoli did not run the APA exercise classes, but did intermittently observe classes to ensure program adherence.
Participants were evaluated at baseline and at 6 months. Comorbidities were assessed with the Cumulative Illness Rating Scale.25 The Mini Mental State Examination26 was used to screen for dementia, and the Hamilton Rating Scale for Depression27 was used to screen for depression. Stroke impairments were assessed using the Motricity Index.28,29 Various dimensions of mobility were measured using the SPPB,30,31 the 6MTW,32,33 and the Berg Balance Scale.34 Basic activities of daily living (ADL) were profiled using the Barthel Index (BI).35 Participant-rated outcomes related to quality of life were examined using the Stroke Impact Scale (SIS).36 Caregiver burden was measured using the Caregiver Strain Index.37
Oral glucose tolerance tests (OGTTs)38 were performed on 19 subjects in the APA-stroke intervention group at baseline and after 6 months in the APA program. Subjects with diabetes (fasting glucose 125 mg/dL or 2-hour postprandial glucose 200 mg/dL) or hyperglycemia (2-hour postprandial glucose >150) were excluded from analysis (22 of the original 49 APA participants), as were 6 subjects who refused OGTT. OGTT was performed only for the subjects in the APA group, not for control subjects.
For each outcome variable and subject, a within-subject change (6 months minus baseline) was computed. The mean within-subject change in the APA group was compared with the mean within-subject change in the control group using analysis of variance adjusted for age, sex, and initial values of the outcome variables. Values at baseline in the APA intervention and control groups were compared using χ2 tests for dichotomous variables and the paired 2-tailed t test for continuous variables. All data are expressed as mean ± standard error.
Results
A total of 93 people met participation criteria at baseline, 49 in the APA intervention group and 44 in the control group. Seventy-eight subjects completed the study (40 in the intervention group and 38 in the control group; see Table 1). Two subjects died (1 patient in each group). Serious ACEs that caused prolonged immobilization, and subsequent exclusion from the study, were observed in 4 subjects, 1 in the intervention group and 3 in the control group. No serious ACEs occurred during the APA exercise classes. Two subjects from the control group did not attend the follow-up assessments, and 7 subjects in the APA intervention group dropped the exercise program (4 for transportation problems and 3 for other reasons). Thus, 40 (82%) participants of the APA group and 38 (86%) of the control group completed the 6-month study.
Table 1. Study Population.
| Status | APA Intervention Group | APA Control Group |
|---|---|---|
| Eligible subjects | 49 | 44 |
| Deaths | 1 | 1 |
| Abandonment due to adverse clinical events | 1 | 3 |
| Refusal | 0 | 2 |
| Interrupted APA | 7 | |
| Evaluated subjects | 40 | 38 |
Abbreviation: APA, Adaptive Physical Activity.
At baseline (Table 2), demographic and most other characteristics of the groups were not significantly different. Most notably there was no significant difference between the 2 groups in the primary outcome measure, 6MTW gait velocity. The mean velocity for the 6MTW was 51.1 ± 3.0 cm/s for the APA group and 54.5 ± 2.6 cm/s for the control group. Distances traversed in the 6MTW were comparable with those reported in previous studies39 and were not significantly different at baseline between groups. Additionally, there was no difference in upper limb function. The Motricity Index of the upper limb on the stroke-affected side was 63.7 ± 3.5 for the APA group and 60.6 ± 4.2 for the control group. There was no significant difference in the BI, 79.5 ± 2.6 for the APA group and 85.4 ± 2.0 for the control group.
Table 2. Comparison of Participant Demographic and Other Characteristics for Intervention and Control Groups at Baseline.
