Abstract
OBJECTIVE
To assess the effectiveness of structured blood glucose testing in poorly controlled, noninsulin-treated type 2 diabetes.
RESEARCH DESIGN AND METHODS
This 12-month, prospective, cluster-randomized, multicenter study recruited 483 poorly controlled (A1C ≥7.5%), insulin-naïve type 2 diabetic subjects from 34 primary care practices in the U.S. Practices were randomized to an active control group (ACG) with enhanced usual care or a structured testing group (STG) with enhanced usual care and at least quarterly use of structured self-monitoring of blood glucose (SMBG). STG patients and physicians were trained to use a paper tool to collect/interpret 7-point glucose profiles over 3 consecutive days. The primary end point was A1C level measured at 12 months.
RESULTS
The 12-month intent-to-treat analysis (ACG, n = 227; STG, n = 256) showed significantly greater reductions in mean (SE) A1C in the STG compared with the ACG: −1.2% (0.09) vs. −0.9% (0.10); Δ = −0.3%; P = 0.04. Per protocol analysis (ACG, n = 161; STG, n = 130) showed even greater mean (SE) A1C reductions in the STG compared with the ACG: −1.3% (0.11) vs. −0.8% (0.11); Δ = −0.5%; P < 0.003. Significantly more STG patients received a treatment change recommendation at the month 1 visit compared with ACG patients, regardless of the patient’s initial baseline A1C level: 179 (75.5%) vs. 61 (28.0%); <0.0001. Both STG and ACG patients displayed significant (P < 0.0001) improvements in general well-being (GWB).
CONCLUSIONS
Appropriate use of structured SMBG significantly improves glycemic control and facilitates more timely/aggressive treatment changes in noninsulin-treated type 2 diabetes without decreasing GWB.
Self-monitoring of blood glucose (SMBG) is widely recognized as a core component of effective diabetic self-management (1–3). Although most evidence indicates that SMBG contributes to good glycemic control among type 1 (4,5) and type 2 diabetic (6,7) patients, it remains uncertain whether SMBG use is efficacious in insulin-naïve type 2 diabetic patients. Current evidence in this latter population is mixed, with some studies pointing to significant glycemic benefits resulting from SMBG use (8–10), while others have shown no significant benefits (11–13). Given the growing cost of current type 2 diabetic care, it is important to determine whether resources devoted to SMBG in the insulin-naïve population are justified and are effectively applied.
Inconsistent findings seen in studies of insulin-naïve type 2 diabetic patients may be due, in part, to differences in key design issues, such as subject selection criteria (e.g., whether or not patients had poor glycemic control at study entry), critical content differences in the actual SMBG intervention (e.g., whether physicians were privy to patient SMBG data), fidelity of treatment delivery (e.g., the same physicians cared for patients from multiple study groups), and/or intervention adherence (e.g., whether patients actually completed the SMBG study protocol as directed). A review of these issues was published previously (14). We developed a comprehensive, structured SMBG intervention package that addresses these design issues and encourages patients and physicians to work collaboratively to collect, interpret, and appropriately use structured SMBG data. Our study was designed to investigate the effect of this intervention on glycemic control in poorly controlled, insulin-naïve type 2 diabetic patients compared with enhanced usual care. Additionally, we assessed the effect of this intervention on SMBG frequency, timing and intensity of treatment modification, and general well-being (GWB).
RESEARCH DESIGN AND METHODS
The Structured Testing Program (STeP) is a 12-month, cluster-randomized, multicenter comparison between poorly controlled (A1C ≥7.5%), noninsulin-treated type 2 diabetic patients using structured SMBG in conjunction with enhanced usual care (structured testing group [STG]) and an active control group (ACG) that received enhanced usual care only. Enhanced usual care included quarterly clinic visits that focused specifically on diabetes management, free blood glucose meters and strips, and office point-of-care A1C capability.
Patients were recruited from primary care practice sites across the eastern U.S., which were stratified to STG or ACG. This included both small and large practices serving communities with a range of patient education, social class, and ethnicity that reflected the diversity of primary care settings in the U.S. The use of a stratified, cluster-randomized design ensured that physicians cared for patients from one study group only. Each site generated a list of all patients who met age, diagnosis, and A1C inclusion criteria from their patient databases or chart review. Participating physicians reviewed the list and eliminated patients whom they felt should not participate in the study (e.g., dementia, psychosis, recent emotional trauma). Patients were then randomly selected from the list using a study-defined protocol until the predetermined sample size was reached.
