Abstract
OBJECTIVE
Cystic fibrosis (CF)-related diabetes (CFRD) is associated with declining pulmonary function and increased mortality. During oral glucose tolerance testing (OGTT), CFRD is defined by 2-h plasma glucose (PG2). We hypothesized PG elevations during OGTT resolving by 2 h, not meeting CFRD criteria, influence pulmonary function in CF. Thus we investigated the frequency of elevated 1-h OGTT PG (PG1) and its relationship with pulmonary function.
RESEARCH DESIGN AND METHODS
Retrospective review of OGTTs was performed between August 2005 (annual screening initiation) and June 2008 at Children’s Hospital of Philadelphia CF Center. First-time, well state OGTTs (PG0, PG1, PG2) were analyzed. Additional data collected were: percent predicted forced expiratory volume in 1 s (FEV1), BMI percentile, lung bacterial colonization, age, and sex. OGTTs were categorized as normal (PG2 <140 mg/dL), impaired glucose tolerance (IGT) (PG2 140–199 mg/dL), CFRD (PG2 ≥200 mg/dL), and indeterminate glycemia (INDET) (PG1 ≥200 mg/dL and PG2 <140 mg/dL). Frequency of PG1 ≥140 but <200 mg/dL was also noted. Multivariable linear regression was used to assess associations between percent predicted FEV1, BMI percentile, and OGTT PG.
RESULTS
OGTTs (101) were available (59 male/42 female; age 5.8–22 years, percent predicted FEV1 = 94.5 ± 18%, BMI percentile = 52 ± 25%). With the use of PG2, 91 OGTT were normal, eight were IGT, and two were CFRD. With the use of PG1 (n = 89), 39 OGTT were normal, 36 were PG1 ≥140 <200 mg/dL, and 14 were PG1 ≥200 mg/dL. PG1 was negatively associated with percent predicted FEV1, adjusting for BMI percentile (P = 0.009, R2 0.13). Percent predicted FEV1 was not associated with PG0, PG2, age, sex, or lung bacterial colonization.
CONCLUSIONS
PG elevations at nontraditional OGTT times are common in CF. The association of increasing PG1 with worse pulmonary function suggests early PG abnormalities may be deleterious or an early marker for worsening disease and will be missed if CFRD diagnosis focuses on PG2.
With improved survival among individuals with cystic fibrosis (CF), diabetes mellitus has emerged as a significant comorbidity. The CF Foundation Patient Registry 2008 Annual Data Report, which reflects data from over 25,000 patients at U.S.-accredited CF care centers, reports that 20% of individuals with CF will have CF-related diabetes (CFRD) by the third decade (1). Higher prevalence of 40–50% has been reported in adults with CF followed at the University of Minnesota, where routine annual screening has been in place since the early 1990s (2). CFRD is associated with worse clinical status, as indicated by reduced pulmonary function; increased frequency of pulmonary exacerbations; increased prevalence of important sputum pathogens such as Pseudomonas aeruginosa and Burkholderia cepacia; and worsening nutritional status, among others (3). Further highlighting its clinical significance, CFRD is associated with increased morbidity and up to a sixfold greater mortality rate (2,4).
CFRD is hypothesized to result primarily from insulin deficiency, although some element of insulin resistance is likely present as well. Before the development of frank diabetes, insulin secretion is delayed and blunted (5). The earliest changes in glucose tolerance involve variable postprandial hyperglycemia. Progressive decline in insulin secretion leads to impaired glucose tolerance (IGT) and, ultimately, CFRD.
The oral glucose tolerance test (OGTT) is recommended for screening purposes (6), and the diagnostic criteria for CFRD are similar to those for other forms of diabetes. However, the appropriateness of using conventional thresholds derived from epidemiologic studies in non-CF patients for the diagnosis of CFRD has been questioned (7). In type 2 diabetes, glycemic thresholds defining diabetes are based on glucose concentrations associated with microvascular complications, such as diabetic retinopathy and nephropathy (8). Although microvascular complications are relevant in CF (9), the paradigm for defining glucose abnormalities in CF is shifting to focus on outcomes more immediately relevant to CF, such as nutritional status and pulmonary function. This paradigm shift is underscored by a recent randomized control trial in which insulin treatment was associated with improved nutritional status in adults with CFRD without fasting hyperglycemia (10).
In 2009, the North American CFRD Consensus Committee restructured glucose tolerance categories in CF, based on the fasting and 1- and 2-h plasma glucose during an OGTT (6): normal glucose tolerance (NGT), IGT, CFRD, and indeterminate glycemia (INDET). This new category of INDET occurs in individuals with normal fasting and 2-h plasma glucose but who have 1-h plasma glucose concentrations greater than 200 mg/dL (6). The clinical relevance of this INDET category with regard to clinical outcome in CF is not known.
