Abstract
Background
Cognitive deficits impede stroke recovery. Aerobic exercise (AEX) improves cognitive executive function (EF) processes in healthy individuals, although the learning benefits after stroke are unknown.
Objective
To understand AEX-induced improvements in EF, motor learning, and mobility poststroke.
Methods
Following cardiorespiratory testing, 38 chronic stroke survivors were randomized to 2 different groups that exercised 3 times a week (45-minute sessions) for 8 weeks. The AEX group (n = 19; 9 women; 10 men; 64.10 ± 12.30 years) performed progressive resistive stationary bicycle training at 70% maximal heart rate, whereas the Stretching Exercise (SE) group (n = 19; 12 women; 7 men; 58.96 ± 14.68 years) performed stretches at home. Between-group comparisons were performed on the change in performance at “Post” and “Retention” (8 weeks later) for neuropsychological and motor function measures.
Results
Vo2max significantly improved at Post with AEX (P = .04). AEX also improved motor learning in the less-affected hand, with large effect sizes (Cohen’s d calculation). Specifically, AEX significantly improved information processing speed on the serial reaction time task (SRTT; ie, “procedural motor learning”) compared with the SE group at Post (P = .024), but not at Retention. Also, at Post (P = .038), AEX significantly improved predictive force accuracy for a precision grip task requiring attention and conditional motor learning of visual cues. Ambulation and sit-to-stand transfers were significantly faster in the AEX group at Post (P = .038), with balance control significantly improved at Retention (P = .041). EF measurements were not significantly different for the AEX group.
Conclusion
AEX improved mobility and selected cognitive domains related to motor learning, which enhances sensorimotor control after stroke.
Keywords: Aerobic exercise, Rehabilitation, Cognition, Executive function, Motor learning, Stroke
Approximately 50% to 75% of all stroke survivors have residual cognitive and/or motor disabilities that prevent them from living independently at home.1,2 Information processing speed and executive function (EF) processes (a set of cognitive control processes involved in attention, planning, judgment, decision making, and other higher-order functions) are significantly impaired for more than 60% of these individuals.3,4 Such cognitive deficits hinder the sensorimotor learning required for successful physical rehabilitation and stroke recovery. For example, reduced information processing is associated with poor motor function5,6 and interferes with daily tasks that rely on fast cognitive processing of sequentially ordered movements, such as dressing, toileting, or transfers. 7 Previous studies indicate that attention, decision making, and other cognitive control processes are necessary for balance,8 ambulation,9 driving,10 and reaching/grasping,11 all motor functions that are affected by stroke. Overall, impaired cognition reduces the likelihood of independent living poststroke.1,12
Recently, aerobic exercise (AEX) has been shown to be beneficial for improving EF13–15 for healthy adults, in addition to improving cardiac function, balance, and coordination.16 Yet no studies have examined the effects of AEX on cognition after hemiparetic stroke, or considered if lower extremity AEX promotes generalized sensorimotor learning for motor performance across limbs, such as the nonaffected upper extremity. We performed a randomized clinical pilot study in a small sample of individuals with chronic stroke to determine whether AEX would produce changes in cognitive function and motor learning in this group. The specific hypothesis was that bicycle exercise in individuals with hemiparetic stroke would durably improve EF and motor learning and translate to improved fine motor control of the upper extremity.
Methods
We performed a blinded pilot study. Chronic stroke survivors were randomized to 2 different intervention groups. Research personnel who performed test assessments were blinded to group assignment. Adults who experienced stroke at least 6 months prior to this study were recruited from the Stroke Database at the University of Kansas Medical Center and also the community at large. In all, 76 individuals were screened for the study of whom 36 were excluded because they did not meet the inclusion criteria (n = 17), refused to participate (n = 4), or were unable to travel to the laboratory for regular training sessions (n = 15), thus yielding 40 individuals who met the criteria and agreed to participate in the study. Two participants dropped out of the study after enrollment (1 woman; 1 man) and their data are not included in the analyses, decreasing the sample to 38 individuals (21 women, 17 men). On enrollment, the participants were randomized to either a Stretching Exercise (SE) group (n = 19; 12 women; 7 men; 58.96 ± 14.68 years) or Aerobic Exercise (AEX) group (n = 19; 9 women; 10 men; 64.10 ± 12.30 years; see Table 1). Participants within both groups experienced their stroke injury 4.9 ± 3.3 years prior to entering the study (SE = 5.11 ± 3.53 years; AEX = 4.62 ± 3.21 years.). All participants gave their informed consent to enter this pilot study, which was approved by The Kansas University Medical Center Human Subject Review Committee. Procedures for this study were followed in accordance with institutional guidelines.
Table 1.