Inclusion criteria were: duration of type 2 diabetes >1 year; aged ≥25 years; A1C level 7.5–12.0%; currently treated by diet, exercise, oral diabetes medication, and/or injectable incretin mimetic; able to read and write English without assistance; and had not participated in any other research protocol within the last 30 days. Exclusion criteria were: type 1 diabetes; managed with insulin at the start of the study; C-peptide level ≤0.50 ng/mL; used systemic oral or inhaled steroids more than 14 days within the last 3 months; treated with chemotherapy or radiation therapy; pregnant or breast-feeding; or had severe depression or other severe psychological conditions.
The study protocol was approved by the Copernicus Group (Central Institutional Review Board) and is in compliance with the Helsinki Declaration (15). Written informed consent was obtained from all subjects.
Procedures
The study’s duration was 12 months with patient visits occurring at initial screening and baseline followed by visits at months 1, 3, 6, 9, and 12. At screening, investigators recorded demographics, collected relevant medical history, performed physical examinations, collected laboratory samples (e.g., A1C, lipids), and documented all current medications. Patients completed the STeP questionnaire, which included measures of self-care, diabetes-related distress, depression, and GWB. A description of these measures was previously published (14). A baseline visit was scheduled within 14 days. At the baseline visit, laboratory results were reviewed. Patients in both arms received a free blood glucose meter and test strips (Accu-Chek Aviva meter system; Roche Diagnostics, Indianapolis, IN), and they were instructed in their use.
At all subsequent visits (months 1, 3, 6, 9, and 12), ACG and STG clinic staff collected laboratory samples, recorded changes in medications, and performed brief physical examinations. Point-of-care A1C equipment (A1CNow+ test kit; Bayer Healthcare, Tarrytown, NY) was provided to all practices for clinical use only to assure that differential availability of the equipment did not affect outcomes. Patients in both groups brought their meters to each subsequent visit for electronic data uploading; physicians and clinic staff were blinded to these data and all other study-collected measures. Patients also reported all changes made to their diabetes regimen since their last visit. All patients completed the STeP questionnaire and a post-visit questionnaire to record physician discussion of SMBG results and recommendations for pharmacologic and lifestyle changes that occurred during the visit.
Intervention
STG participants used the Accu-Chek 360° View blood glucose analysis system (Roche Diagnostics), a validated tool (16) that enabled patients to record/plot a 7-point SMBG profile (fasting, preprandial/2-h postprandial at each meal, bedtime) on 3 consecutive days prior to each scheduled study visit (months 1, 3, 6, 9, and 12), to document meal sizes and energy levels, and to comment on their SMBG experiences. STG participants received training in the use of the Accu-Chek system, including instructions for how to identify problematic glycemic patterns and how best to address such problems through changes in physical activity, portion sizes, and/or meal composition. STG patients and physicians reviewed the completed form at each of the scheduled visits and noted areas of needed medication and lifestyle change. Completion of the Accu-Chek system was prompted via a telephone call from their physician’s office one week prior to their next appointment. ACG subjects did not receive the Accu-Chek system. ACG patients were instructed to use their meter following their physicians’ recommendations but received no additional SMBG prompting, training, or instruction.
STG physicians/staff received training on interpreting the structured data and were provided with an algorithm that described various pharmacologic/lifestyle treatment strategies that could be used in response to the specific SMBG patterns identified. Physicians were free to select from these options based on patient/physician preferences. All STG physicians were contacted regularly over the 12 months of the study to ensure consistency of the intervention over time. ACG physicians and staff received no additional training. STeP Study tools and resources are available at www.behavioraldiabetes.org/studies/STeP-Study.html.
Measurements
The primary end point was change in A1C from screening to 12 months. A1C analysis was conducted by a central laboratory (Covance, Indianapolis, IN) using the Variant II and Variant II Turbo hemoglobin testing systems (Bio-Rad Laboratories, Hercules, CA).