The goals of this study were to investigate the frequency of abnormal 1-h plasma glucose during an OGTT and to explore the relationship between the 1-h plasma glucose (PG1), percent predicted forced expiratory volume in 1 s (FEV1), and BMI percentile in a pediatric CF population. We hypothesized that elevations in PG1 would be both common and negatively associated with percent predicted FEV1 and BMI percentile. Our data suggest that elevations of PG1 are in fact associated with worse clinical outcomes in CF and provide a foundation for further investigation regarding optimal management of CFRD.
RESEARCH DESIGN AND METHODS
Subjects
In August 2005, The Children's Hospital of Philadelphia CF Center initiated annual CFRD screening in children over age 6–8 years using a standard OGTT (75 g/kg dextrose, maximum dose 75 g) after an overnight fast. This retrospective chart review is limited to OGTT performed for the first time in individual subjects between August 2005 and June 2008 in the well state, defined as no hospitalizations or oral or intravenous glucocorticoid exposure within 6 weeks before OGTT. Patients that had undergone lung transplant or had a previous diagnosis of CFRD were excluded. Patients who were unable to cooperate with pulmonary function testing because of young age or behavioral/developmental issues were excluded from the analyses examining associations between OGTT and pulmonary function.
Data
Data were collected from clinical records and Port CF and included OGTT plasma glucose concentrations, percent predicted FEV1 and percent predicted forced vital capacity (FVC) according to the Wang-Hankinson reference equations (REF), and BMI percentile. Sputum cultures to assess bacterial colonization were performed on spontaneously expectorated sputum (when available) or by cough swabs. All of these evaluations were performed as part of standard clinical care at the time of the OGTT.
OGTT results were categorized according to PG2 as normal (PG2 <140 mg/dL), IGT (PG2 140–199 mg/dL), and CFRD (PG2 ≥200 mg/dL), as well as according to PG1 as INDET (PG1 ≥200 mg/dL but PG2 <140 mg/dL or PG1 ≥140 mg/dL but <200 mg/dL).
Statistical methods
Means and SD were calculated for continuous variables; proportions were used to characterize categorical data. Simple linear regression was used to test the associations between OGTT PG, as a continuous variable, and pulmonary function tests, BMI percentile, and other clinical factors such as sex, age, and sputum bacterial colonization. Multivariable linear regression was then used to test the associations between OGTT PG and percent predicted FEV1 after adjustment for BMI percentile. The regression models were assessed further through the Shapiro-Wilk test of normality of the residuals and the Cook-Weisberg test for heteroscedasticity.
Data analysis was performed using Stata 9 (Stata Corporation, College Station, TX). Two-sided tests of hypotheses were used, and a P < 0.05 was considered statistically significant.
This study was approved by the Institutional Review Board at The Children's Hospital of Philadelphia.
RESULTS
During 2005 through 2008, first-time OGTT were performed in 101 children with CF. Complete OGTT data (PG0, PG1, PG2) were available in 89 subjects.
Clinical characteristics of the 101 (42F) are listed in Table 1. Mean age was 12.5 ± 3.9 years. Mean BMI percentile was 52 ± 25%, and mean percent predicted FEV1 was 94.5 ± 18%. Mean PG0, PG1, and PG2 were 86 ± 9 mg/dL, 148 ± 51 mg/dL, and 102 ± 32 mg/dL, respectively.
Table 1.
Patient characteristics
| Characteristic | All (n = 101) |
|---|---|
| Female/male | 42/59 |
| Age (years) | 12.5 ± 3.9 |
| White | 99 (98%) |
| BMI percentile | 52% ± 25 |
| Percent predicted FEV1 | 94.5% ± 18.3 |
| Percent predicted FVC | 98.7% ± 16.8 |
| MSSA or MRSA | 23 |
| Burkholderia cepacia | 4 |
| Pseudomonas aeruginosa | 24 |
Data are mean ± SD. MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
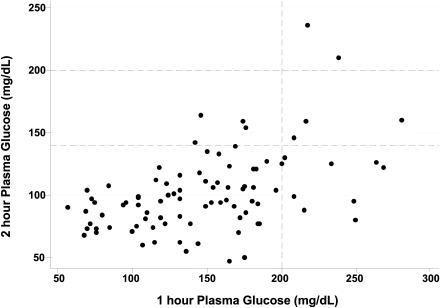
Of the 101 who underwent OGTT, 14 (14%) had PG1 ≥200 mg/dL. Of this group, nine (64%) had normal PG2 (<140 mg/dL) and, until recently, would have been labeled as having normal glucose tolerance. Two of these 14 had CFRD (PG2 ≥200 mg/dL), and an additional three of these 14 had IGT (Fig. 1). An additional 36 had PG1 ≥140 but <200 mg/dL.