Means and Standard Deviations for Between-Group Comparisons at Baselinea
| Mean (SD) | |||
|---|---|---|---|
| Variable | Control (n = 19) | Intervention (n = 19) | P Value |
| Age | 58.96 (14.68) | 64.10 (12.30) | .42 |
| MMSE | 29.00 (1.41) | 28.17 (2.15) | .21b |
| Orpington Total | 2.63 (0.87) | 2.82 (1.00) | .72b |
| Vo2max | 14.67 (5.42) | 14.76 (4.23) | .92b |
| WCST (no. of rules learned) | 2.32 (1.80) | 1.74 (1.66) | .31 |
| Stroop task (interference – baseline) | −27.95 (8.18) | −25.42 (9.11) | .37 |
| Trail-making B-A | 57.95 (43.92) | 75.63 (47.77) | .19b |
| SRTT repeated | −141.50 (113.99) | −95.42 (70.09) | .04b |
| SRTT random | −108.65 (117.96) | −73.77 (126.55) | .40 |
| PGFM | 9.77 (3.05) | 9.40 (3.23) | .62b |
| FM total | 79.42 (35.54) | 75.63 (35.10) | .77b |
| BB control | 38.89 (14.70) | 40.37 (9.69) | .95b |
| GUG fast speed | 25.10 (37.96) | 17.42 (17.57) | .70b |
Abbreviations: MMSE, Mini-Mental State Examination; WCST, Wisconsin Card Sorting Task; SRTT, serial reaction timed task; PGFM, predictive grip force modulation; FM, Fugl-Meyer sensorimotor test; BB, Berg Balance Scale; GUG, Get Up and Go test.
Two-sample T test is used if normality assumption was satisfied. Otherwise Wilcoxon rank sum test is used (see footnote b).
P values calculated using Wilcoxon rank sum test.
The inclusion criteria were (a) single ischemic stroke occurring at least 6 months prior to enrollment; (b) residual hemiparetic deficits in either the upper or lower extremity; (c) Mini-Mental Status Examination (MMSE)17 score of >23, including a perfect score on the 3-step command to ensure comprehension of our protocol; and (d) adequate cardiac function to adhere to the protocol as determined by their personal physician. The exclusion criteria were (a) regularly performing >20 minutes of cardiovascular exercise 3 times a week in their daily routine; (b) alcohol consumption >2 oz liquor, 8 oz wine, or 24 oz beer per day; (c) cardiac history of unstable angina, recent myocardial infarction within the last 3 months, congestive heart failure, significant heart valve dysfunction, or unstable hypertension; (d) other neurological diseases; and (e) hospitalization within the past 3 months or medical conditions that would prevent adherence to the exercise protocol, such as symptomatic peripheral arterial occlusive disease, chronic pain conditions, organ failure, active cancer, unstable diabetes mellitus, or untreated depression.
The SE group performed 45 minutes of upper and lower extremity stretching activities, 3 times per week for 8 weeks (24 sessions) at home. During the 8-week session, a physical therapist contacted participants in the SE group each week to answer questions about their exercise program. Using a stationary bicycle, participants in the AEX group performed progressive, resistive AEX at a target level equal to 70% maximal (max) heart rate (HR) for 45 minutes (based on Karvonen’s formula18), 3 times per week for 8 weeks under the supervision of a physical therapist and exercise physiologist. All AEX sessions included a 5-minute warm-up and cool-down period. Target HR was determined from the metabolic stress test performed at baseline, described in detail below. In week 1, participants bicycled to tolerance for 10-minute to 20-minute duration at 40% to 50% maxHR, but then progressed to the 70% maxHR in week 2. AEX intensity was systematically progressed for each participant by increasing resistance to maintain conditioning at 70% maxHR. Vital signs and scores from the Borg Perceived Exertion Scale19 were recorded for each individual during every session.
All participants underwent a medical exam, metabolic stress test, cognitive assessment, and motor performance assessments at 3 time points: (a) “Baseline,” or the time period prior to the SE or AEX activities; (b) “Post,” 8 weeks after Baseline, when the SE or AEX activities were completed; and (c) “Retention,” 8 weeks after completing the SE or AEX activities (ie, 16 weeks from Baseline). Participants performed a series of neuropsychological and motor tests that were presented in a randomized order at each time point and are described here. Wisconsin Card Sorting Task (WCST) measured rule learning and resistance to perseveration.20 The WCST required participants to sort 64 cards based on color, shape, or number of items, without explicit knowledge of the “rules,” which would ensure the correct answer. Once they make several correct responses, the rule changed and they must learn a new sorting rule. Thus, they were required to simultaneously maintain several sets of rules within working memory and be able to switch rules based on feedback. Stroop task21 measured selective attention and resistance to interference. Performance in a baseline condition (naming the color in a series of colored Xs) was compared with an interference condition (naming the color of ink for a series of color words, eg, “red” printed in blue ink). In both cases, the dependent measure was the number of colors correctly named within 45 seconds. Trail-Making Task (A, B)22 assessed visual search ability, working memory, and attention switching. Using the less-affected hand, participants were required to draw connecting lines between either randomly arranged numbers on a page (trails A), or alternating numbers/letters (trails B). The dependent measure in each condition was the time to complete the task within a 5-minute maximum time limit.