Treatment intensification was calculated using information entered into patient medical records at each clinic visit. These included recommended pharmacologic modification (defined as the initiation of a new medication, increase or decrease in the dose of an existing medication, or termination of an existing medication) and recommended lifestyle modification (defined as any change in diet, exercise, or other self-care behavior). The total number of visits with medication or lifestyle modifications and the time to the first treatment change was recorded for all patients.
Frequency of SMBG for all patients was calculated from blood glucose meter data that were uploaded electronically by the site coordinator directly to a web server at each study visit via the Accu-Chek Smart Pix device (Roche Diagnostics).
GWB was measured using the WHO-5 Well-Being Index assessment tool (17), a widely used, five-item questionnaire with a total score range of from 0–100 (higher scores indicating more positive well-being). Findings regarding other patient-reported outcomes will be presented in subsequent reports.
Statistical analysis
The study was designed to have a 90% power to detect a difference of 0.5% in A1C levels. This was determined using a two-sample t test (two-sided, α = 0.05), assuming a common SD of 1.5%. The estimate of SD in A1C values was inflated from 1.15 to 1.50 because of the clustering effect (18,19). We required a total of 408 patients (204 per study arm) to achieve the specified statistical power. A larger STG sample was initially recruited to account for potentially greater attrition expected in this group over time.
The analysis of change in A1C and other dependent variables was performed using linear mixed models (LMM) analysis with SAS PROC MIXED (20,21). LMM allows for comparisons between groups across study waves over time, along with analyses of moderator and mediator variables within the same analytic frame (20,21). Control variables in all analyses included: baseline dependent variable (A1C); patient age, gender, and race (white/nonwhite) as fixed effects; and practice site and subject as random effects. Missing data were estimated using maximum likelihood methods (22). Based on the mixed model, the least-square estimates of the group differences were obtained and tested for statistical significance. Additional analyses of patient attrition at each step in the protocol also were undertaken.
LMM was performed in two ways using values from all study visits across the 12 months. In the first approach, the analysis focused on the intent-to-treat (ITT) population, which was defined as ACG and STG subjects who completed the baseline and at least one postbaseline visit. The second approach was a per protocol (PP) analysis, which included all ACG and STG patients who adhered to the study protocol. Adherence in the ACG was defined as those who completed the study (with ≥4 visits) and did not use structured SMBG records that were similar to the Accu-Chek 360° View blood glucose analysis system intervention tool. Adherence in the STG was defined as those who completed at least 80% of all blood glucose values on the intervention tool, brought their completed tool to the clinic visit, and reported that their physicians looked at the tool and discussed the results (via the Post-Visit Questionnaire) at ≥4 of the 5 clinic visits.
RESULTS
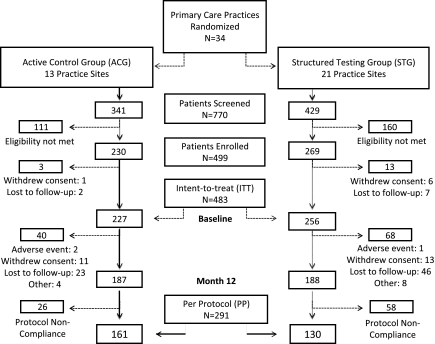
We recruited 34 primary care practices that were then randomized with stratification to ACG (n = 13) or STG (n = 21) (Fig. 1). Of the 770 patients screened, 499 patients were eligible and enrolled in the study. Of these, 7 patients (ACG, n = 1; STG, n = 6) withdrew consent, and 9 patients (ACG, n = 2; STG, n = 7) were lost to follow-up. The remaining 483 patients (ACG, n = 227; STG, n = 256) were included in the ITT cohort. During the study, 15 patients discontinued, 24 withdrew consent, and 69 were lost to follow-up, all primarily because of time or other life demands. Dropouts were slightly younger (P < 0.02), more likely to be African American (P < 0.02), had a higher A1C (P < 0.01), and had fewer comorbid conditions at baseline (P < 0.02). Characteristics of the dropouts were not significantly different between the two study groups. An additional 84 patients (ACG, n = 26; STG, n = 58) were excluded from the PP analyses because of protocol nonadherence. Thus, the PP cohort included 161 (71%) ACG patients and 130 (51%) STG patients.
