Abstract
OBJECTIVE
To test the effect of an automated system providing real-time estimates of HbA1c, glucose variability, and risk for hypoglycemia.
RESEARCH DESIGN AND METHODS
For 1 year, 120 adults with type 1 diabetes (69 female/51 male, age = 39.1 [14.3] years, duration of diabetes 20.3 [12.9] years, HbA1c = 8.0 [1.5]), performed self-monitoring of blood glucose (SMBG) and received feedback at three increasingly complex levels, each continuing for 3 months: level 1—routine SMBG; level 2—adding estimated HbA1c, hypoglycemia risk, and glucose variability; and level 3—adding estimates of symptoms potentially related to hypoglycemia. The subjects were randomized to feedback sequences of either levels 1-2-3 or levels 2-3-1. HbA1c, symptomatic hypoglycemia, and blood glucose awareness were evaluated at baseline and at the end of each level.
RESULTS
For all subjects, HbA1c was reduced from 8.0 to 7.6 from baseline to the end of study (P = 0.001). This effect was confined to subjects with baseline HbA1c >8.0 (from 9.3 to 8.5, P < 0.001). Incidence of symptomatic moderate/severe hypoglycemia was reduced from 5.72 to 3.74 episodes/person/month (P = 0.019), more prominently for subjects with a history of severe hypoglycemia (from 7.20 to 4.00 episodes, P = 0.008) and for those who were hypoglycemia unaware (from 6.44 to 3.71 episodes, P = 0.045). The subjects’ ratings of the feedback were positive, with up to 89% approval of the provided features.
CONCLUSIONS
Feedback of SMBG data and summary SMBG-based measures resulted in improvement in average glycemic control and reduction in moderate/severe hypoglycemia. These effects were most prominent in subjects who were at highest risk at the baseline.
Intensive insulin treatment attempting to approximate near-normal levels of glycemia markedly reduces chronic complications of type 1 diabetes (1,2) but may risk potentially life-threatening severe hypoglycemia (SH)—a result of imperfect insulin replacement (3). Consequently, hypoglycemia has been identified as the primary barrier to optimal diabetes management (4). Thus, people with type 1 diabetes mellitus face a lifelong behaviorally controlled optimization problem: to maintain strict glycemic control without increasing their risk for hypoglycemia (5). Handling these complex and demanding aspects of diabetes management requires motivation, goal setting, and accurate assessment of risk of hypoglycemia. It is therefore reasonable to expect that feedback that can be motivating (e.g., tracking progress in HbA1c on a weekly basis) and at the same time informative about risks and symptoms of hypoglycemia could be a useful tool assisting the improvement of glycemic control. Such feedback should be available in patients’ natural environment and should use routine data, such as self-monitoring of blood glucose (SMBG).
Most contemporary home SMBG devices provide accurate blood glucose (BG) readings and are capable of storing a significant number of BG readings (typically >150). The current study gauges the utility of three levels of SMBG-based feedback to patients with type 1 diabetes: level 1—routine SMBG alone; level 2—routine SMBG plus SMBG-based estimated of HbA1c, risk for hypoglycemia, and glucose variability; and level 3—adding symptom ratings and the evaluation of symptoms presumably related to hypoglycemia in level 2 feedback.
The theoretic background for this approach can be traced to previous interventions based on BG feedback that have been shown to improve clinical outcomes in type 1 diabetes. For example, it is well documented that the avoidance of low BG events (<70 mg/dL) for a few weeks can improve symptom perception and reverse hypoglycemia unawareness (6,7). Second, our group has shown in several studies that blood glucose awareness training (BGAT)—a psycho-behavioral intervention for people with type 1 diabetes based on systematic feedback about BG levels and accuracy of symptom perception—has positive effects. These include improvement in low BG detection, knowledge, decision-making, and reduced events, such as SH (8–10). The assumption of these interventions is that providing patients with systematic feedback provides information needed to make adjustments in diabetes management that improve self-regulation.