| Variables | APA Intervention Group (Mean ± SE) | APA Control Group (Mean ± SE) | P |
|---|---|---|---|
| Age (years) | 66.8 ± 1.4 | 70.0 ± 1.7 | .15 |
| Gender | |||
| Male | 25 | 29 | .29 |
| Female | 15 | 9 | |
| Body mass index | 27.4 ± 0.6 | 26.2 ± 0.6 | .15 |
| Number of falls (annually) | 0.45 ± 0.18 | 0.63 ± 0.23 | .53 |
| Time since stroke (years) | 4.2 ± 0.8 | 3.5 ± 0.5 | .46 |
| Hemiparesis | |||
| Right | 14 | 19 | .27 |
| Left | 26 | 19 | |
| Cumulative Illness Rating Scale comorbidity index | 0.75 ± 0.12 | 0.58 ± 0.12 | .32 |
| Number of pharmaceuticals (number of drugs) | 5.1 ± 0.4 | 5.2 ± 0.4 | .80 |
| MMSE corrected for age and education | 23.6 ± 0.9 | 24.3 ± 0.9 | .55 |
| Systolic BP (standing) | 130.3 ± 2.7 | 135.8 ± 3.1 | .18 |
| Diastolic BP (standing) | 77.0 ± 1.8 | 81.8 ± 1.3 | .04 |
| Motricity Index | |||
| Upper limb paretic side | 63.7 ± 3.5 | 60.6 ± 4.2 | .59 |
| Upper limb non–paretic side | 97.7 ± 1.4 | 98.7 ± 1.0 | .56 |
| Berg Balanced Scale total | 39.9 ± 1.7 | 44.7 ± 1.7 | .04 |
| SPPB | |||
| Gait | 1.78 ± 0.16 | 2.21 ± 0.20 | .09 |
| Repeated chair stand | 1.18 ± 0.13 | 1.84 ± 0.20 | .007 |
| Balance | 1.80 ± 0.26 | 2.68 ± 0.25 | .02 |
| Summary performance score | 4.75 ± 0.45 | 6.74 ± 0.54 | .006 |
| Six-Minute Timed Walk | |||
| Distance | 184.0 ± 11.8 | 194.4 ± 9.2 | .47 |
| Mean velocity (cm/s) | 51.1 ± 3.0 | 54.5 ± 2.6 | .47 |
| SIS | |||
| Strength | 35.8 ± 3.2 | 38 ± 3.2 | .63 |
| Memory and thinking | 73.4 ± 3.7 | 75.8 ± 3.7 | .69 |
| Emotions | 56.4 ± 2.3 | 58.4 ± 1.8 | .47 |
| Communication | 74.6 ± 4.1 | 88.3 ± 2.9 | .01 |
| ADL/IADL | 79.3 ± 4.5 | 66.3 ± 3.6 | .03 |
| Mobility | 68.9 ± 2.6 | 80.7 ± 3.2 | .006 |
| Hand function | 32.5 ± 5.1 | 38 ± 6.5 | .50 |
| Participation/role | 59.4 ± 3.3 | 61.5 ± 4.3 | .70 |
| Stroke Recovery Scale | 52.3 ± 3.7 | 54.7 ± 3.6 | .63 |
| Hamilton Depression Scale | 11.8 ± 1.2 | 10.7 ± 0.9 | .17 |
| Barthel Index | 79.5 ± 2.6 | 85.4 ± 2.0 | .08 |
| Caregiver Stress Index | 5.1 ± 0.5 | 4.8 ± 0.6 | .71 |
Abbreviations: APA, Adaptive Physical Activity; SE, standard error; MMSE, Mini-Mental State Examination; BP, blood pressure; SPPB, Short Physical Performance Battery; SIS, Stoke Impact Scale; ADL, activities of daily living; IADL, instrumental activities of daily living.
At baseline there were a few significant differences between the groups. The control group was significantly less impaired than the APA-stroke group in balance and gait: Berg Balance Scale 39.9 ± 1.7 for the APA group and 44.7 ± 1.7 for the control group; SPPB summary performance score 4.75 ± 0.45 for the APA group and 6.74 ± 0.54 for the control group (P < .04 and .006, respectively). The APA group had significantly lower scores on 2 SIS items, communication and mobility (P < .01 and .006, respectively). The APA group had significantly higher scores than the control group for SIS activities of daily living and instrumental activities of daily living (P < .03).
Six-Month Outcomes
Six-month outcomes are reported in Table 3. The performance of the control group on the 6MTW test declined by 4.7 cm/s, whereas the performance of the APA group increased by 7.1 cm/s. This difference in changes between groups was significant, P < .0001. Similarly, performance on the SPPB declined by 0.84 for the control group, whereas the performance on the SPPB increased by 1.58 in the APA group. Berg Balance scores declined for the control group by 1.5, whereas they increased by 5.1 in the APA group. The differences in changes between the control and APA groups for both SPPB and Berg scores were significant at P < .00004. On the SIS, only participation scores showed significant between-group changes. SIS participation in the control group declined by 9.2, whereas it increased by 11.6 in the APA group (difference in changes between groups was significant, P < .00015). The Motricity Index of the paretic upper extremity improved by 7.4 in the APA group, whereas the value for the control group declined slightly by 2.1. This difference in changes between groups was significant at P < .05. Changes in the BI, other SIS scores, and Index of Caregiver Stress failed to reach statistical significance.