Figure 1.
Consort diagram.
Site and patient characteristics are summarized in Table 1. Patient demographic and disease-related characteristics at baseline between the two study groups differed only by age and ethnicity. These differences were controlled in all subsequent analyses. There were no intervention-related adverse events. Over the 12 months, no severe hypoglycemic events were reported. The incidence of hypoglycemia (<70 mg/dL), based on downloaded meter data, was 1.9% in the ACG and 1.8% in the STG (P = NS). There were no significant differences between the groups in number of total visits (scheduled study visits plus follow-up visits) over the 12 months (ACG = 5.1 [2.2]; STG = 4.9 [2.6], P = 0.56)
Table 1.
Baseline characteristics of practice sites and patients with type 2 diabetes by randomization group
| Practice sites | All sites | ACG | STG | P |
|---|---|---|---|---|
| n | 34 | 13 | 21 | |
| Physician age: mean (SD) age (years) | 44.8 (7.7) | 43.3 (6.4) | 45.7 (8.4) | 0.3867 |
| Gender: male | 27 (79.4) | 11 (84.6) | 16 (76.2) | 0.5549 |
| Years in practice: mean (SD) (years) | 13.1 (7.9) | 11.3 (7.2) | 14.1 (8.3) | 0.3441 |
| Type of practice | 0.4289 | |||
| Primary care | 27 (79.5) | 10 (76.9) | 17 (81.0) | |
| Multispecialty care | 6 (17.6) | 2 (15.4) | 4 (19.0) | |
| Primary care/multispecialty care | 1 (2.9) | 1 (7.7) | 0 (0.0) | |
| Number of type 2 diabetic patients: mean (SD) | 1,084 (1,483) | 1,250 (2,023) | 978 (1,065) | 0.6276 |
| Primary location of practice | 0.3024 | |||
| Rural setting | 10 (29.4) | 2 (15.4) | 8 (38.1) | |
| Suburban | 17 (50.0) | 9 (69.2) | 8 (38.1) | |
| Urban | 6 (17.6) | 2 (15.4) | 4 (19.0) | |
| Urban and suburban | 1 (3.0) | 0 (0.0) | 1 (4.8) |
| Patients | All patients | ACG | STG | P |
|---|---|---|---|---|
| n | 483 | 227 | 256 | |
| Patient age: mean (SD) age (years) | 55.8 (10.7) | 57.0 (11.2) | 54.8 (10.1) | 0.0197 |
| Gender: male | 257 (53.2) | 122 (53.7) | 135 (52.7) | 0.8243 |
| Ethnicity | 0.0004 | |||
| African American | 150 (31.1) | 72 (31.7) | 78 (30.5) | |
| Caucasian | 305 (63.1) | 152 (67.0) | 153 (59.8) | |
| Other | 28 (5.8) | 3 (1.3) | 25 (9.8) | |
| Highest level of education | 0.1002 | |||
| No college | 253 (52.7) | 114 (50.9) | 139 (54.3) | |
| Some college | 98 (20.4) | 40 (17.9) | 58 (22.7) | |
| College graduate | 129 (26.9) | 70 (31.3) | 59 (23.0) | |
| A1C: mean (SD) A1C (%) | 8.9 (1.2) | 8.9 (1.2) | 8.9 (1.2) | 0.8751 |
| BMI: mean (SD) BMI (kg/m2) | 35.1 (7.3) | 35.1 (6.7) | 35.0 (7.8) | 0.8851 |
| Diabetes duration: mean (SD) (years) | 7.6 (6.1) | 7.7 (6.1) | 7.5 (6.1) | 0.6547 |
Values are n (percentages) unless stated otherwise.