The computational background for the estimates of level 2 feedback has been presented in a series of studies showing that specific risk analysis of SMBG data could capture long-term trends toward increased risk for hypoglycemia (11,12) and identify 24-h periods of increased risk for hypoglycemia (13,14). These analyses were based on recognition of a specific asymmetry of the BG measurement scale and on a nonlinear transformation correcting this asymmetry (15,16). In essence, we have developed and tested algorithms using SMBG to evaluate glycemic control (17) and glucose variability (18) in type 1 diabetes. The computational background for the symptom estimates of level 3 feedback can be traced to the Stochastic Model of Self-Regulation Behavior, which gives a mathematic description to the feedback pattern internal condition: symptom perception/awareness, appraisal, and self-regulation decision (19).
On the basis of these theoretic and computational developments, we hypothesized that readily available automated feedback of summary parameters of glycemic control, such as estimated HbA1c, risk for hypoglycemia, and hypoglycemic symptom specificity, would enhance the control of type 1 diabetes. To test this hypothesis, we designed a feedback system residing in a handheld computer (HHC) given to patients with type 1 diabetes in their natural environment. We then evaluated the efficacy of this system for improving HbA1c and reducing risks for hypoglycemia.
RESEARCH DESIGN AND METHODS
Subjects
A total of 120 adults with type 1 diabetes were recruited through regional advertising. Inclusion criteria were age ≥18 years, type 1 diabetes defined by the American Diabetes Association criteria or physician judgment, and willingness to participate in the study for up to 12 months performing SMBG four to five times per day. The demographic and biometric characteristics of the participants are presented in Table 1.
Table 1.
Demographic, biometric, and diabetes history characteristics of participants
| Group A | Group B | All subjects | P level A vs. B | |
|---|---|---|---|---|
| Age (years) – mean (SD) | 40.65 (14.84) | 37.61 (13.78) | 39.15 (14.35) | 0.248 |
| Gender (male/female) | 26/33 | 25/36 | 51/69 | 0.735 |
| Baseline HbA1c – mean (SD) | 7.96 (1.49) | 8.02 (1.49) | 7.99 (1.48) | 0.846 |
| Duration of diabetes (years) | 20.93 (13.03) | 19.61 (12.89) | 20.28 (12.92) | 0.577 |
| No. of severe hypoglycemic episodes in the past year | 1.23 (2.25) | 1.07 (2.55) | 1.15 (2.396) | 0.714 |
| Percentage of subjects reporting SH in the past year | 0.34 (0.48) | 0.31 (0.46) | 0.32 (0.47) | 0.650 |
| Hypoglycemia awareness (aware/unaware) | 35/26 | 38/21 | 73/47 | 0.434 |
| No. of SMBG readings per day reported retrospectively at screening | 4.92 (2.1) | 4.46 (1.55) | 4.69 (1.84) | 0.172 |
| No. of SMBG readings per day during level 1 feedback | 4.89 (2.03) | 4.75 (1.37) | 4.82 (1.73) | 0.684 |
| No. of SMBG readings per day during level 2 feedback | 5.34 (2.49) | 4.76 (1.36) | 5.09 (2.07) | 0.135 |
| No. of SMBG readings per day during level 3 feedback | 5.02 (2.58) | 4.40 (1.40) | 4.74 (2.13) | 0.137 |
Of the 120 individuals enrolled in the study, 97 completed the entire 1-year protocol. The observed 19.2% attrition rate was lower than the anticipated 25% attrition rate used to power the study. Thirteen subjects dropped out during the first phase of the study (SMBG alone; see next paragraph), three subjects dropped out during the second phase of the study, and seven subjects dropped out during the third phase of the study.
Procedure
The study was approved by the institutional review board of the University of Virginia and registered as a clinical trial (institutional review board 12126, Clinical Trials.gov ID NCT00315939). After recruitment, subjects signed consent forms, completed background questionnaires, and were randomized into group A or group B matched by gender, age, and baseline HbA1c. As seen in Table 1, there were no significant between-group differences on history of SH, hypoglycemia awareness, and frequency of SMBG: Group A began with routine SMBG alone (level 1), followed sequentially by levels 2 and 3. Group B began with level 2, followed by level 3 and then level 1. Each level continued for 3 months and proceeded as follows: level 1 was routine SMBG. Subjects were given LifeScan OneTouch UltraSmart meters (LifeScan Inc., Milpitas, CA) and free strips, and asked to perform SMBG four to five times per day. No additional instructions about the timing of SMBG or the interpretation of the data were given. No changes to treatment were recommended. At each visit, the subject was only asked about any health concerns or any new medications or change in insulin. This information was recorded but not used for feedback. Thus, level 1 should be regarded as a control condition, which was different from routine SMBG only because subjects were enrolled in a study and given free test strips.