Motor learning tests were also performed. Serial Reaction Timed Task (SRTT; see Figure 1) indexed the capacity for change in an implicit learning skill, or learning without conscious awareness. 23,24 Participants made key presses with their less-affected hand to perform a 12-element sequence of colored circles as they appeared on a computer screen. Sequences were composed of either random stimuli or repeated stimuli. Faster response times (milliseconds) occurred if the participant learned the repeated sequence and could anticipate the upcoming response. Predictive Grip Force Modulation (PGFM) assessed conditional learning ability25 of the relationship between color and weight, information that then could be used to predictively program grip forces used to lift a test object. As previously described,26–29 the novel test object (Figure 2, top panel) has two 6-axis force plates, which measure the horizontal grip force (GF), vertical lift force (LF), and anterior–posterior shear force (SF). The mean GF and LF measures are the focus of this study. Prior to lifting the test object with their less affected hand,26 participants observed the experimenter randomly insert a colored weight (250 g, 500 g, 780 g) into the test object in one condition whereas in another condition the weights were inserted into the object without participants’ awareness (total of 84 lifts across 2 conditions).27 The color–weight association was randomized across time points to ensure a new conditional memory was formed at each assessment. For each lift of the 500-g object weight, the mean peak grip force (pkGF) was measured at a time just prior to somatosensory feedback regarding weight information or at the moment the object lifted from the table.28,29 Thus, pkGF that was accurately matched for the object weight indicated feed-forward or predictive control of the muscular forces.30 Fugl-Meyer Sensorimotor Test (FM)31 scores assessed overall motor function of the involved arm and leg (0–124). Berg Balance Scale (BB)32 scores measured balance and coordination during standing and sitting (0–56). Get Up and Go (GUG)33 measured the participant’s time to rise from a chair, walk 3.048 meters, and return to the chair as fast as possible. Vo2max (mL/kg/min) measures of maximal oxygen uptake to determine aerobic capacity were obtained from the metabolic stress test performed at each time point.
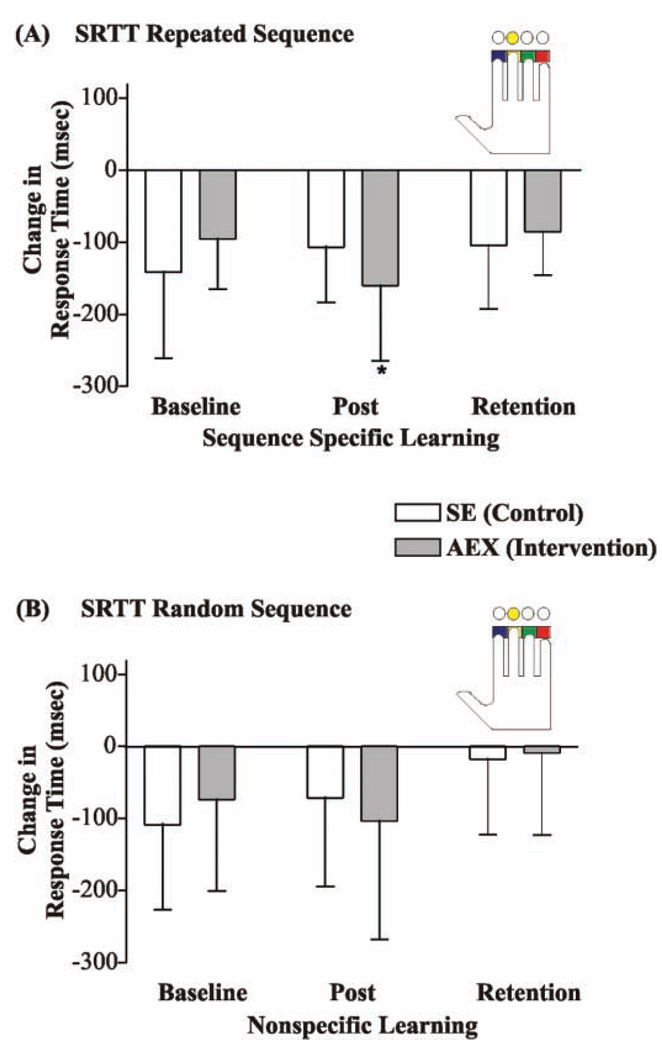
Figure 1. Change in Serial Reaction Timed Task (SRRT) Performance Between Groups.
Note: Using a blocked design, the colored circles appeared one at a time in either a randomized or repeated sequence. Once the colored circle appeared on the computer screen, participants were instructed to press the corresponding key as fast as possible (see inset). To index learning, the change in the mean response time was calculated for the random and also repeated sequences. Compared with the stretching exercise (SE) group, the aerobic exercise (AEX) group made significantly more change in the response time (ie, were faster; P = .024) at “Post” for the repeated sequence (sequence-specific learning) but not for the random sequence (nonspecific learning).