Table 3. Values of Outcome Variables at Baseline and Change (6-Month Value – Baseline Value) Seen Over the 6-Month Study.
| Variable (Range) | APA Intervention Group | Control Group | Pa (Between-Group Change P) | ||||
|---|---|---|---|---|---|---|---|
| Baseline (Mean ± SE) | Change (Mean ± SE) | Within-Group P | Baseline (Mean ± SE) | Change (Mean ± SE) | Within-Group P | ||
| 6MTW gait velocity (cm/s) | 51.1 ± 2.9 | 7.1 ± 1.7 | <.0001 | 54.5 ± 2.6 | −4.7 ± 1.9 | .015 | <.0001 |
| Motricity Index, paralyzed side (0–100) | 63.7 ± 3.5 | 7.4 ± 2.1 | <.03 | 60.6 ± 4.2 | −2.1 ± 4.2 | .65 | <.05 |
| SPPB, summary score (0–12) | 4.75 ± 0.45 | 1.58 ± 0.35 | <.0002 | 6.74 ± 0.54 | −0.84 ± 0.29 | <.04 | <.00004 |
| Berg Balance Score (0–54) | 39.9 ± 1.7 | 5.1 ± 1.5 | <.0001 | 44.7 ± 1.7 | −1.5 ± 1.7 | .07 | <.00015 |
| Barthel Index (0–100) | 79.5 ± 2.6 | 3.9 ± 1.7 | <.02 | 85.4 ± 2.0 | 0.7 ± 1.0 | .28 | .385 |
| Hamiltonb (0–54) | 11.8 ± 1.1 | 4.4 ± 0.9 | <.01 | 10.7 ± 0.9 | 0 ± 0.8 | .88 | <.003 |
| SIS communication (0–100) | 74.6 ± 4.1 | 7.2 ± 3.0 | <.05 | 88.3 ± 2.9 | 1.2 ± 2.0 | .75 | .25 |
| SIS mobility (0–100) | 68.9 ± 2.6 | 6.8 ± 2.5 | .055 | 80.7 ± 3.2 | −2.4 ± 2.1 | .70 | .10 |
| SIS participation (0–100) | 59.4 ± 3.3 | 11.6 ± 3.3 | <.0007 | 61.5 ± 4.3 | −9.2 ± 3.2 | <.007 | <.00015 |
| Caregiver Strain (0–13) | 5.1 ± 0.5 | −1.6 ± 0.5 | <.02 | 4.9 ± 0.6 | −1.4 ± 0.6 | .07 | .09 |
Abbreviations: APA, Adaptive Physical Activity; SE, standard error; 6MTW, 6-Minute Timed Walk; SPPB, Short Physical Performance Battery; SIS, Stoke Impact Scale.
Change in measures between baseline and 6 months between APA and control groups are compared using analysis of variance with correction for age, sex, and initial values of the outcome measures.
Including only subjects with Hamilton Depression Scores ≥8 at baseline.
In assessing depression, we included only those individuals with depressive symptoms at baseline (Hamilton Rating Scale for Depression ≥ 8; APA [n = 15], Control [n = 13]). Participants in the APA group improved by an average score of 4.4 at 6 months, whereas the scores of the participants in the control group were unchanged. The difference in change scores between the APA and control groups were significant at P < .003.
Of the 40 participants who completed the intervention, 28 (70%) attended more than 60% of the classes and 12 (30%) attended less than 60% of the classes. For individuals who attended less than 60% of the sessions, the average change in walking speed was 5.5 cm/s, when compared with 8.4 cm/s for individuals who attended more than 60% of the sessions. Although there was a positive relationship between attendance and change in velocity, the strength of the relationship was modest. Attendance explained 10.5% of the variance in change in walking speed (R2 = .105 using an ordinary least squares regression, excluding outliers, with attendance as a continuous independent variable and change in walking speed as a dependent variable). The low R2 value suggests that other factors such as comorbidities, age, and severity of stroke may be as important as attendance rates (or may be confounders with attendance) in influencing change in walking speed. A larger study population will be required to address this question.