Level 2 retained level 1, but an HHC was given to the subjects, programmed to estimate HbA1c (20), risk for hypoglycemia (Low BG Index) (11), and glucose variability (Average Daily Risk Range) (18) using previously published algorithms. The subjects were asked to carry the HHC and enter all their glucose readings when performing SMBG. The estimates of HbA1c were updated weekly, and the estimates of risk for hypoglycemia and glucose variability were updated at each SMBG entry. Detailed instructions were provided on the meaning of these different types of glucose feedback; the study staff was available to answer any questions.
Level 3 retained level 2, but the HHC asked subjects to provide symptom ratings when BG was low (<3.9 mmol/L) and at an equal number of matching euglycemic readings. From these data, the HHC estimated a set of potentially significant symptoms of hypoglycemia for each individual, using an iterative algorithm following a previously published symptom significance estimation procedure (19). The patient manual for the HHC program is provided in supplementary data.
With this design, the study was split into three sequential 3-month blocks of time: In the first 3 months, subjects from group A served as controls to group B assessing the effectiveness of SMBG alone versus level 2 feedback; the subsequent 3 months contrasted level 2 with level 3 feedback, and in the last 3 months contrasted level 3 feedback with SMBG alone.
Metrics
HbA1c was assessed at the University of Virginia laboratory at baseline and after each level of feedback. To assess hypoglycemic symptoms and accuracy of low BG detection, four 1-month HHC assessments were performed, including one at baseline and one during the last month of each of the levels of feedback. To eliminate assessment bias, these assessments were identical throughout all stages of the study. Each assessment required subjects to complete 70 HHC trials over a month, with each trial asking them to rate their symptoms and estimate BG on the basis of symptoms before SMBG.
In parallel, diaries of SH were completed using an automated Internet-based system, previously used for other studies (21). Once subjects logged in, they were able to see which diaries needed to be completed and then proceed through the diary questions. Responses were stored on our Health Insurance Portability and Accountability Act-compliant server. SH was defined as “Blood glucose so low that you could not treat yourself because you were stuporous or unconscious.” The system also recorded episodes of moderate hypoglycemia (MH), defined as “Blood glucose so low that you could not continue with your activities because you could not think clearly or lost your coordination, but you could still treat yourself.”
Ratings of feedback
After completing level 2, subjects were given the opportunity to evaluate the feedback presented to them by the HHC. Questions about HbA1c feedback included Q1: “Do you think this feature will help you lower your HbA1c levels in the long run?” and Q2: “Do you want to see your HbA1c insight on a weekly basis?” Questions regarding glucose variability feedback included Q3: “Do you feel this feature helped you manage your diabetes?” and Q4: “Do you want to be presented with the Variability insight number on a weekly basis?” Questions regarding risk for hypoglycemia included Q5: “Would you look at this feature on a regular basis?” and Q6: “Do you feel more comfortable knowing you will be warned of your risk of hypoglycemia?” Each question could be answered “Yes,” “No,” or “Not sure.”
Statistical analysis
The primary outcomes of this study were HbA1c and frequency of SH/MH as recorded independently from SMBG using the Internet system. The data for subjects who dropped out during the study were handled according to the intention-to-treat principle, using “dropout” as a factor in the analysis and adjusting significance levels accordingly. Repeated-measures ANOVA was used to compare HbA1c and SH data, as well as the data from the four HHC assessments of the study. Group variables defined at the baseline were used in the analyses, including study groups A and B; HbA1c group: <8.0 vs. >8.0 baseline HbA1s; SH group: history versus no history of SH; and hypoglycemia awareness group: aware versus unaware subjects. Secondary analyses included average BG, BG range, and metrics of frequency of hypo- and hyperglycemic episodes recorded by SMBG. Subjects’ ability to estimate their BG accurately and detect hypoglycemia on the basis of symptoms was assessed by the Accuracy Index, defined as percentage in A minus C, D, E zones on the Clarke Error-Grid Analysis (22,23), and by the percent detection of BGs <3.9 mmol/L.
