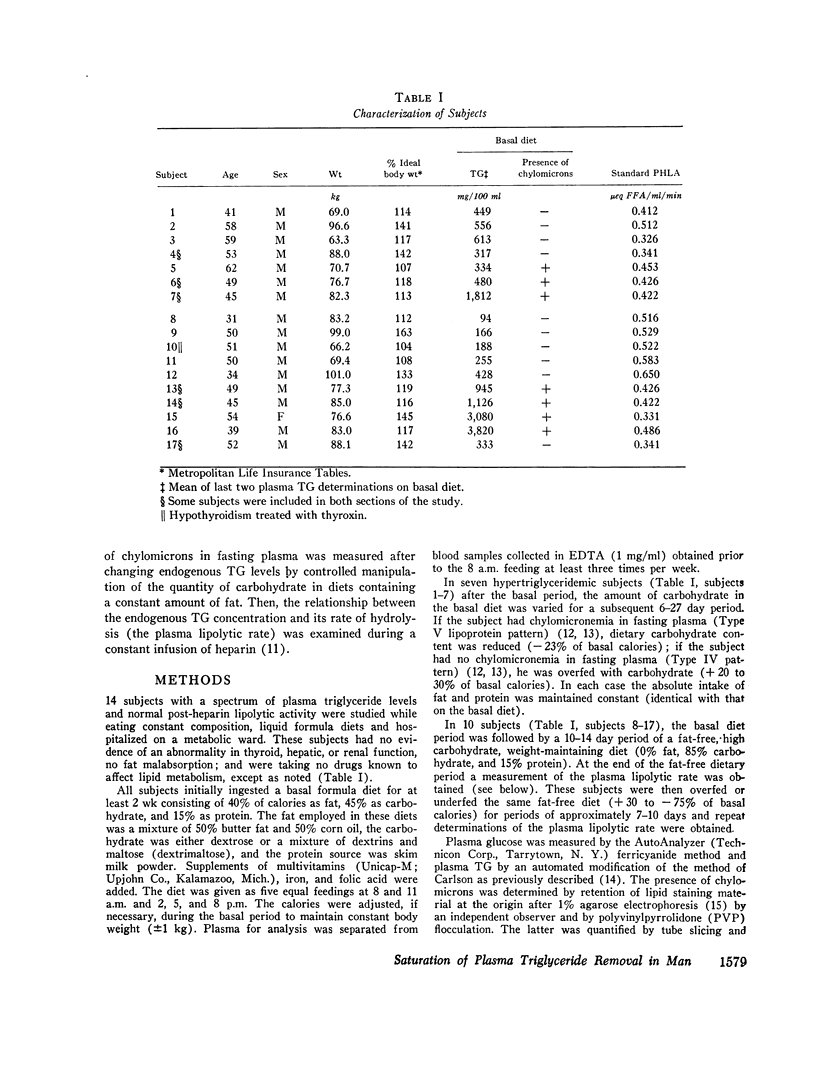
Abstract
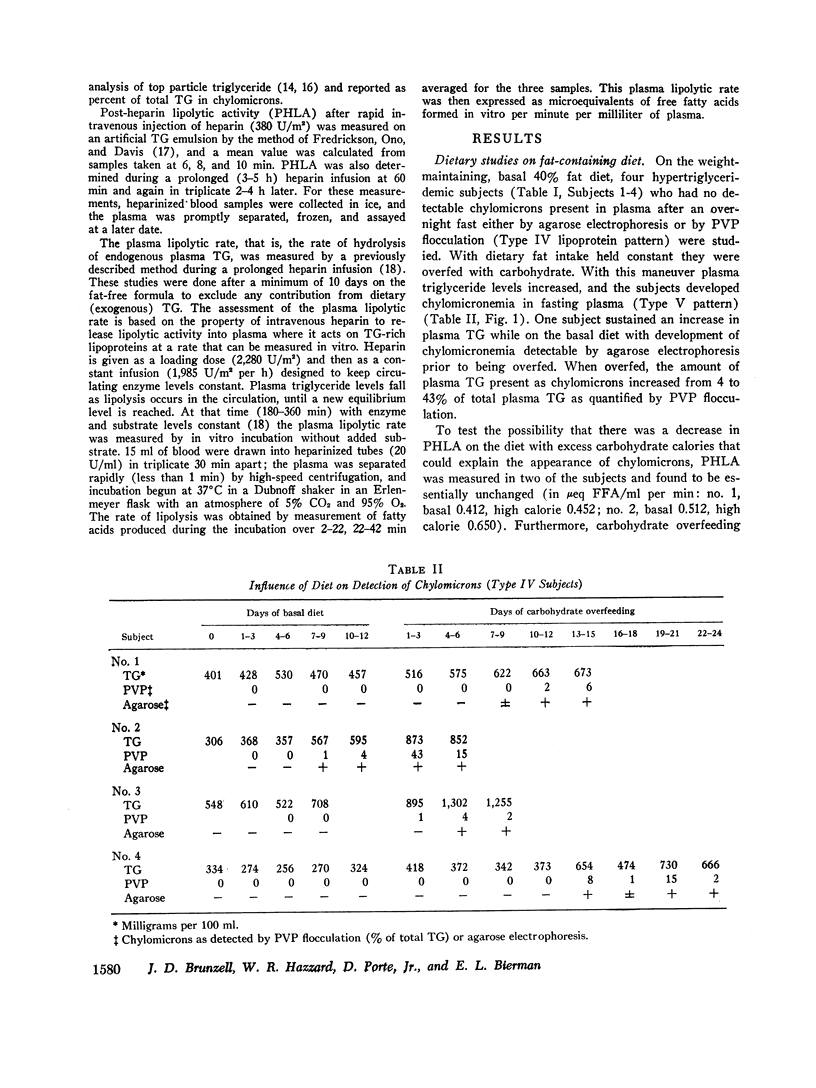
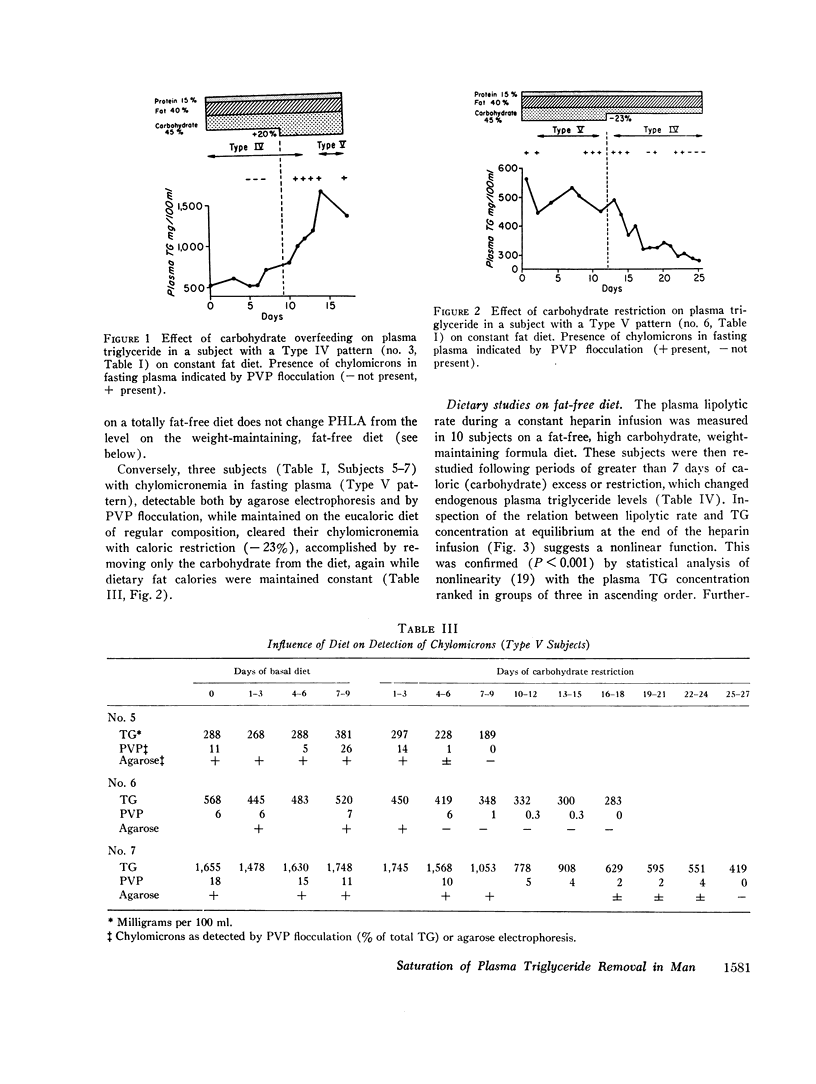
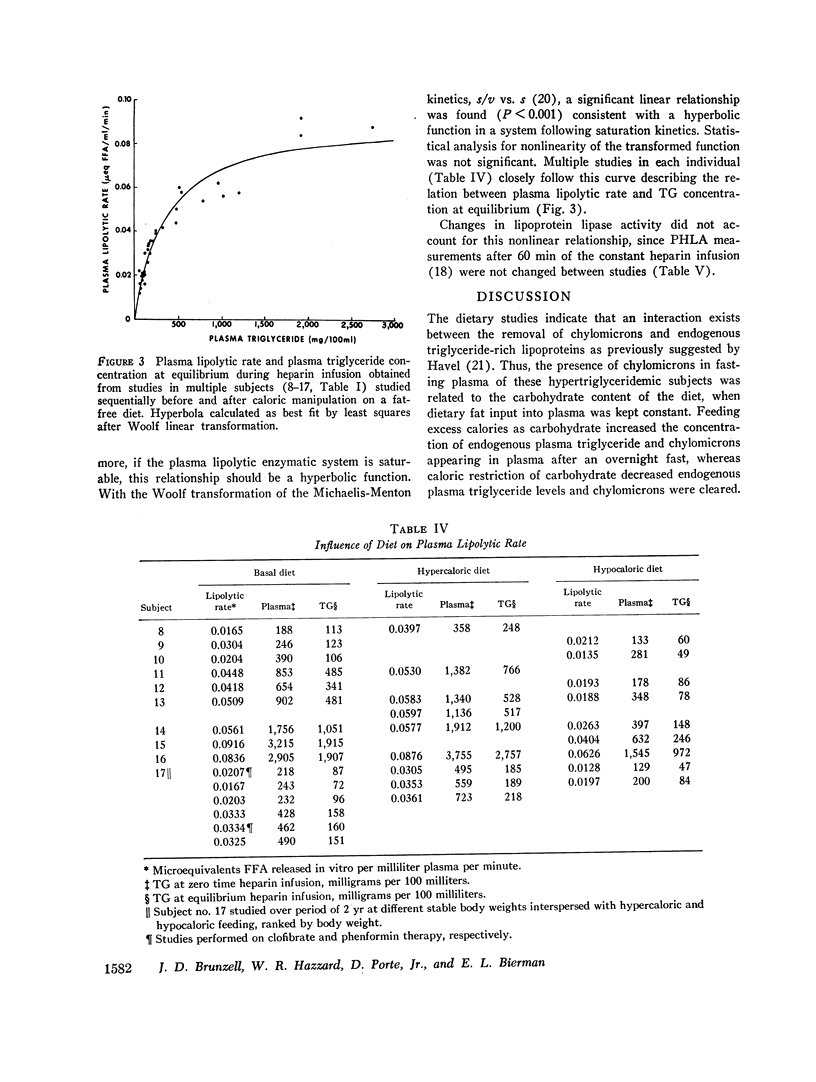
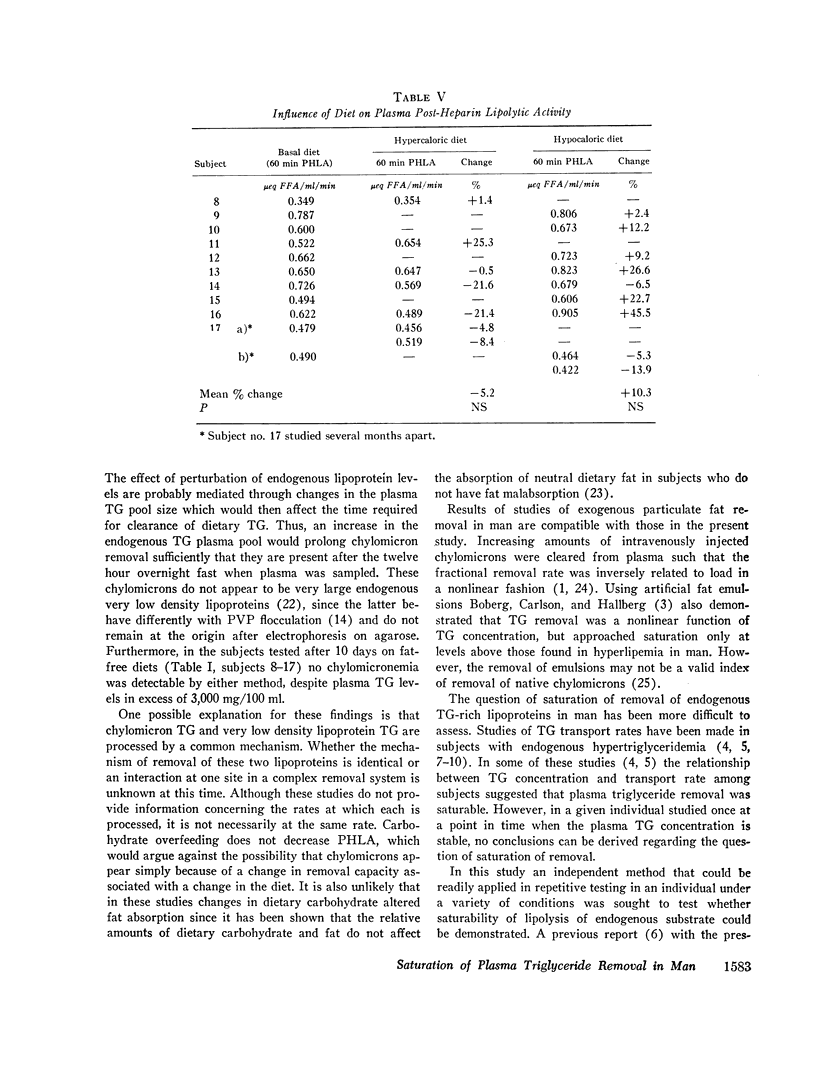
Hypertriglyceridemic subjects were fed diets in which dietary fat calories were held constant, but carbohydrate calories were varied. Three subjects with fasting chylomicronemia (Type V) were given less carbohydrate and four subjects without fasting chylomicronemia (Type IV) were fed diets with more calories as carbohydrate. The restricted carbohydrate intake led to disappearance of chylomicronemia in those subjects who had chylomicronemia on a normal diet (Type V to IV). In those subjects without chylomicronemia, chylomicronemia appeared in response to increased carbohydrate intake (Type IV to V). Thus chylomicron concentrations in plasma were altered even though fat intake and presumably chylomicron input into plasma was kept constant. These findings provide evidence for saturation of chylomicron removal mechanisms by alteration of endogenous triglyceride-rich lipoprotein concentrations. They suggest that chylomicrons compete with very low density lipoproteins for similar