To examine the functional significance of the changes in gait velocity over time, we classified subjects into 3 groups according to gait velocity40—severely disabled (<40 cm/s), limited community ambulators (40-80 cm/s), and full community ambulators (>80 cm/s). Table 4 presents this analysis. In the APA group, the number of subjects severely limited declined from 18 to 10, limited ambulators increased from 17 to 22, and the number of full ambulators increased from 5 to 8. In contrast, the number of severely limited ambulators in the control group increased from 11 to 16, whereas the number of limited ambulators declined from 25 to 19.
Table 4. Distribution of Subjects by Ambulatory Category.
| Ambulation/Velocity | APA, Before |
APA, After |
Control, Before |
Control, After |
|---|---|---|---|---|
| Severe, <40 | 18 | 10 | 11 | 16 |
| Limited, 40 to 80 | 17 | 22 | 25 | 19 |
| Full community, >80 | 5 | 8 | 2 | 3 |
Abbreviation: APA, Adaptive Physical Activity.
Glucose Studies
Glucose levels at baseline and at 6 months for the APA-stroke intervention group are plotted in Figure 1A, and are essentially overlapping. Insulin secretion, however, changed over the interval, as demonstrated in Figure 1B. The mean insulin secretion (area under the insulin curve) declined by 29% after APA training (P = .01, paired t test). The magnitude of this change is comparable with that observed in chronic stroke patients undergoing treadmill training6 and in healthy elderly individuals in response to endurance training.41 We did not measure caloric intake. However, weight loss was not a factor in the APA group. This group actually gained an average of 1.2 kg during the study.
Figure 1. Oral Glucose Tolerance Test (OGTT).
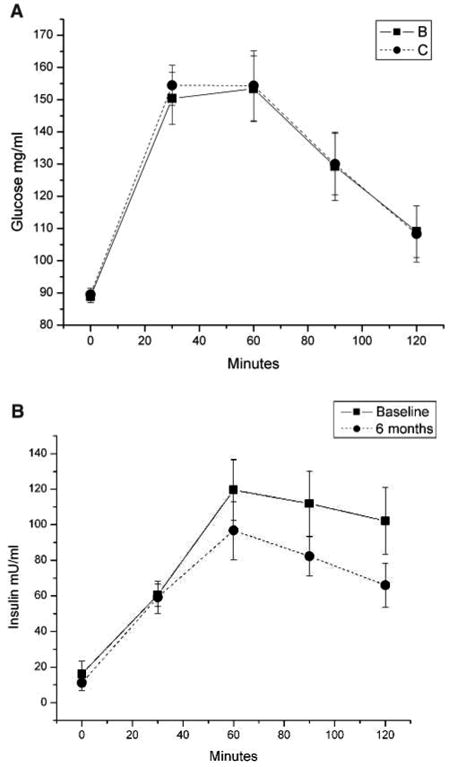
Note: A, Adaptive Physical Activity (APA) participants' serum glucose tolerance test. Diabetic and pre-diabetic patients excluded. OGTT was performed only on the subjects in the APA group, not on control subjects. B, APA participants' serum insulin in the oral glucose tolerance test. Diabetic and pre-diabetic patients excluded. OGTT was performed only on the subjects in the APA group, not on control subjects
Discussion
A major objective of this study was to assess the effectiveness of APA-stroke in a community-based population of patients with moderate hemiparetic disability. The main outcome was gait velocity, a measure strongly associated with both indoor and outdoor disability.42,43 A clear-cut increase in gait velocity in the APA group was paralleled by the results of the other measures of function and physical performance, for example, SPPB, Berg Balance Score, and the Motricity Index. We observed an opposite pattern in the control group, where gait velocity declined during the study period, along with the other measures of function and physical performance. The rapid rate of decline experienced by the control group in this 6-month study is worth noting. Moreover, the level of community participation measured with patient self-report using the SIS parallels the results obtained with the functional measures—increases in the intervention group and declines in the control group. These findings are consistent with the notion that functional limitations in patients with chronic disability are exacerbated by a sedentary lifestyle and that they can be reversed by participation in appropriately designed long-term, regular exercise programs.