Figure 1.
Plasma glucose excursions during OGTT.
Simple linear regression analysis was performed to test for associations between percent predicted FEV1, percent predicted FVC, and BMI percentile and age, sex, PG0, PG1, and PG2 as well as between BMI percentile and pulmonary function tests. As shown in Table 2, percent predicted FEV1 and percent predicted FVC were associated with both BMI percentile and PG1 but not age, sex, PG0, PG2, or colonization status in this unadjusted analysis. BMI percentile was also associated with PG1.
Table 2.
Unadjusted associations between OGTT and pulmonary function and BMI
| Variable |
Partial β-coefficient (95% CI) |
P value |
R2 |
|
|---|---|---|---|---|
| Dependent |
Independent |
|||
| Unadjusted | ||||
| Percent predicted FEV1 | PG1 | −0.11 (−0.18 to −0.04) | <0.0003 | 0.10 |
| BMI percentile | 0.19 (0.05–0.33) | 0.007 | 0.07 | |
| Percent predicted FVC | PG1 | −0.06 (−0.13 to 0.005) | 0.07 | 0.04 |
| BMI percentile | 0.19 (0.06–0.32) | 0.004 | 0.08 | |
| BMI percentile | PG1 | −0.11 (−0.21 to −0.005) | 0.04 | 0.05 |
| Adjusted for BMI percentile | ||||
| Percent predicted FEV1 | 0.13 | |||
| PG1 | −0.10 (−0.17 to −0.02) | 0.009 | ||
| BMI percentile | 0.14 (−0.01 to 0.29) | 0.067 | ||
| Percent predicted FVC | 0.1 | |||
| PG1 | −0.05 (−0.11 to 0.02) | 0.2 | ||
| BMI percentile | 0.18 (0.04–0.32) | 0.01 | ||
We performed multivariable linear regression analyses to adjust for potential confounders (Table 2); PG1 remained negatively associated with percent predicted FEV1 even after adjustment for BMI percentile. This relationship did not persist for percent predicted FVC after adjustment for BMI percentile. According to the regression model, after adjustment for BMI percentile, percent predicted FEV1 was reduced by 1% for every 10 mg/dL increase in PG1.
CONCLUSIONS
In children with CF undergoing routing OGTT for CFRD screening, the 1-h PG is commonly elevated despite normal fasting and 2-h PG concentrations. In fact, until the recent introduction of the INDET category of glucose tolerance in CF, ∼10% of our center’s pediatric CF population would have been labeled as having normal glucose tolerance despite an elevated PG1, or 1-h PG. More importantly and likely clinically relevant, our data suggest that the 1-h OGTT PG is negatively associated with percent predicted FEV1 even after adjustment for BMI percentile. In contrast, fasting and 2-h PG concentrations, parameters upon which we typically rely to define abnormal glucose, were related to neither BMI percentile nor pulmonary function. These data suggest that clinically important abnormal PG elevations may be missed if glucose tolerance testing omits evaluation of PG1 and optimal therapy may require additional focus on normalizing PG1.
A similar model for redefining glucose abnormalities predicated on nontraditional outcomes can be seen with gestational diabetes. The original definitions of glucose abnormalities acquired during pregnancy were based upon subsequent development of diabetes and not upon predictive value for adverse pregnancy outcomes. Recently, the Hyperglycemia and Adverse Pregnancy Outcome study identified an association between maternal glucose concentrations and markers of perinatal complications, even at glucose concentrations below those traditionally accepted as diagnostic of gestational diabetes (11). Additionally, the finding of improved pregnancy outcomes with treatment of mild gestational diabetes (12) emphasizes importance of the 1-h PG. An abnormal PG1 predicts postpartum metabolic dysfunction (13) and has been associated with increased risk for adverse fetal outcomes such as large for gestational age, neonatal hypoglycemia, increased rate of cesarean section, and shoulder dystocia (11).