A1C findings
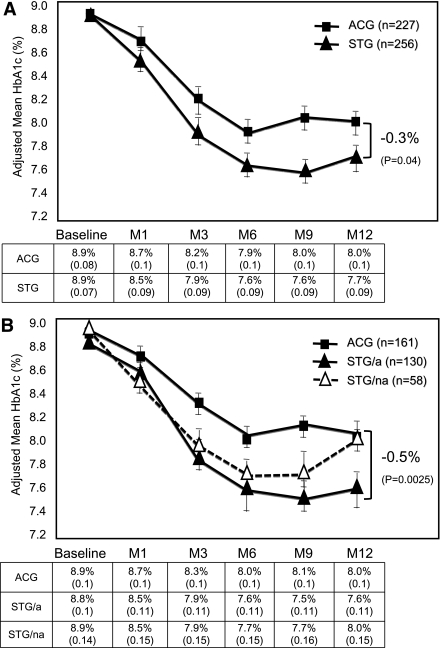
ITT analysis revealed that both groups showed significant reductions in A1C levels; however, STG subjects evidenced significantly greater mean (SE) reductions in A1C than ACG subjects over the 12 months: −1.2% (0.09) vs. −0.9% (0.10); Δ = −0.3%; P = 0.04 (Fig. 2A). PP analysis revealed an even greater mean (SE) A1C reduction among those STG subjects who adhered to the intervention compared with ACG subjects: −1.3% (0.11) vs. −0.8% (0.11); Δ = −0.5%; P < 0.003 (Fig. 2B). It is noteworthy that A1C reductions in nonadherent STG subjects were not significantly different than reductions seen in ACG subjects.
Figure 2.
A: ITT analysis: mean (± SE) A1C over 12 months (M) in patients with noninsulin-treated type 2 diabetes according to randomization group.B: PP analysis: mean (± SE) A1C over 12 months (M) in patients with noninsulin-treated type 2 diabetes, comparing ACG patients, adherent STG (STG/a) patients, and nonadherent STG (STG/na) patients.
Seven-point blood glucose profile findings
STG subjects showed significantly lower average preprandial and postprandial glucose levels at all meals and at bedtime from month 1 to month 12 (in all cases, P < 0.001). More importantly, there was a significant drop from month 1 to month 12 in preprandial to postprandial glucose excursions at all meals: breakfast (44 to 35 mg/dL, P < 0.005), lunch (25 mg/dL to 17 mg/dL, P < 0.03), and supper (34 to 26 mg/dL, P < 0.05). Measurements of mean amplitude of glucose excursions indicated significant (P = 0.0003) mean (SE) reductions in glycemic variability among STG subjects from 38.5 mg/dL (0.9) at month 1 to 34.3 mg/dL (1.0) at month 12. There were no changes in these findings when insulin-using patients were excluded from the analyses.
Changes in treatment
ITT analysis showed that patients in both study groups who received a treatment change recommendation (pharmacologic and/or lifestyle) at the month 1 visit experienced significantly (P < 0.0005) greater reductions in A1C than patients who did not receive a treatment change recommendation at the month 1 visit. However, significantly more STG patients received a treatment change recommendation at the month 1 visit compared with ACG patients, regardless of the patient’s baseline A1C level: 179 (75.5%) vs. 61 (28.0%); P < 0.0001. Of note, almost twice as many STG patients were started on intermediate or long-acting insulin than ACG patients between the month 1 and month 12 visits: 42 vs. 23; P = 0.046. ITT analyses excluding patients who began insulin during the study period also indicated significant decreases in A1C for both the ACG and the STG, with STG patients still demonstrating significantly greater reductions in A1C by month 12 than ACG patients: −1.3% (0.10) vs. −1.0% (0.10); Δ = −0.3%; P = 0.03. Similar between-group differences occurred using last observation carried forward analysis (last A1C before insulin start carried forward): −1.0% (0.10) vs. −0.7% (0.10); Δ = −0.3%; P = 0.03. Thus, the observed group difference in glycemic outcomes was not accounted for by those patients who started insulin during the course of the study.
The mean (SD) number of scheduled visits at which a treatment change was recommended was significantly higher in STG patients than in ACG patients: 2.7 (1.5) vs. 1.1 (1.0); P < 0.0001. PP analyses showed that the mean (SD) number of clinic visits where treatment change recommendations occurred was almost three times greater in STG patients than in ACG patients: 3.1 (1.4) vs. 1.1 (1.0), P < 0.0001.
SMBG frequency
ITT analyses showed that the mean (SD) number of daily blood glucose tests, even when including the 7-point Accu-Chek 360° View blood glucose analysis system profiles for the STG, was significantly lower for the STG than for the ACG at month 6 (0.97 [0.81] vs. 1.21 [1.00], P = 0.007); month 9 (0.85 [0.72] vs. 1.11 [0.84], P = 0.001); and month 12 (0.77 [0.69] vs. 1.05 [0.80], P < 0.0001). PP analysis showed no significant between-group differences in SMBG frequency.