The magnitude of change in gait velocity in this study was modest, but within the range of effects achieved by more intensive rehabilitation interventions (4-20 cm/s).44 These changes did have functional significance. Although the proportion of subjects classified as severely limited ambulators decreased from 45% to 25% in the APA group, this number increased from 29% to 42% in the control group.
Metabolic syndrome is a risk factor for adverse outcome in patients with cardiovascular disease. After myocardial infarction, patients with metabolic syndrome have a risk of major cardiovascular events of 2.6 times that of patients without the metabolic syndrome.45 Whether treatment of the metabolic syndrome with medications or drugs will lower this risk is unknown. However, it is interesting to note that the magnitude of the decline in insulin secretion with exercise observed in the current study is comparable with that observed with oral hypoglycemic agents.46
Sustained improvement in community activities with exercise training in stroke patients has been difficult to demonstrate. A recent meta-analysis18 found a medium-size effect of gait training on gait speed and walking distance for stroke patients, while cardiorespiratory and leg strengthening programs produced no effects. However, no convincing evidence of improvement in ADL or health-related quality of life was observed. Several characteristics of the trials included in the meta-analysis merit further scrutiny. First, only 1 study lasted 6 months, and another 19 weeks. All the remaining studies were from 4 to 12 weeks in duration. Second, only limited long-term follow-up of the subjects was attempted in these studies. Ada et al16 found that gains in walking speed, as compared with control, were maintained 3 months after cessation of their 4-week program, whereas in a more recent study of a 12-week exercise program, Mead et al47 found essentially no difference between treatment and control groups 4 months after the program ended. In another more recent study, Studenski et al48 found that a 12-week home exercise program significantly improved physical function and social participation as measured by the SF-36 and SIS, but these gains receded after 6 months. A randomized study of a treadmill aerobic exercise training intervention found a significant increase (17%) in absolute Vo2 peak. Similar findings were noted in measures of gait, with the treadmill-trained group improving 4-fold compared with the physical therapy–exercise control group32; however, as with earlier studies, gains from the laboratory-based treadmill studies have not been demonstrated to translate into ongoing ambulatory activity in the home and community.
The improvements demonstrated on performance measures were not reflected in the BI (a measure of ADLs) or on the Index of Caregiver Stress. The known ceiling effects of the BI49 may have diminished the sensitivity to the APA intervention, but it is also likely that the improvements in balance and gait had only very limited impact on the majority of domains in the BI (feeding, bathing, grooming, dressing, bowel, bladder, toilet use, transfers, mobility, and stairs). Many of the caregivers for the participants enrolled in the APA study still provided transportation to the gymnasiums, so it may be that, from their point of view, the increases in mobility did not result in a striking increase in independence, either in performing ADLs or in requirements for transportation assistance. We were surprised that the SIS mobility scores did not achieve statistical significance between APA and control groups. The scores for this measure in the APA group had a very wide spread, and subjects were required to be able to perform the first 4 items to enter the study, thus introducing a substantial floor effect into this measurement.
Important Characteristics of the APA-Stroke Model
To promote fitness, enhance motor learning, disrupt patterns of learned nonuse, and optimize stroke mobility recovery, exercise behaviors must be integrated into everyday lives of stroke survivors. We believe the following characteristics of the APA-stroke model contribute to its success in this regard. First, the APA-stroke exercise intervention was specifically designed for stroke survivors, based on empirical evidence from previous research.32 Second, the group support demonstrated in the community-based APA-stroke program provided social support50 and may have contributed to improved self-efficacy and outcomes expectations.50-54 Participation was further reinforced by physician referral.55
It is also noteworthy that the local health authority worked closely with the gym instructors as part of chronic disease management. When a participant missed more than 3 consecutive classes or if the instructor observed something about a participant that raised concern, he or she was expected to contact the local health authority. The participant then received a telephone call from a physical therapist and, if appropriate, was referred to his or her physician or scheduled for a consultation with the rehabilitation service.
