Abstract
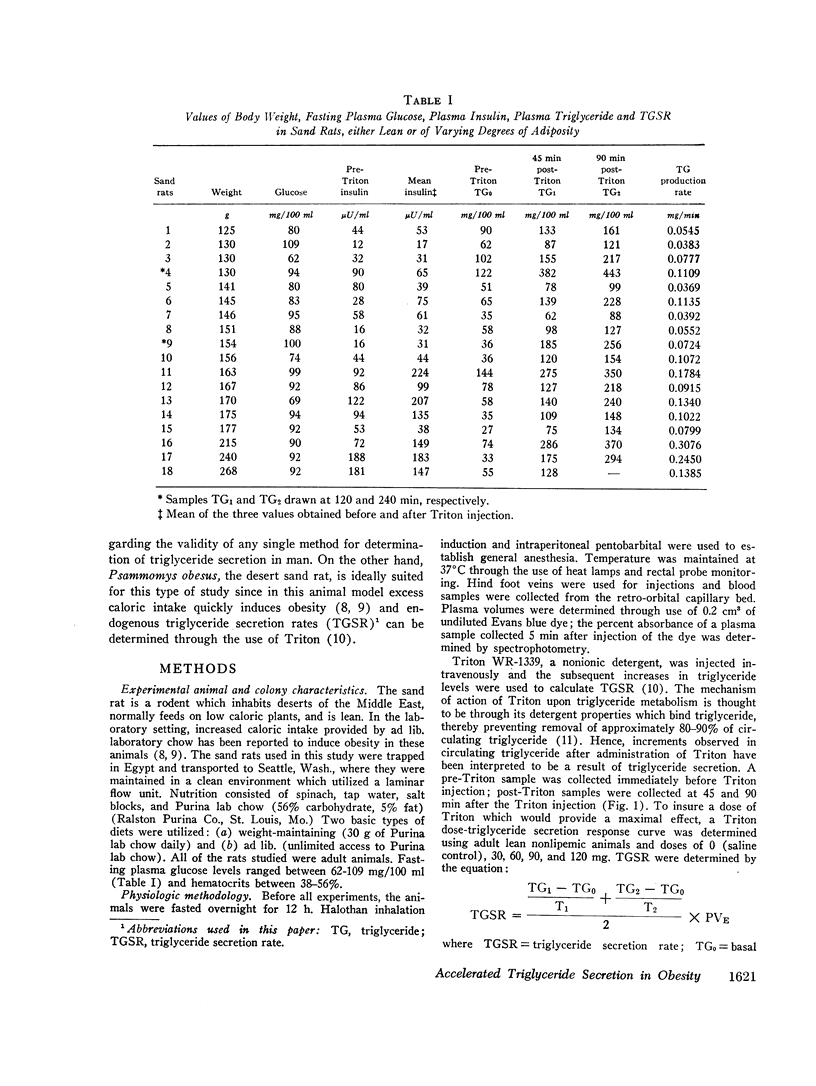
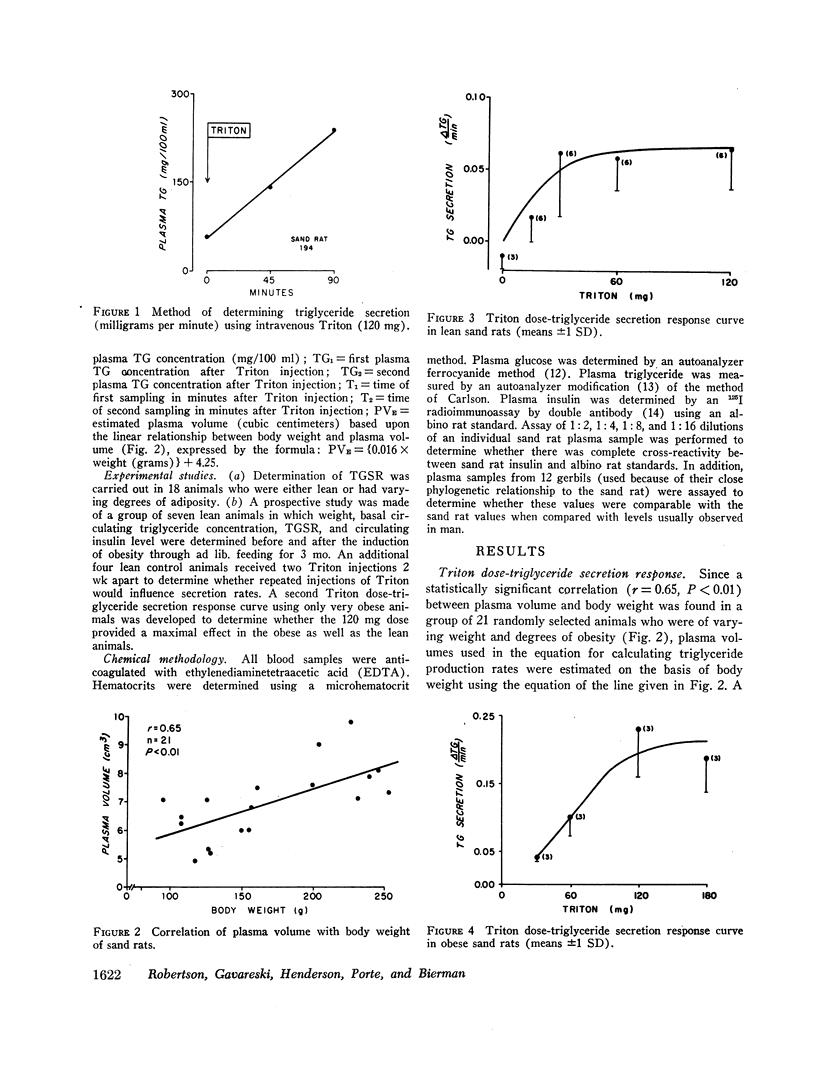
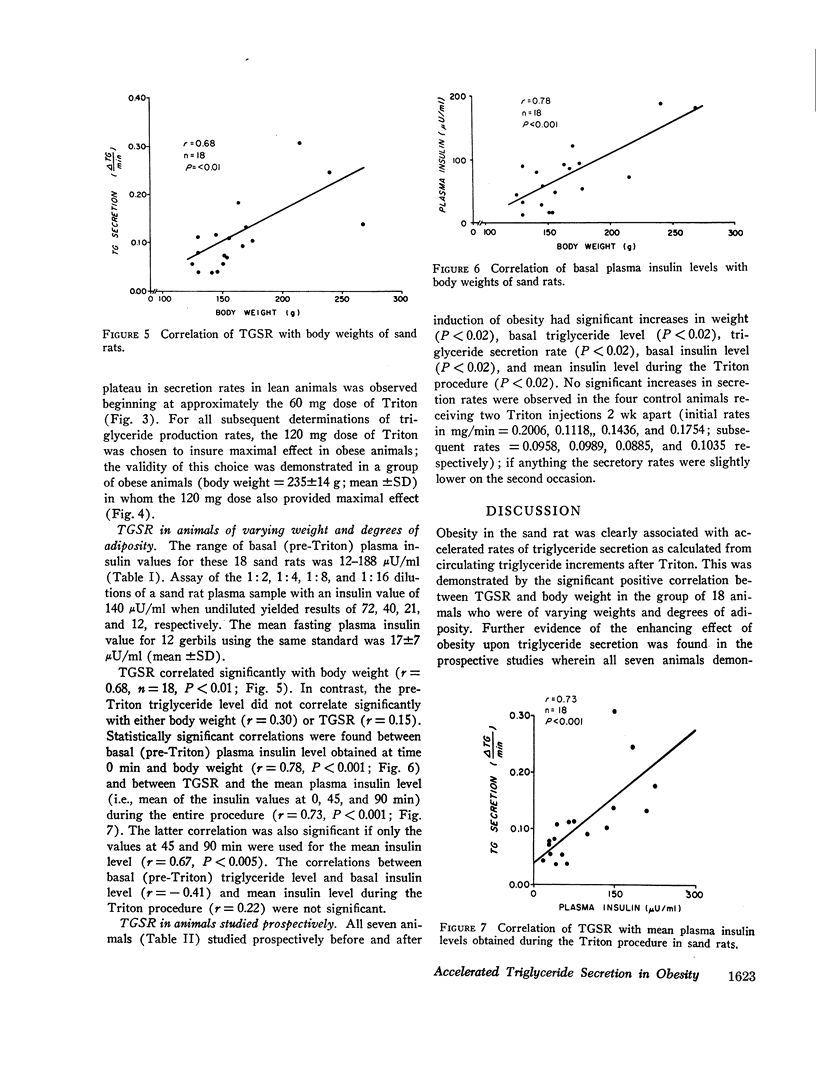
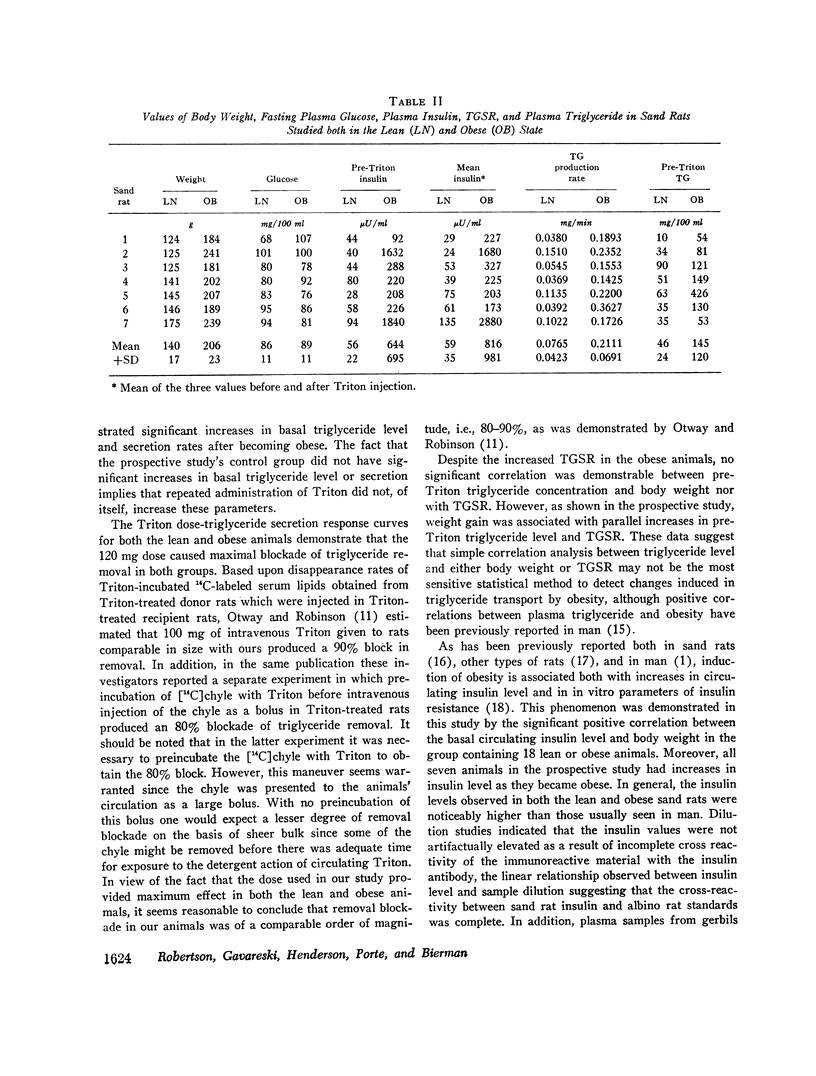
A new animal model was developed to determine the effect of obesity upon endogenous triglyceride secretion. Desert sand rats (Psammomys obesus), rodents which become spontaneously obese and hyperinsulinemic when given ad lib. chow, were given intravenous Triton to allow in vivo measurement of triglyceride secretion rates (TGSR). In a group of 18 fasted animals of varying body weight and degrees of obesity, TGSR correlated significantly with body weight (r=0.68, P < 0.01) indicating that obesity was associated with accelerated endogenous release of triglyceride. In these same animals, basal plasma insulin levels correlated significantly with body weight (r=0.78, P < 0.001) and TGSR correlated significantly with mean plasma insulin levels (r=0.73, P < 0.001), suggesting that hyperinsulinemia may have been the mechanism through which obesity enhanced TGSR. No correlation was found between basal