General well-being
There was a significant increase in GWB over the study period in both the ACG (P < 0.007) and the STG (P < 0.0001), as assessed by the WHO-5 (16), with no significant between-group differences over time. In the ACG, mean (SD) WHO-5 scores rose from 58.0 (20.7) at study start to 62.0 (20.8) at month 12. In the STG, mean (SD) WHO-5 scores rose from 57.3 (23.6) at study start to 65.5 (21.3) at month 12. PP analyses revealed that adherent STG subjects reported a significantly greater improvement in GWB than adherent ACG subjects (P < 0.04).
CONCLUSIONS
We found that programmatic, structured SMBG contributes to significant improvement in glycemic control in insulin-naïve type 2 diabetic patients compared with patients who did not receive structured SMBG. Further, this significant between-group difference occurs even though there is a significant A1C reduction of 0.9% achieved by the ACG during the 12-month study period. Glycemic improvement was even greater in STG patients who adhered to the protocol. Of note, patients in the structured SMBG group also show improvement in mean amplitude of glucose excursions and in 7-point blood glucose profiles over the 12-month period.
We suspect that the significant A1C improvement in the ACG over time is due mainly to the heightened attention paid to study subjects, the use of free meters and strips, the requirement that subjects bring their meters to all study visits, and the more frequent than usual medical visits. Thus, the observed differences between the STG and the ACG may be a conservative estimate compared with what might be obtained with similar patients in most clinical settings.
We also found significant differences between the STG and the ACG in the frequency and intensity of the treatment change recommendations made by physicians. This suggests that when patients bring structured SMBG information to clinic visits, and when physicians know how to interpret and respond to SMBG information, timely and appropriate treatment changes are more likely to occur than in cases in which structured SMBG data are not available, as occurred in the ACG.
Another possible explanation is that the treatment changes made by the STG physicians, and the resulting improvements in A1C, occurred because only the STG physicians were trained on a treatment algorithm and were encouraged to follow it. However the PP analyses show that the glycemic advantage occurred only among the STG patients who adhered to the intervention. Therefore, physician training alone does not sufficiently explain these findings.
Additionally, the greater improvement in A1C over time in the STG than in the ACG occurred with less (ITT) SMBG frequency. This finding has important policy implications, suggesting that it may be appropriate to shift the current focus from SMBG quantity (testing frequency) to SMBG quality (meaningful test results that contribute to positive action), utilizing protocols that place more emphasis on when patients test and how they and their physicians organize and make clinically relevant use of the resulting data.
Study strengths and weaknesses
We used a comprehensive approach to address the design limitations of previous studies (23): a cluster-randomized design; an A1C inclusion criterion of ≥7.5%; a highly structured protocol that led to actionable outcomes for both patients and physicians; and a set of PP analyses to determine the effects of protocol completion.
Several limitations are noteworthy. First, the study did not include a third study arm that would have assessed the effect of the increased attention paid to both study groups over the 12-month period. Thus, the enhanced usual care received by the ACG resulted in a conservative estimate of between-group differences. Second, we could not determine if the treatment changes initiated by the physicians and the patients were clinically appropriate; our measures examined the number of changes, rather than the type of change recommended. Third, we could not determine how many of the recommended treatment changes actually occurred. Fourth, there was more attrition of the STG patients than the ACG patients over the 12-month period (though this difference was not statistically significant), suggesting that some patients may have found the structured SMBG intervention too burdensome, which may have biased the findings.
Our findings demonstrate that appropriate use of SMBG in poorly controlled, insulin-naïve type 2 diabetic patients can be efficacious and clinically meaningful. This suggests that most patients and physicians will make appropriate use of SMBG data when there is a well-defined testing protocol, a tool for organizing the data, and the knowledge to interpret the data. It is evident that physicians can be trained to use well-organized SMBG data and to collaborate with patients to make timely and effective treatment decisions to improve glycemic control. Integrating SMBG into a collaborative program of care may lead to improved glycemic control without increasing strip consumption.