Elevations of PG1 are also associated with abnormalities in inflammatory markers and metabolic characteristics in patients without a diagnosis of diabetes. Adults with PG1 >155 mg/dL had significant increases in inflammatory markers and lipid ratios, higher white blood cell count and fibrinogen levels, and worse insulin sensitivity (14). Moreover, subjects with normal glucose tolerance as defined by fasting and 2-h PG, but who had PG1 >155 mg/dL, had a fivefold greater risk of type 2 diabetes than those subjects with PG1 <155 mg/dL (15). These findings further highlight the importance of understanding the role of hyperglycemia that occurs earlier than the 2-h mark.
Both CFRD and IGT are associated with greater declines in pulmonary function (16). Glucose abnormalities occurring earlier than 2 h during a OGTT have also been associated with declines in weight in the year preceding the OGTT (17). More concerning, CFRD is associated with increased mortality (2). Recent data from the University of Minnesota suggest, however, that early diagnosis and treatment of CFRD can improve survival (2). In a 1-year randomized control trial of CFRD without fasting hyperglycemia, insulin treatment improved BMI percentile, and, although not statistically significant, trends in reversing declines in pulmonary function were suggested.
Current recommendations for the treatment of blood glucose abnormalities in CF rely on the 2-h blood glucose concentration, which is defined as abnormal based upon its ability to predict development of microvascular complications. In the setting of CF, worsening pulmonary function and poor nutritional status are the more clinically relevant causes of significant morbidity and mortality. Given that current median predicted survival for people with CF is only ∼37 years, pulmonary function and nutritional status eclipse microvascular disease as clinically significant causes of morbidity and mortality in CF. Thus our findings of worse percent FEV1 in the setting of increasing PG1 but not PG2 suggest earlier and not typically assessed glucose abnormalities may have implications for pulmonary status and that reliance on PG2 may miss an important impetus for intervention.
In this study, a negative association between PG1 and baseline percent FEV1 was identified; however, this association does not differentiate between 1) a causal effect and 2) glucose abnormalities and worsening percent FEV1, both arising as manifestations of worsening CF disease. In fact, PG1 may only be an early marker of pulmonary decline. It is important to also note that although the model is statistically significant, the R2 was low; an association between glucose abnormalities is therefore suggested, but the clinical impact of this association has yet to be determined. The finding of increasing PG1 predicting worse percent FEV1 in subsequent years would provide support for this causal effect, while an intervention study targeting the 1-h OGTT blood glucose would provide important insights into the role of glucose/insulin in nutritional status and pulmonary function.
We have identified a significant association between elevated PG1 and decreased percent predicted FEV1 in children with CF. These findings support a new paradigm for the approach to PG abnormalities in CF by defining PG abnormalities by risk of pulmonary function decline and nutritional status compromise rather than microvascular complications, as well as support further investigation of glycemic control defined by PG1 in CF.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH K23-RR021973 to A.K.) and the Cystic Fibrosis Foundation (to A.K. and R.C.R.). No potential conflicts of interest relevant to this article were reported.
J.B. wrote the article, contributed to discussion, and researched data. S.D. researched data and contributed to discussion. R.M. researched data. R.C.R. researched data, contributed to discussion, and reviewed and edited the article. A.K. researched data, contributed to discussion, wrote the article, and reviewed and edited the article.
The authors thank The Children’s Hospital of Philadelphia Cystic Fibrosis Center, Division of Pulmonary Medicine, and Division of Endocrinology and Diabetes.
References
- 1.Cystic Fibrosis Foundation. Patient Registry 2008 Annual Data Report Bethesda, MD, 2008.
- 2.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–687 [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Hardin D, Rodman D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999;45:61–73 [DOI] [PubMed] [Google Scholar]
- 5.Lanng S, Thorsteinsson B, Røder ME, et al. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (Copenh) 1993;128:207–214 [DOI] [PubMed] [Google Scholar]
- 6.Moran A. Evidence based guidelines for cystic fibrosis related diabetes. Diabetes throughout the lifespan symposium. In 23rd Annual North American Cystic Fibrosis Conference Minneapolis, MN, 15–17 October 2009 [Google Scholar]
- 7.Moran A. Cystic fibrosis-related diabetes: an approach to diagnosis and management. Pediatr Diabetes 2000;1:41–48 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32(Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Pekow P, Grover P, et al. Cystic Fibrosis Related Diabetes Therapy Study Group Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis-related diabetes therapy trial. Diabetes Care 2009;32:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 12.Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab 2010;95:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardini G, Dicembrini I, Cresci B, Rotella CM. Inflammation markers and metabolic characteristics of subjects with 1-h plasma glucose levels. Diabetes Care 2010;33:411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2000;162:891–895 [DOI] [PubMed] [Google Scholar]
- 17.Hameed S, Morton JR, Jaffé A, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 2010;33:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]