RESULTS
Frequency of self-monitoring of blood glucose
As presented in Table 1, the retrospectively assessed frequency of SMBG during the screening visit baseline was 4.69 readings/subject/day, which was not statistically different from the subsequently observed frequency of SMBG. No between-group differences were observed. However, the frequency of SMBG in subjects who had baseline HbA1c ≥8.0 was 4.26 → 4.40 → 4.33 → 4.1 readings/day at baseline and during feedback levels 1, 2, and 3, respectively, which was consistently lower than in subjects with HbA1c <8.0: F = 8.8, P = 0.004.
HbA1c
Throughout the study, for all subjects regardless of attrition, HbA1c was reduced from 7.99 (1.48) at baseline to 7.69 (1.30) after feedback level 1, to 7.69 (1.33) after feedback level 2, to 7.58 (1.08) after feedback level 3 (F = 5.6, P = 001). Attrition was a significant factor for the analysis of HbA1c—subjects who dropped out had significantly higher HbA1c at baseline than those who completed the study, 9.07 (2.05) vs. 7.73 (1.19), P < 0.001. By retaining the last known HbA1c for those who quit the study, these averages were adjusted to 7.99 → 7.80 → 7.85 → 7.83, and the significance of the feedback was assessed after this adjustment: F = 5.6, P = 0.001.
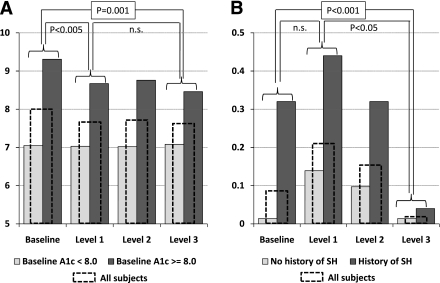
As presented in Fig. 1A, the reduction in HbA1c was entirely accounted for by those subjects who had baseline HbA1c ≥8.0. For the rest of the subjects, HbA1c level did not change, which resulted in a significant interaction: F = 7.0, P < 0.0005.
Figure 1.
A: Reduction of A1C (HbA1c) throughout the study. Level 1 feedback (SMBG alone) accounts for most of the improvements in HbA1c. The beneficial effects of the study are confined to those with baseline HbA1c >8.0; the rest of the subjects did not change their average glycemic control. The included significance levels refer to comparisons involving all subjects. B: Incidence of severe hypoglycemic was significantly reduced as a result of the study. However, the initial feedback from SMBG alone increased SH, and only more extensive feedback (levels 2 and 3) was able to reduce the incidence of SH. The included significance levels refer to comparisons involving all subjects. Although HbA1c can be improved by intensive SMBG, simultaneous improvement in both HbA1c and incidence of SH requires more extensive feedback to the patients. SH, severe hypoglycemia.
Additional analyses revealed that the order of feedback initiation did not have significant effect on the progression of HbA1c improvement: The largest decrease was registered after the first treatment session, regardless of whether this session included level 1 or 2 feedback: HbA1c for group A changed from 7.99 to 7.76, whereas for group B the change was from 8.0 to 7.9 (F = 0.9, NS) from the beginning to the end of study.
Severe and moderate hypoglycemia
During the baseline monitoring period, the participants reported 0.09 SH episodes/person/month, whereas during the last monitoring period (after feedback level 2) there were 0.02 SH episodes/person/month. This difference was significant: F = 15.3, P < 0.001. This effect was most prominent for subjects who reported a history of SH (from 0.32 to 0.04 SH episodes/subject/month, P < 0.001) and for those who reported hypoglycemia unawareness at baseline (from 0.25 to 0.03 SH episodes/subject/month, P = 0.001) (Fig. 1B). However, the progression of the improvement in SH regulation was substantially different from the progress in HbA1c. As seen in Fig. 1A, HbA1c decreased immediately after the first level of feedback and retained similar levels thereafter. In contrast, as seen in Fig. 1B, SH increased (although not statistically significantly) from baseline to level 1 (SMBG alone), and then gradually decreased throughout the more extensive feedback periods. There were no between-group differences: In group A, SH episodes decreased from 0.09/subject/month to 0.023 episodes/subject/month; in group B, this change was from 0.09 to 0.019 episodes.