Acknowledgments
Funding for the study was provided by Roche Diagnostics, Indianapolis, Indiana. W.H.P., D.A.H., and C.G.P. have worked as consultants for Roche Diagnostics and Abbott Diabetes Care. L.F., C.H.S., and Z.J. have worked as consultants for Roche Diagnostics. B.P., M.S., and R.S.W. are employed by Roche Diagnostics. No other potential conflicts of interest relevant to this article were reported.
The study conception/protocol was by W.H.P., L.F., M.S., and R.S.W. Subject training was by D.A.H., C.H.S., and C.G.P. The clinical operations study manager was B.P. The statistical analysis was by Z.J. The manuscript development was by W.H.P., L.F., and C.G.P. The manuscript was reviewed by an independent Publication and Analysis Committee, which had full and independent access to all study data and independent responsibility for the development, writing, and oversight of all manuscripts. The committee is comprised of W.H.P.; L.F.; M.S.; R.S.W.; Richard Bergenstal, MD; Frank Snoek, PhD; and David Owens, MD. All authors read and approved the final manuscript.
Parts of this study were presented in poster form at the 70th Scientific Sessions of the American Diabetes Association (ADA), Orlando, Florida, 25–29 June 2010; the 45th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September–2 October 2009; and the 69th Scientific Sessions of the ADA, New Orleans, Louisiana, 5–9 June 2009.
The authors thank the participating clinicians and patients for their assistance and commitment to this research effort.
Footnotes
Clinical trial reg. no. NCT00674986, clinicaltrials.gov.
See accompanying editorial, p. 527.
References
- 1.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 2009;32(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007;13(Suppl. 1):1–68 [DOI] [PubMed] [Google Scholar]
- 3.IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes. International Diabetes Federation 2005. Available from www.idf.org. Accessed 10 April 2010
- 4.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ 1999;319:83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, McKitrick C, Larkin M, Schaffran R, Singer DE. Glycemic control in diabetes mellitus: have changes in therapy made a difference? Am J Med 1996;100:157–163 [DOI] [PubMed] [Google Scholar]
- 7.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med 2001;111:1–9 [DOI] [PubMed] [Google Scholar]
- 8.Guerci B, Drouin P, Grangé V, et al. ASIA Group Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab 2003;29:587–594 [DOI] [PubMed] [Google Scholar]
- 9.Schwedes U, Siebolds M, Mertes G, SMBG Study Group Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care 2002;25:1928–1932 [DOI] [PubMed] [Google Scholar]
- 10.Barnett AH, Krentz AJ, Strojek K, et al. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab 2008;10:1239–1247 [DOI] [PubMed] [Google Scholar]
- 11.Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ 2007;335:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Kane MJ, Bunting B, Copeland M, Coates VE, ESMON study group Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ 2008;336:1174–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med 2005;118:422–425 [DOI] [PubMed] [Google Scholar]
- 14.Polonsky W, Fisher L, Schikman C, et al. The value of episodic, intensive blood glucose monitoring in non-insulin treated persons with type 2 diabetes: design of the Structured Testing Program (STeP) study, a cluster-randomised, clinical trial [NCT00674986]. BMC Fam Pract 2010;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 16.Polonsky WH, Jelsovsky Z, Panzera S, Parkin CG, Wagner RS. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin-treated type 2 diabetes. Diabetes Technol Ther 2009;11:283–291 [DOI] [PubMed] [Google Scholar]
- 17.Bech P, Olsen LR, Kjoller M, Rasmussen NK. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res 2003;12:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littenberg B, MacLean CD. Intra-cluster correlation coefficients in adults with diabetes in primary care practices: the Vermont Diabetes Information System field survey. BMC Med Res Methodol 2006;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ, The Diabetes Care From Diagnosis Research Team Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. BMJ 1998;317:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SAS Institute. SAS/STAT User's Guide. 8 ed. Cary, NC, SAS Institute, Inc., 1999 [Google Scholar]
- 21.Fitzmaurice G, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ, John W. Wiley and Sons, 2004 [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. Hoboken, NJ, John W. Wiley and Sons, 2002 [Google Scholar]
- 23.Klonoff DC, Bergenstal R, Blonde LS, et al. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Tech 2008;2:1030–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]