Community-Based Implementation of Exercise Programs for Stroke
A major barrier to participation in exercise programs for individuals with chronic stroke is the absence of accessible and appropriate exercise programs in the community. Prior to APA, efforts to implement evidence-based exercise interventions for people with chronic stroke in the community settings have not been widespread.14,15 APA-stroke was designed for implementation in community settings. It does not require costly equipment or room modifications and is taught by exercise professionals who have been hired by local gyms and received specialized APA-stroke instructor training. A major factor in sustainability and expansion of APA-stroke is that participants pay for the courses and cost is relatively low.
To the best of our knowledge, the Italian APA-stroke program is the largest of its kind in the world.56
After completion of the APA-stroke evaluation, the local health authority serving Empoli has continued to expand the program. From October 2005 to May 2008, the number of participants grew to 257 in 27 classes in 17 gyms, distributed in 11 out the 15 municipalities served by the local health authority. Because of differences in the financing of health services in Italy and other countries, community implementation of APA-stroke outside of Italy will require different organizational structures. For example, in the United States, new models that foster coordination between the health system (rehabilitation and physicians) and community-based organizations that offer exercise programs (such as Office on Aging Senior Centers, YMCAs, and private gyms) could provide a network of community-based APA-stroke programs with significant geographic access.
Community Safety
Previous research in an outpatient setting has demonstrated that stroke survivors with severe disabilities and comorbidities can safely accomplish exercise training using a treadmill equipped with a safety harness, and the exercise can induce positive functional and physiologic changes, even years following a stroke.32 The APA-study contributes new evidence that stroke survivors with appropriate medical screening can safely exercise in small groups in a community-based exercise program that includes an intervention specifically designed to improve ambulatory function. However, given the mixed results of previous exercise studies for stroke,19 and the vulnerability of the stroke population to falls,13 we recommend that any modification to this program be subjected to tests of safety and efficacy in a controlled setting before introduction to the community. Furthermore, ongoing monitoring to ensure that exercise protocols are being followed is important to safety as well as efficacy.57
Limitations
The major limitation of this study was lack of random assignment of subjects to APA-exercise versus control interventions. We note the robustness of the statistical findings and that at baseline our APA and control groups were similar on most major characteristics examined. However, the possibility of unmeasured selection bias must be considered. Additionally, our sample size was modest and did not cover the entire spectrum of impairments that cause functional limitations and disability after stroke. Moreover, examiners were not blinded to subject group assignment. Finally, the effects of socialization in the experimental group cannot be separated from the effects of the exercise. A pilot study is currently underway to test a more aerobically intense version of APA-stroke, and a large randomized trial will begin shortly to test the Empoli model of APA-stroke protocol versus an attention control featuring socialization and seated exercise.
Functional gains obtained through participation in a program like APA-stroke may lead to improved health outcomes and reduced health care costs. However, larger scale studies will be required to build on our pilot study.
Acknowledgments
This study was funded by the Istituto Superiore di Sanità within the research project “Obtaining Optimal Functional Recovery and Efficient Managed Care for the Chronic Stroke Population” (convenzione n. 530/F20/2) within the framework of the 2003 Memorandum of Understanding between the US National Institutes of Health and Italy's Ministry of Health. This work was also supported by an Intramural Research Program of the National Institute of Child Health and Human Development and the Clinical Center of the National Institutes of Health. The opinions presented in this report reflect the views of the authors and not those of the National Institutes of Health or the US Public Health Service.
We appreciate the support of Enrico Roccatto, MD, Medical Director, and Alessandro Reggiani, General Director, of Azienda Unità Sanitaria Locale 11 di Empoli.
The authors wish to acknowledge the contribution of the Italian AFA-Stroke Study Group: From the Dipartimento della Riabilitazione, Azienda Unità Sanitaria Locale 11, Empoli—Teresa Bertelli, PT, Sara Corsi, PT, Lucia Farsetti, MD, Antonella Notarelli, MD, Adriana Gerini, MD; From the Dipartimento Diagnostica di Laboratorio, Azienda Unità Sanitaria Locale 11, Empoli—Antonio Buggiani, MD, Sergio De Cesaris, MD, Mario Checchi, MD; From the Reparto di Riabilitazione Neurologica ed ortopedica, Casa di Cura Ulivella e Glicini, Firenze—Dimitri Bartoli, PT; From the Unità Operativa Recupero e Rieducazione Funzionale, Azienda Unità Sanitaria Locale 5, Pisa—Elisabetta Geri, MD, Patrizia Lupi, PT, Patrizia Salvadori, PT; From the Laboratorio di Neuropsichiatria, IRCCS Fondazione Santa Lucia, Roma—Gianfranco Spalletta, MD.