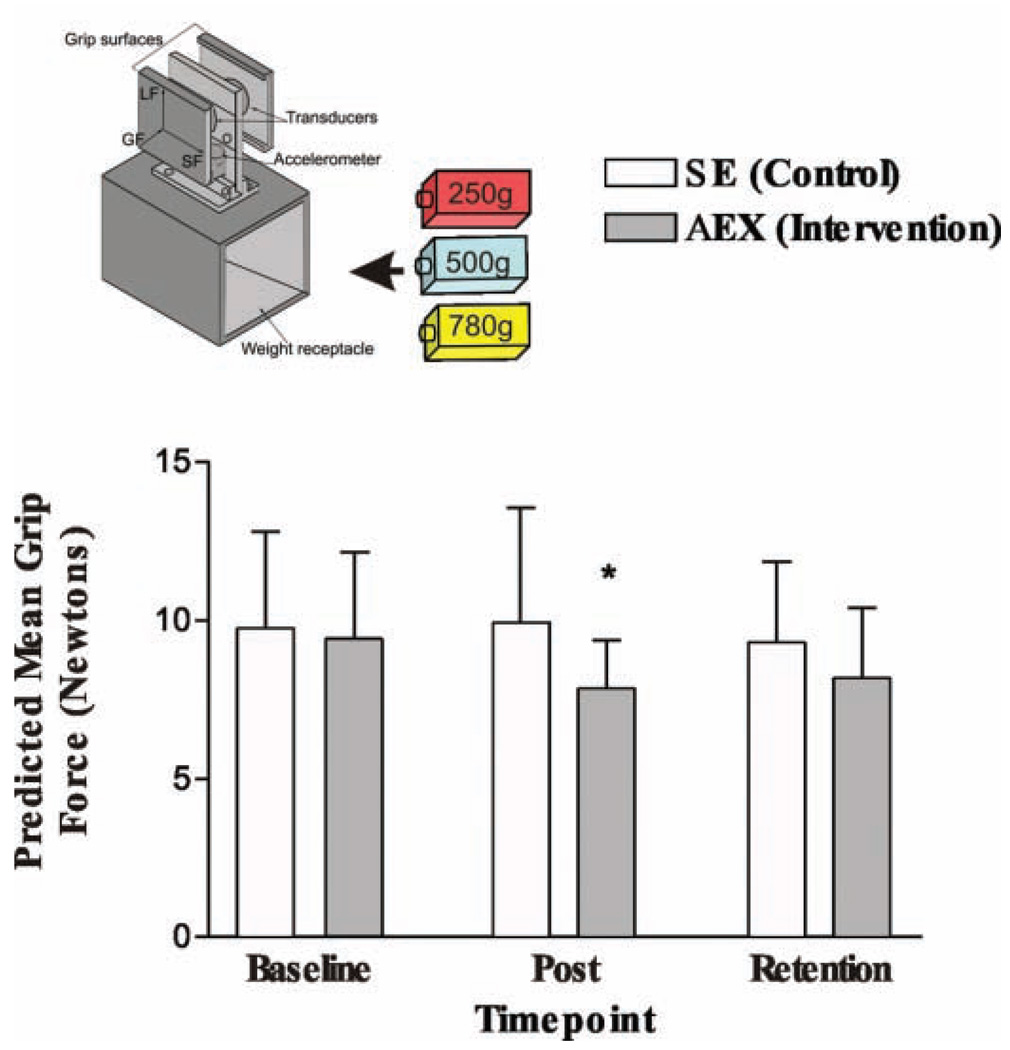
Figure 2. Change in Grip Force Accuracy (Predictive Grip Force Modulation [PGFM]) Between Groups.
Note: The novel test object (top panel) has two 6-axis force plates that measure the horizontal grip force (GF), vertical lift force (LF), and anterior–posterior shear force (SF) applied to the object during precision grip. Prior to each lift, participants observed a colored weight (see inset) that was randomly inserted into the novel test object by the experimenter. Thus, the visual color cue indicated the weight of the object for the subsequent lift. Generating low predictive grip forces (prior to somatosensory feedback) to lift the object with the 500-g weight suggest that individuals learned the color–weight relationship. At “Post,” the aerobic exercise (AEX) group significantly decreased (P < .038) their predicted mean grip forces (measured at lift-off of the object) when lifting the object (500-g weight) compared with the stretching exercise (SE) group.
Within each group, the means and standard deviations were calculated for each variable. Data were analyzed by calculating the change in performance at Post (Post – Baseline) and at Retention (Retention – Baseline) time points for each outcome measure. Performance changes were compared between SE and AEX groups. Depending on the normality distribution for each variable, the change scores were compared using either Wilcoxon rank sum tests or 2-sample T tests with α = .05. Effect sizes (Cohen’s d) were calculated for the statistically significant between-group differences. At Baseline, comparisons were performed for each variable to determine between-group differences. The Baseline scores were compared using either the Wilcoxon Rank Sum test or the Two Sample T test (α = .05), depending on the normality distribution for each variable.
Results
For each group, the means and standard deviations of each variable at Baseline are reported in Table 1. Between-group comparisons at Baseline confirmed that the groups were similar with the exception of SRTT Repeated test (P = .04), which revealed that the participants randomized to the AEX group had significantly slower response times throughout the repeated portion of the test compared with the SE group (see Table 1).