Episodes of MH also decreased, from 5.63/person/month in the beginning of the study to 3.72/person/month at the end (F = 5.3, P = 0.02). This effect was more prominent in subjects with a history of SH (from 6.88 to 3.96 episodes/subject/month) and in those who reported hypoglycemia unawareness (from 6.19 to 3.68 episodes/subject/month), but the influence of history of SH and hypoglycemia unawareness was not statistically significant. Subjects from study group B had a more significant reduction in MH: from 5.4 to 2.2 episodes, compared with 5.8 → 4.9 episodes for group A (F = 8.8, P < 0.01).
SMBG-based markers of glycemic control
Several markers of glycemic control derived from SMBG data improved as a result of the feedback provided to the patients. Specifically, in subjects with baseline HbA1c ≥8, average glucose was reduced from 196.4 to 187.2 mg/dL (F = 6.4, P = 0.013), the percent SMBG value >180 mg/dL was reduced from 50.1 to 45.8% (F = 8.23, P = 0.005), and the BG range was reduced from 183.8 to 168.1 mg/dL (F = 8.85, P = 0.04). Here, all significance levels reflect the interaction effect (baseline vs. end of study) X (low-high baseline HbA1c). A multivariate test of significance including all three variables resulted in F = 21.0, P < 0.001, indicating significant overall reduction in hyperglycemia, which was consistent with the observed reduction in HbA1c.
Blood glucose awareness and subjective detection of hypoglycemia
The last stage of the study permitted the direct comparison of level 1 versus level 3 feedback (which included symptom ratings), used to assess the effectiveness of level 3 feedback on subjects’ awareness of their BG level. Subjects’ Accuracy Index increased from 21 to 24% from pre- to post-level 3, whereas the Accuracy Index on SMBG alone decreased from 31 to 26% (F = 6.2, P = 0.015). Similarly, the percent detection increased from 35 to 44% from pre- to post-level 3 and decreased from 44 to 41% from pre- to post-SMBG: F = 3.0, P < 0.05, one-tail. These data suggest that the reduction in MH/SH may be accompanied, or prompted by, a better subjective BG evaluation.
Subjective ratings of feedback
For HbA1c feedback (Questions Q1 and Q2 under research design and methods), there were 82% and 89% “Yes” responses, respectively, and no difference between participants with HbA1c <8.0/>8.0 (80% vs. 84%, respectively). For glucose variability feedback (Questions Q3 and Q4), 61% and 75% of participants responded “Yes,” with significantly more “Yes” responses in those with HbA1c >8.0 (79% vs. 71% for Q4), indicating that this feedback was important for subjects in poor glycemic control. For risk of hypoglycemia feedback (Questions Q5 and Q6), 51% and 42% of participants responded “Yes.” However, among those with history of SH, the utility of this feedback was rated much higher, with 85% and 70% “Yes” responses, indicating that hypoglycemia risk feedback was important for subjects at higher risk for SH.
CONCLUSIONS
The challenge for any treatment designed to improve glycemic status in type 1 diabetes is to find a way to reduce the frequency of hyperglycemia (and thereby improve HbA1c) while simultaneously avoiding an increase in the frequency of hypoglycemia (and thereby not worsening risk for SH). These findings demonstrate that an automated bio-behavioral feedback intervention, delivered in the field, via an HHC, may be able to achieve this goal. Notably, this type of automated intervention was most effective for those patients most at risk for poor glucose control and problematic SH, reducing HbA1c in those with baseline HbA1c >8.0, and reducing the incidence of SH among those with a history of SH. Although the improvement in HbA1c was less than the 1% decrease typically accepted as clinically meaningful, it is worth noting that the reduction for patients in poor control was comparable to the degree of improvement achieved by contemporary studies using continuous glucose monitoring (CGM) (24). The reduction in frequency of SH was clinically significant.