removal mechanisms. The relationship between endogenous triglyceride concentration and the lipolytic activity in plasma following heparin was then evaluated with the use of long-term heparin infusions to release and maintain lipolytic activity in the circulation. 10 subjects were placed on fatfree diets to remove circulating dietary fat. The plasma lipolytic rate during the heparin infusion was measured consecutively on different days in individuals whose triglyceride concentrations were varied by either increasing or decreasing calories. The lipolytic rate was curvilinearly related to the plasma triglyceride concentrations. This curvilinear relationship followed Michaelis-Menton saturation kinetics over a wide range of triglyceride concentrations on fat-free, high-carbohydrate diets, in multiple studies in a group of individuals. These studies suggest that endogenous and exogenous triglyceride compete for a common, saturable, plasma triglyceride removal system related to lipoprotein lipase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIERMAN E. L., HAMLIN J. T., 3rd A preparation of C14-labeled triglyceride in plasma as a tracer form plasma particulate fat. Proc Soc Exp Biol Med. 1962 Mar;109:747–750. doi: 10.3181/00379727-109-27326. [DOI] [PubMed] [Google Scholar]
- BIERMAN E. L., PORTE D., Jr, O'HARA D. D., SCHWARTZ M., WOOD F. C., Jr CHARACTERIZATION OF FAT PARTICLES IN PLASMA OF HYPERLIPEMIC SUBJECTS MAINTAINED ON FAT-FREE HIGH-CARBOHYDRATE DIETS. J Clin Invest. 1965 Feb;44:261–270. doi: 10.1172/JCI105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont J. L., Carlson L. A., Cooper G. R., Fejfar Z., Fredrickson D. S., Strasser T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull World Health Organ. 1970;43(6):891–915. [PMC free article] [PubMed] [Google Scholar]
- Bierman E. L., Brunzell J. D., Bagdade J. D., Lerner R. L., Hazzard W. R., Porte D., Jr On the mechanism of action of atromid-S on triglyceride transport in man. Trans Assoc Am Physicians. 1970;83:211–224. [PubMed] [Google Scholar]
- Boberg J., Carlson L. A., Hallberg D. Application of a new intravenous fat tolerance test in the study of hypertriglyceridaemia in man. J Atheroscler Res. 1969 Mar-Apr;9(2):159–169. doi: 10.1016/s0368-1319(69)80051-7. [DOI] [PubMed] [Google Scholar]
- DILUZIO N. R., RIGGI S. J. THE RELATIVE PARTICIPATION OF HEPATIC PARENCHYMAL AND KUPFFER CELLS IN THE METABOLISM OF CHYLOMICRONS. J Reticuloendothel Soc. 1964 Jul;1:248–263. [PubMed] [Google Scholar]