In Table 2, the mean performance levels and between-group statistical result after Intervention are reported for the dependent variables. Bicycling significantly improved Vo2max for the AEX group at Post (P = .040; effect size d = 0.65) compared with the control SE group, but not at Retention. AEX did not affect executive function performance; no significant group differences were observed at Post or Retention on any of the EF measures (WCST, Stroop Task, and Trail-making). AEX significantly improved scores and demonstrated large effect sizes for complex motor learning tasks. Between-group differences in learning on the SRTT repeated sequence task were observed; response time was significant at Post (P = .024; effect size d = 0.91), but not at Retention (Figure 1, Table 2). No between-group differences were observed for SRTT random sequence response time at either Post or Retention. Between-group difference in the PGFM used to lift the object was significant at Post (P = .038; effect size d = −0.79) (Figure 2; Table 2), but not at Retention. Significant functional gain from AEX was also observed in GUG at Post (P = .038; effect size d = −0.70) but not at Retention. Also, a trend toward significance in BB Control was observed at Post (P = .053) and then reached significance at Retention (P = .041; effect size d = −0.70; see Table 2).
Table 2.
Means and Standard Deviations for Assessments at Each Time Point With Between-Group Comparisons of the Intervention Effects
| Mean (Standard Deviation) | Between-Group Comparison, P Value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control SE | Intervention AEX | |||||||
| Baseline | Post | Retention | Baseline | Post | Retention | Post | Retention | |
| Variable | (n = 19) | (n = 19) | (n = 19) | (n = 19) | (n = 19) | (n = 19) | ||
| Vo2max | 14.67 (5.42) | 14.39 (4.99) | 14.62 (5.80) | 14.76 (4.23) | 15.47 (5.13) | 15.00 (5.12) | .040 | .400 |
| WCST (no. of rules learned) | 2.32 (1.80) | 1.89 (1.85) | 1.95 (1.93) | 1.74 (1.66) | 1.74 (1.69) | 1.53 (1.61) | .140 | .620 |
| Stroop task (interference –baseline) | −27.95 (8.18) | −27.16 (10.41) | −27.42 (8.30) | −25.42 (9.11) | −27.37 (8.77) | −25.89 (8.14) | .128 | .661 |
| Trail-making B-A | 57.95 (43.92) | 57.05 (46.41) | 54.32 (53.39) | 75.63 (47.77) | 67.68 (53.83) | 51.22 (35.97) | .280 | .207 |
| SRTT repeated | −141.50 (113.99) | −106.80 (76.25) | −104.50 (87.91) | −95.42 (70.09) | −160.14 (104.66) | −86.00 (59.910) | .024 | .190 |
| SRTT random | −108.65 (117.96) | −71.47 (122.49) | −17.73 (104.63) | −73.77 (126.55) | −103.36 (164.44) | −8.82 (113.79) | .1965 | .5890 |
| PGFM color cues | 9.77 (3.05) | 10.10 (3.62) | 9.28 (2.61) | 9.40 (3.23) | 7.86 (1.53) | 8.18 (2.25) | .038 | .146 |
| FM total | 79.42 (35.54) | 81.42 (36.80) | 80.52 (35.72) | 75.63 (35.10) | 77.84 (34.85) | 76.39 (33.93) | .352 | .202 |
| BB control | 38.89 (14.70) | 39.05 (14.27) | 38.79 (14.11) | 40.37 (9.69) | 41.68 (9.62) | 42.06 (9.87) | .053 | .041 |
| GUG fast speed | 25.10 (37.96) | 29.11 (45.26) | 25.74 (35.84) | 17.42 (17.57) | 15.26 (14.82) | 16.78 (18.32) | .038 | .334 |
Abbreviations: SE, stretching exercise; AEX, aerobic exercise; WCST, Wisconsin Card Sorting Task; SRTT, Serial Reaction Timed Task; PGFM, predictive grip force modulation; FM, Fugl-Meyer sensorimotor test; BB, Berg Balance Scale; GUG, Get Up and Go test.
Discussion
Following lower extremity bicycle exercise, chronic stroke survivors in the AEX group significantly improved motor learning, which is required for daily function. Study participants were presented 2 distinct skilled motor tasks that required them to cognitively comprehend and process visual cues for the motor action. In the SRTT, participants were given either random sequences (to assess nonspecific learning) or repeated sequences (to assess sequence-specific learning). Information processing for the repeated sequence was significantly faster for the AEX group, indicating that AEX participants more rapidly predicted the upcoming key press and subsequent finger response (SRTT; Figure 1). In PGFM, both groups used precision grip to repeatedly grasp and lift a novel object. The colored weights that were inserted into the object cavity between lifts presented the participants with the opportunity to predictively program their grip forces based on learning color–weight relationship. The AEX group significantly improved their predictive force modulation to grasp and lift the test object (Figure 2) compared with SE. Collectively, these findings provide evidence that even a brief 2-month intervention of lower extremity AEX training produces generalized cognitive benefits, including improved implicit and conditional motor learning demonstrable in the less-affected hand of chronic stroke survivors.