Studies have demonstrated the efficacy of behavioral interventions, such as BGAT, in improving glucose control, including reductions in HbA1c and the frequency of SH episodes (9,10). However, these interventions are labor- and resource-intensive, typically involving multiple face-to-face training sessions usually led by expert personnel who have to undergo specialty training in the delivery of the treatment program. Although BGAT has been operationalized as an Internet-based intervention (25), issues of wide-scale dissemination and long-term sustainability remain a challenge. Web-based systems can offer comprehensive content, immersive experience, and great avenues for training and information delivery. However, a simple automated bio-behavioral feedback system, such as the one developed and tested in this study, may provide a more effective alternative, or complementary, method for delivering and disseminating this type of intervention. Such a system has low computational demand and can be built directly in contemporary SMBG devices, which have adequate computing power to handle the calculations needed for the estimation of HbA1c, glucose variability, risks for hypoglycemia, and associated symptoms. The advantages of such a system would include the following: 1) real-time feedback available immediately after each SMBG reading, without the need to transfer SMBG data elsewhere; 2) real-time goal setting based on estimates of HbA1c or risk for hypoglycemia and opportunities for immediate treatment adjustment if an increased risk for hypoglycemia is indicated; and 3) educational experience allowing the person to relate self-treatment behaviors to changes in weekly or monthly markers of glycemic control.
This last point is particularly important because the current systems generally allow for relating a treatment action to its immediate consequences captured by an SMBG reading, but not to longer-term effects that are reflected by summary characteristics. The results of this study support the notion that knowledge of longer-term consequences of diabetes management behaviors is beneficial: Routine SMBG alone (level 1) was useful for reducing HbA1c in those patients with poor control, which suggests that simply increasing awareness of frequent hyperglycemia can sometimes be beneficial for this patient group. However, this level of feedback was not effective in reducing risk of SH, which did not occur until after both level 2 and 3 feedback was received. We can therefore speculate that higher-level data processing (and perhaps behavioral responses to data feedback) is needed to anticipate and prevent hypoglycemic episodes. In addition, by comparing level 3 with level 1 feedback, we found that subjects’ awareness of their BG levels decreased when real-time feedback about their symptoms was discontinued, which indicates that continuous engagement with feedback would be needed to maintain the intervention effect.
In addition to the objectively measured positive effect of feedback, the participants’ opinions about the perceived benefits of the feedback were positive. As would be expected, real-time estimation of HbA1c had the highest approval rating of all estimates included in the feedback, because HbA1c is the best known metric of glycemic control, with 89% of the participants wanting this feedback on a weekly basis. Feedback about glucose variability was also well accepted, with 75% of all patients (and 79% of those with high baseline HbA1c) wanting this feedback weekly. However, the positive ratings for variability were lower than those of HbA1c feedback, indicating that more in-depth training or patient education would be needed to clarify the meaning and the importance of glucose variability for maintaining glycemic control. The feedback about risk for hypoglycemia was rated highly by those with a history of SH, 81% of whom stated that they would look at this feature regularly. The rest of the patients, however, found little benefit in this type of feedback, indicating that such a feature should be offered only to those in need of risk assessment. Although these positive responses are promising, future population-based studies are needed to determine whether patients would use and benefit from this type of automated feedback in clinical settings.
More research is also obviously needed to test additional types of automated feedback that might further improve glucose control and reduce SH risk, as well as other ways to optimize the effectiveness of these interventions. Furthermore, this study did not clarify a relevant question: how participants used the feedback at each level. Subjects were given written instructions on the meaning of the presented parameters (e.g., estimated HbA1c, risk for hypoglycemia), as well as general advice on measures that could be taken to improve HbA1c or reduce risks for hypoglycemia, but were not asked to change their treatment on the basis of the presented parameters. Moreover, there were no specific recommendations about any changes in insulin dosing. Therefore, we can assume that providing glucose profile feedback on certain longer-term summary characteristics that go beyond the information contained in a single SMBG reading was useful. A similar effect was recently reported in a large trial of CGM; it was unclear how subjects interpreted CGM information, but the effect of receiving CGM feedback was significant (24). It is therefore evident that future research needs to focus on how patients are using additional glucose feedback and what types of changes are occurring in diabetes management to improve outcome.