- Eaton R. P. Synthesis of plasma triglycerides in endogenous hypertriglyceridemia. J Lipid Res. 1971 Jul;12(4):491–497. [PubMed] [Google Scholar]
- FREDRICKSON D. S., LEES R. S. A SYSTEM FOR PHENOTYPING HYPERLIPOPROTEINEMIA. Circulation. 1965 Mar;31:321–327. doi: 10.1161/01.cir.31.3.321. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K., DAVIS L. L. LIPOLYTIC ACTIVITY OF POST-HEPARIN PLASMA IN HYPERGLYCERIDEMIA. J Lipid Res. 1963 Jan;4:24–33. [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Feb 2;276(5):273–concl. doi: 10.1056/NEJM196702022760507. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTEL P. J., DENBOROUGH M. A., O'DEA J. Disposal of human chylomicrons administered intravenously in ischemic heart disease and essential hyperlipemia. Circ Res. 1962 May;10:786–791. doi: 10.1161/01.res.10.5.786. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Polymorphism of plasma triglyceride kinetics in normal human adult subjects. Acta Med Scand. 1971 Jul-Aug;190(1-2):49–59. doi: 10.1111/j.0954-6820.1971.tb07395.x. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- O'Hara D. D., Porte D., Jr, Williams R. H. Use of constant composition polyvinylpyrrolidone columns to study the interaction of fat particles with plasma. J Lipid Res. 1966 Mar;7(2):264–269. [PubMed] [Google Scholar]
- Porte D., Jr, Bierman E. L. The effect of heparin infusion on plasma triglyceride in vivo and in vitro with a method for calculating triglyceride turnover. J Lab Clin Med. 1969 Apr;73(4):631–648. [PubMed] [Google Scholar]
- Quarfordt S. H., Frank A., Shames D. M., Berman M., Steinberg D. Very low density lipoprotein triglyceride transport in type IV hyperlipoproteinemia and the effects of carbohydrate-rich diets. J Clin Invest. 1970 Dec;49(12):2281–2297. doi: 10.1172/JCI106448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Hill D. B., Gross R. C., Farquhar J. W. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 1965 Nov;44(11):1826–1833. doi: 10.1172/JCI105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Jones A. L., Krauss R. M., Shafrir E. A biochemical and morphologic study of very low density lipoproteins in carbohydrate-induced hypertriglyceridemia. J Clin Invest. 1971 Jun;50(6):1355–1368. doi: 10.1172/JCI106615. [DOI] [PMC free article] [PubMed] [Google Scholar]