The fact that the motor learning improvements occurred using the less-affected hand does not diminish the implication of our results. The faster response times induced through AEX in the SRTT are importantly related to generalizable hemiparetic motor learning poststroke. Rapid sequence-specific learning is impaired following stroke,24,34 yet essential for relearning complex motor tasks such as inserting a key into a lock and then opening a door.35 Performing movements in a specific sequential order is necessary in order to successfully perform many daily tasks. The cognitive-motor interactions used during SRTT activate motor regions, parietal cortex, basal ganglia, and the cerebellum.35,36 These same regions are frequently affected by stroke and are the targets of activity-dependent neuroplasticity during task-specific neurorehabilitation performed with the hemiparetic limbs.37
We recently reported that accurate force modulation is not possible with repetitive task-specific practice after middle cerebral artery stroke, most likely due to faulty sensorimotor integration.28 AEX induced an increase in fingertip force accuracy in PGFM, presumably through accelerated learning of the conditional color–weight relationship. The AEX group used the color–weight information to predictively generate muscular forces that were seemingly unrelated to somatosensory information gained from the previous lift of the object.25,38,39 These visuomotor transformations influence motor planning and movement execution40 for driving or other complex motor skills.25 For example, predictive grasp force is modulated according to visual cues, behavioral experience, and memories related to a particular object. Therefore, grasping a gallon of milk uses different muscular force than grasping the same container when it is empty of milk. Such context-specific visual cues dominate skilled motor behavior during daily life, including motor actions from the hemiparetic limbs after stroke. The frontal cortex, hippocampal system, and basal ganglia are active during conditional motor learning.25
Ploughman et al41 recently reported that a single session of treadmill exercise improved grasping function in the hemiparetic hand but not cognitive function for chronic stroke survivors. Here, we demonstrate that under specific contexts, prolonged exercise improves both motor and cognitive function poststroke. Participants performed the SRTT and PGFM tests with the hand ipsilateral to the side of the lesion, unaffected with spasticity or hemiparesis, suggesting that benefits were due to learning rather than hemiparetic biomechanical improvements. However, prolonged bicycling durations may be required to maintain these learning abilities because both SRTT and PFGM effects were diminished at Retention.
In healthy adults, AEX promotes important hormonal, structural, and functional changes within the brain that are associated with learning.42,43 This pilot study provides initial evidence that select cognitive domains subserving motor learning are improved with AEX after stroke, whereas EF-related tests (Trails, WCST, and Stroop Effect) were unaffected by exercise. The EF tests chosen for this study may lack the sensitivity required to detect change in the stroke population. Or, specific baseline cognition levels may be necessary for EF improvements to occur following brain injury. A recent meta-analysis indicates the magnitude of EF change is associated with the AEX duration, exercise modality and level of fitness.14 Our AEX group significantly improved their Vo2max, yet remained in the “sedentary” category of aerobic fitness, which suggests that the 8-week intervention was inadequate. 18 But, larger studies are needed to define the scope of exercise-mediated effects on EF and determine if age, gender, stroke severity, and lesion location influence treatment response.
Although previous studies have demonstrated AEX improves cardiovascular fitness, indices of insulin sensitivity and glucose tolerance, and mobility after stroke,18,44–49 this is the first report that examines the benefits for cognitive and motor learning. On the basis of this study, where does AEX therapy “fit” in relationship to traditional task-specific stroke rehabilitation? Motor learning and memory processes are important for independent daily function after stroke.50,51 Improved motor learning increases the likelihood that individuals with stroke will successfully navigate complex situations within their environment. 52 As such, AEX therapy may be an important adjunct therapy that assists task-specific rehabilitation.
Although our findings are promising that AEX can improve elements of motor learning in chronic stroke, the results must be cautiously interpreted. The brief interaction we provided to the SE group potentially may have impacted their subsequent test performance. Generally, cognition and fitness abilities were similar between groups at Baseline. Although the SE group demonstrated better performance on the SRTT Repeated test compared with the AEX group, the small sample size may have lacked adequate power to detect true differences. It is possible that recruitment from a sample of convenience may have introduced selection bias. Or, the wide ranges in age and severity of impairments may have confounded the treatment response. Furthermore, the brief AEX training regimen produced only modest fitness gains. Larger randomized studies are necessary to determine the dose and temporal profile of exercise that durably improves cognitive function and motor learning across the spectrum of age and impairment level within chronic stroke survivors.
In conclusion, this pilot study provides initial evidence that AEX improves the speed of information processing, motor learning, implicit memory, and motor function for chronic stroke survivors. Specifically, the cognitive benefits of a bicycle training program extended beyond improving lower extremity mobility to also improving sensorimotor learning and cognitive performance. Hence, AEX may function to augment sensorimotor recovery and motor control through cognitive effects, even years after the hemiparetic stroke. Of the 750 000 Americans who suffer stroke each year,53 more than 60% have cognitive impairments that hinder motor recovery,10,54–58 which underscores the need for further study to determine the temporal profile and dose intensity of AEX to optimize long-term outcomes after stroke. Likely, AEX training will become an important part of contemporary stroke rehabilitation programs.