Acknowledgments
This study was supported by grants R01-DK51562 and R01-DK085623 from the National Institutes of Health. OneTouch UltraSmart meters and strips were provided by LifeScan, Inc., Milpitas, CA.
B.K. has received grant support and patent royalties from LifeScan, Inc., a manufacturer of self-monitoring devices. No other potential conflicts of interest relevant to this article were reported.
B.P.K. directed the development and the execution of all clinical trials, the data analysis, the interpretation of the results, and the writing of this article. P.M. was in charge of subject recruitment and follow-up, and the overall completeness of the large volumes of data collected in this study; S.A. completed all screening exams and oversaw patient care and insulin administration during the study; J.S.H. was in charge of database maintenance, data cleaning, and certain statistical analyses; L.M.R. oversaw the Internet-based collection of hypoglycemia diaries; L.G.-F. contributed to the development of the behavioral–intervention algorithms used by this study, and to the interpretation of the results. All authors contributed to the writing of this article.
Footnotes
Clinical trial reg. no. NCT00315939, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1366/-/DC1.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Engl J Med 1993;329:978–986 [DOI] [PubMed] [Google Scholar]
- 2.Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes 1994;43:313–317 [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 4.Cryer PE. Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes 1994;43:1378–1389 [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 6.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 7.Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993;42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 8.Cox DJ, Gonder-Frederick LA, Polonsky W, Schlundt D, Julian D, Clarke WL. A multicenter evaluation of blood glucose awareness training-II. Diabetes Care 1995;18:523–528 [DOI] [PubMed] [Google Scholar]
- 9.Cox DJ, Gonder-Frederick LA, Polonsky W, Schlundt D, Kovatchev B, Clarke W. Blood glucose awareness training (BGAT-2): long-term benefits. Diabetes Care 2001;24:637–642 [DOI] [PubMed] [Google Scholar]
- 10.Kinsley BT, Weinger K, Bajaj M, et al. Blood glucose awareness training and epinephrine responses to hypoglycemia during intensive treatment in type 1 diabetes. Diabetes Care 1999;22:1022–1028 [DOI] [PubMed] [Google Scholar]
- 11.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke WL. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev BP, Straume M, Cox DJ, Farhy LS. Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes. Theor Med 2001;3:1–10 [Google Scholar]
- 13.Kovatchev BP, Cox DJ, Farhy LS, Straume M, Gonder-Frederick LA, Clarke WL. Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J Clin Endocrinol Metab 2000;85:4287–4292 [DOI] [PubMed] [Google Scholar]
- 14.Cox DJ, Gonder-Frederick LA, Ritterband L, Clarke WL, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care 2007;30:1370–1373 [DOI] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care 1997;20:1655–1658 [DOI] [PubMed] [Google Scholar]
- 16.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke WL. Transforming the Blood Glucose Scale: statistical and clinical implications (Abstract). Diabetes 1997;46(Suppl. 1):A268 [Google Scholar]
- 17.Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick LA, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther 2003;5:817–828 [DOI] [PubMed] [Google Scholar]
- 18.Kovatchev BP, Otto E, Cox DJ, Gonder-Frederick LA, Clarke WL. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Schlundt D, Clarke WL. Stochastic model of self-regulation decision making exemplified by decisions concerning hypoglycemia. Health Psychol 1998;17:277–284 [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP, Cox DJ, Straume M, Farhy LS. Association of self-monitoring blood glucose profiles with glycosylated hemoglobin. In Methods in Enzymology. 321: Numerical Computer Methods, Part C. Johnson M, Brand L, Eds. New York, Academic Press, 2000, p. 410–417 [DOI] [PubMed] [Google Scholar]
- 21.Cox DJ, Ford D, Gonder-Frederick LA, et al. Driving mishaps among individuals with type 1 diabetes: a prospective study. Diabetes Care 2009;32:2177–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987;10:622–628 [DOI] [PubMed] [Google Scholar]
- 23.Cox DJ, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL. Understanding error grid analysis. Diabetes Care 1997;20:911–912 [DOI] [PubMed] [Google Scholar]
- 24.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 25.Cox DJ, Ritterband L, Magee J, Clarke W, Gonder-Frederick L. Blood glucose awareness training delivered over the internet. Diabetes Care 2008;31:1527–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]