From the United States, we wish to thank Dr Pamela Duncan, Duke University, for sharing the SIS in Italian, and faculty and staff from the University of Maryland Claude D. Pepper Older Americans Independence Center (NIH-NIA P30AG28747) and the University of Maryland, Baltimore County, especially Suzanna Roettger, MA.
Footnotes
For reprints and permission queries, please visit SAGE's Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Lind K. A synthesis of studies on stroke rehabilitation. J Chronic Dis. 1982;35:133–149. doi: 10.1016/0021-9681(82)90114-x. [DOI] [PubMed] [Google Scholar]
- 3.Kelley-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 4.Ivey FM, Ryan AS, Hafer-Macko CE, et al. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovasc Dis. 2006;22:368–371. doi: 10.1159/000094853. [DOI] [PubMed] [Google Scholar]
- 5.van de Port IG, Kwakkel G, Bruin M, Lindeman E. Determinants of depression in chronic stroke: a prospective cohort study. Disabil Rehabil. 2007;29:353–358. doi: 10.1080/09638280600787047. [DOI] [PubMed] [Google Scholar]
- 6.Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: a preliminary report. Stroke. 2007;38:2752–2758. doi: 10.1161/STROKEAHA.107.490391. [DOI] [PubMed] [Google Scholar]
- 7.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 8.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 9.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African American group of stroke survivors. Med Sci Sports Exerc. 2000;32:1900–1996. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clin Rehabil. 2004;18:640–651. doi: 10.1191/0269215504cr779oa. [DOI] [PubMed] [Google Scholar]
- 11.Pang M, Eng J, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke. Chest. 2005;127:495–501. doi: 10.1378/chest.127.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivey FM, Hafer-Macko CE, Macko RF. Exercise training for cardiometabolic adaptation after stroke. J Cardiopulm Rehabil Prev. 2008;28:2–11. doi: 10.1097/01.HCR.0000311501.57022.a8. [DOI] [PubMed] [Google Scholar]
- 13.Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporosis Int. 2005;16:1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng J, Chu KS, Kim CM, Dawson AS, Carswell A, Hepburn KE. A community-based group exercise program for persons with chronic stroke. Med Sci Sports Exerc. 2003;35:1271–1278. doi: 10.1249/01.MSS.0000079079.58477.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang M, Eng J, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ada L, Dean C, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 17.Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:870–874. doi: 10.1016/j.apmr.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Port IG, Wood-Dauphinee S, Lindeman E, Kwakkel G. Effects of exercise training programs on walking competency after stroke: a systematic review. Am J Phys Med Rehabil. 2007;86:935–951. doi: 10.1097/PHM.0b013e31802ee464. [DOI] [PubMed] [Google Scholar]
- 19.Saunders DH, Greig CA, Young A, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2004;(1):CD003316. doi: 10.1002/14651858.CD003316.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil. 2007;29:559–566. doi: 10.1080/09638280600924996. [DOI] [PubMed] [Google Scholar]
- 21.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 22.Stuart M, Chard S, Roettger S. Exercise for chronic stroke: a policy perspective. J Rehabil Res Dev. 2008;45:329–336. doi: 10.1682/jrrd.2007.02.0027. [DOI] [PubMed] [Google Scholar]
- 23.Macko RF, Benvenuti F, Stanhope S, et al. Adapted physical activity improves mobility, function, and quality of life in chronic hemiparesis. J Rehabil Res Dev. 2008;45:323–328. doi: 10.1682/jrrd.2007.02.0025. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 25.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund JL, Vieweg BW. The Hamilton Rating Scale for Depression: a comprehensive review. J Operation Psychiatry. 1979;10:149–166. [Google Scholar]
- 28.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. 1990;53:576–579. doi: 10.1136/jnnp.53.7.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wade DT, Collen FM, Robb GF, Warlow CP. Physiotherapy intervention late after stroke and mobility. BMJ. 1992;304:609–613. doi: 10.1136/bmj.304.6827.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 33.Demers C, McKelvie RS, Negassa A, Yusuf S. RESOLVD Pilot Study Investigators. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142:698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 34.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 35.Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233–238. doi: 10.1177/1545968306294729. [DOI] [PubMed] [Google Scholar]