Acknowledgments
We thank Randolph Nudo, PhD, Steven Wise, PhD, and Jeffrey Burns, MD, for their helpful comments on this manuscript. We also thank Brenda Wessel, PT, Marcio Santos, PT, PhD, Kelli Teson, and Sara Kurtz, PTA, for their technical expertise on the project. We thank the American Heart Association (National Scientist Development Grant) and NIH NICHD NCMRR K01 HD047148-05 funding agencies (B. M. Quaney, principal investigator), KUMC General Clinical Research Center (M01 RR0223940 from the NCRR, a component of NIH), and Center for Biostatistics for their support of this study. B. M. Quaney managed all aspects of this project. L. A. Boyd, J. M. McDowd, J. He, M. S. Mayo, and R. F. Macko assisted in data interpretation and manuscript preparation. L. H. Zahner performed data management and analysis.
Footnotes
References
- 1.Haring HP. Cognitive impairment after stroke. Curr Opin Neurol. 2002;15:79–84. doi: 10.1097/00019052-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hofgren C, Björkdahl A, Esbjörnsson E, Sunnerhagen KS. Recovery after stroke: cognition, ADL function and return to work. Acta Neurol Scand. 2007;115:73–80. doi: 10.1111/j.1600-0404.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 2004;19:1053–1057. doi: 10.1002/gps.1209. [DOI] [PubMed] [Google Scholar]
- 4.Barko-Collo S, Feigin V. The impact of neuropsychological deficits on function stroke outcomes. Neuropsychol Rev. 2006;16:53–64. doi: 10.1007/s11065-006-9007-5. [DOI] [PubMed] [Google Scholar]
- 5.Zinn S, Bosworth HB, Hoenigh HM, Swartzwelder HS. Executive function deficits in acute stroke. Arch Phys Med Rehabil. 2007;88:173–180. doi: 10.1016/j.apmr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Dancause N, Ptito A, Levin MF. Error correction strategies for motor behavior after unilateral brain damage: short-term motor learning processes. Neuropsychologia. 2002;40:1313–1323. doi: 10.1016/s0028-3932(01)00218-4. [DOI] [PubMed] [Google Scholar]
- 7.Walker C, Sunderland A, Sharma J, Walker M. The impact of cognitive impairment on upper body dressing difficulties after stroke: A video analysis of patterns of recovery. J Neurol Neurosurg Psychiatry. 2004;75:43–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Stelmach G, Zelanik H, Lowe D. The influence of aging and attentional demands on recovery from postural instability. Aging. 1990;2:155–161. doi: 10.1007/BF03323910. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff J, Yogev G, Springer S, Simon E, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman L, McDowd JM, Atchley P, Dubinsky R. The role of visual attention in predicting driving impairment in older adults. Psychol Aging. 2005;20:610–622. doi: 10.1037/0882-7974.20.4.610. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan JR. Action-perception coupling judgements of hand-held loads. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and Brain. San Diego, CA: Academic Press; 1996. pp. 415–429. [Google Scholar]
- 12.Saxena SK, Ng TP, Koh G, Yong D, Fong NP. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta Neurol Scand. 2007;115:339–346. doi: 10.1111/j.1600-0404.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 13.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 16.Spirduso WW, MacRae HH, MacRae PG, Prewitt J, Osborne L. Exercise effects on aged motor function. Ann N Y Acad Sci. 1988;515:363–375. doi: 10.1111/j.1749-6632.1988.tb33010.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerety M, Mulrow C, Tuley M, et al. Development and validation of a physical performance instrument for the functionally impaired elderly: the Physical Disability Index (PDI) J Gerontol. 1993;48:M33–M38. doi: 10.1093/geronj/48.2.m33. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109:2031–2041. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 20.Drewe EA. The effect of type and area of brain lesion on Wisconsin card sorting test performance. Cortex. 1974;10:159–170. doi: 10.1016/s0010-9452(74)80006-7. [DOI] [PubMed] [Google Scholar]
- 21.Hicks LE. Some effects of anxiety and cognitive style upon pursuit rotor learning. Br J Soc Clin Psychol. 1975;14:155–168. doi: 10.1111/j.2044-8260.1975.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell WE, Reynolds DM, De Soto CB. Neuropsychological Impairment Scale (NIS): initial validation study using trailmaking test (A & B) and WAIS digit symbol (scaled score) in a mixed grouping of psychiatric, neurological, and normal patients. J Clin Psychol. 1983;39:746–748. doi: 10.1002/1097-4679(198309)39:5<746::aid-jclp2270390517>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Nissan MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- 24.Boyd LA, Quaney BM, Pohl PS, Winstein CJ. Learning implicitly: effects of task and severity after stroke. Neurorehabil Neural Repair. 2007;21:444–454. doi: 10.1177/1545968307300438. [DOI] [PubMed] [Google Scholar]
- 25.Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- 26.Quaney BM, Perera S, Maletsky R, Luchies CW, Nudo RJ. Impaired grip force modulation in the ipsilesional hand after unilateral middle cerebral artery stroke. Neurorehabil Neural Repair. 2005;19:338–349. doi: 10.1177/1545968305282269. [DOI] [PubMed] [Google Scholar]
- 27.Nowak DA, Koupan C, Hermsdorfer J. Formation and decay of sensorimotor and associative memory in object lifting. Eur J Appl Physiol. 2007;100:719–726. doi: 10.1007/s00421-007-0467-y. [DOI] [PubMed] [Google Scholar]
- 28.Quaney B, Nudo RJ, Cole KJ. Can internal models of objects be utilized for different prehension tasks? J Neurophysiol. 2005;93:2021–2027. doi: 10.1152/jn.00599.2004. [DOI] [PubMed] [Google Scholar]
- 29.Quaney B, Rotella DL, Peterson C, Cole KJ. “Sensorimotor memory” for fingertip forces: evidence for a task-independent motor memory. J Neurosci. 2003;23:1981–1986. doi: 10.1523/JNEUROSCI.23-05-01981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- 31.Gladstone D, Danells C, Black S. The Fugl-Meyer Assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 32.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 suppl 2:S7–S11. [PubMed] [Google Scholar]
- 33.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go”test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- 34.Boyd LA, Winstein CJ. Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neurosci Lett. 2001;298:65–69. doi: 10.1016/s0304-3940(00)01734-1. [DOI] [PubMed] [Google Scholar]
- 35.Forkstam C, Petersson K. Towards an explicit account of implicit learning. Curr Opin Neurol. 2005;18:435–441. doi: 10.1097/01.wco.0000171951.82995.c4. [DOI] [PubMed] [Google Scholar]
- 36.Grafton S, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- 37.Dobkin B. Neurobiology of rehabilitation. Ann N Y Acad Sci. 2004;1038:148–170. doi: 10.1196/annals.1315.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrides M. Visuo-motor conditional associative learning after frontal and temporal lesions in the human brain. Neuropsychologia. 1997;32:989–997. doi: 10.1016/s0028-3932(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 39.Ameli M, Dafotakis M, Fink GR, Nowak DA. Predictive force programming in the grip-lift task: the role of memory links between arbitrary cues and object weight. Neuropsychologia. 2008;46:2383–2388. doi: 10.1016/j.neuropsychologia.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Messinger A, Squire LR, Zola SM, Albright TD. Neural correlates of knowledge: stable representation of stimulus associations across variations in behavioral performance. Neuron. 2005;48:359–371. doi: 10.1016/j.neuron.2005.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ploughman M, McCarthy J, Bosse M, Sullivan H, Corbett D. Does treadmill exercise improve performance of cognitive or upper extremity tasks in people with chronic stroke? A randomized cross-over trial. Arch Phys Med Rehabil. 2008;89:2041–2047. doi: 10.1016/j.apmr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 42.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 45.Ivey FM, Womack CJ, Kulaputana O, Dobrovolny CL, Wiley LA, Macko RF. A single bout of walking exercise enhances endogenous fibrinolysis in stroke patients. Med Sci Sports Exerc. 2003;35:193–198. doi: 10.1249/01.MSS.0000048634.89370.06. [DOI] [PubMed] [Google Scholar]
- 46.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 48.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 50.McDowd JM, Filion DL, Pohl PS, Richards LG, Stiers W. Attentional abilities and functional outcomes following stroke. J Gerontol B Psychol Sci Soc Sci. 2003;58:P45–P53. doi: 10.1093/geronb/58.1.p45. [DOI] [PubMed] [Google Scholar]
- 51.Pohjasvaara T, Leskela M, Vataja R, et al. Post-stroke depression, executive dysfunction and functional outcome. Eur J Neurol. 2002;9:269–275. doi: 10.1046/j.1468-1331.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 52.Mussa-Ivaldi F, Bizzi E. Motor learning through the combination of primitives. Philos Trans R Soc Lond B Biol Sci. 2000;355:1755–1769. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heart Disease and Stroke Statistics—2005 Update. Dallas, TX: American Heart Association; 2005. American Heart Association. [Google Scholar]
- 54.Hoffman M, Sacco R, Mohr J, Tatemichi T. Higher cortical function deficits among acute stroke patients: the stroke data bank experience. J Stroke Cerebrovasc Dis. 1997;6:114–120. doi: 10.1016/s1052-3057(97)80226-1. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman M. Higher cortical function deficits after stroke: an analysis of 1000 patients from a dedicated cognitive stroke registry. Neurorehabil Neural Repair. 2001;15:113–127. doi: 10.1177/154596830101500205. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra P, Jager H, Parton A, et al. Spatial working memory capacity in unilateral neglect. Brain. 2005;128:424–435. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- 57.Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Res Cogn Brain Res. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43:917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]