- 36.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale Version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MT. Caregiver Strain Index (CSI) Home Healthc Nurse. 2003;21:197–198. doi: 10.1097/00004045-200303000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Drutz C, Kumar R, et al. Use of the six-minute walk test poststroke: is there a practice effect? Arch Phys Med Rehabil. 2008;89:1686–1692. doi: 10.1016/j.apmr.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Schmid A, Duncan P, Studenski S, et al. Improvements in speed-based gait classification are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 41.Evans EM, Racette SB, Peterson LR, Villareal DT, Greiwe JS, Holloszy JO. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77-87 yr olds. J Appl Physiol. 2005;98:40–45. doi: 10.1152/japplphysiol.00928.2004. [DOI] [PubMed] [Google Scholar]
- 42.Viosca E, Lafuente R, Martinez JL, Almagro PL, Gracia A, Gonzalez C. Walking recovery after an acute stroke: assessment with a new functional classification and the Barthel Index. Arch Phys Med Rehabil. 2005;86:1239–1244. doi: 10.1016/j.apmr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Viosca E, Martinez JL, Almagro PL, Gracia A, Gonzalez C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch Phys Med Rehabil. 2005;86:1234–1238. doi: 10.1016/j.apmr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 45.Tamita K, Katayama M, Takagi T, et al. Impact of newly diagnosed abnormal glucose tolerance on long-term prognosis in patients with acute myocardial infarction. Circ J. 2007;71:834–841. doi: 10.1253/circj.71.834. [DOI] [PubMed] [Google Scholar]
- 46.Forst T, Pfutzner A, Lubben G, et al. Effect of simvastatin and/or pioglitazone on insulin resistance, insulin secretion, adiponectin, and proinsulin levels in nondiabetic patients at cardiovascular risk—the PIOSTAT Study. Metabolism. 2007;56:491–496. doi: 10.1016/j.metabol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Mead GE, Greig CA, Cunningham I, et al. Stroke: a randomized trial of exercise or relaxation. J Am Geriatr Soc. 2007;55:892–899. doi: 10.1111/j.1532-5415.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 48.Studenski S, Duncan P, Perera S, Reker D, Lai SM, Richards L. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. 2005;36:1764–1770. doi: 10.1161/01.STR.0000174192.87887.70. [DOI] [PubMed] [Google Scholar]
- 49.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40:1–8. doi: 10.1682/jrrd.2003.01.0001. [DOI] [PubMed] [Google Scholar]
- 50.Litt MD, Kleppinger A, Judge JO. Initiation and maintenance of exercise behavior in older women: predictors from the social learning model. J Behav Med. 2002;25:83–97. doi: 10.1023/a:1013593819121. [DOI] [PubMed] [Google Scholar]
- 51.Resnick B. Testing a model of exercise behavior in older adults. Res Nurs Health. 2001;24:83–92. doi: 10.1002/nur.1011. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzer RF. Changing risk behaviors and adopting health behaviors: the role for self-efficacy beliefs. In: Bandura A, editor. Self-efficacy in Changing Societies. New York, NY: Cambridge University Press; 1995. pp. 259–289. [Google Scholar]
- 53.Conn VS. Older adults and exercise: path analysis of self-efficacy related constructs. Nurs Res. 1998;47:180–189. doi: 10.1097/00006199-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Sallis JF, Hovell MF, Hofstetter CR. Predictors of adoption and maintenance of vigorous physical activity in men and women. Prev Med. 1992;21:237–251. doi: 10.1016/0091-7435(92)90022-a. [DOI] [PubMed] [Google Scholar]
- 55.Shaughnessy M, Resnick B, Macko RF. Testing a model of exercise behavior following stroke. Rehabil Nurs. 2006;31:15–21. doi: 10.1002/j.2048-7940.2006.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 56.Stuart M, Chard S, Roettger SJ. Exercise for chronic stroke: a policy perspective. J Rehabil Res Dev. doi: 10.1682/jrrd.2007.02.0027. In Press. [DOI] [PubMed] [Google Scholar]
- 57.Bellg A, Borelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]