triglyceride level and either body weight, basal insulin, or TGSR which suggested that individual triglyceride removal rates among the animals may have been variable. To test this hypothesis, seven animals were studied prospectively before and after induction of obesity. There were significant increases (P < 0.02) in all parameters, i.e., weight, plasma insulin level, TGSR, and basal triglyceride level. Thus, when each animal was used as its own control, thereby minimizing the postulated factor of variable individual triglyceride removal, increments in basal triglyceride were shown to accompany the development of obesity, hyperinsulinemia, and accelerated triglyceride secretion. These data from studies in the sand rat offer in vivo evidence that obesity leads to accelerated triglyceride secretion, an effect which may be mediated by hyperinsulinemai, and which can be invoked as one possible mechanism to explain hypertriglyceridemia associated with obesity in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIERMAN E. L., PORTE D., Jr, O'HARA D. D., SCHWARTZ M., WOOD F. C., Jr CHARACTERIZATION OF FAT PARTICLES IN PLASMA OF HYPERLIPEMIC SUBJECTS MAINTAINED ON FAT-FREE HIGH-CARBOHYDRATE DIETS. J Clin Invest. 1965 Feb;44:261–270. doi: 10.1172/JCI105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary and drug treatment of primary hyperlipoproteinemia. Ann Intern Med. 1972 Aug;77(2):267–294. doi: 10.7326/0003-4819-77-2-267. [DOI] [PubMed] [Google Scholar]
- Farquhar J. W., Frank A., Gross R. C., Reaven G. M. Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest. 1966 Oct;45(10):1648–1656. doi: 10.1172/JCI105472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S., Jr, Bozian R. C., Knowles H. C., Jr Interactions of obesity, and glucose and insulin levels in hypertriglyceridemia. Am J Clin Nutr. 1968 Sep;21(9):904–910. doi: 10.1093/ajcn/21.9.904. [DOI] [PubMed] [Google Scholar]
- Hackel D. B., Frohman L., Mikat E., Lebovitz H. E., Schmidt-Nielsen K., Kinney T. D. Effect of diet on the glucose tolerance and plasma insulin levels of the sand rat (Psammomys obesus). Diabetes. 1966 Feb;15(2):105–114. doi: 10.2337/diab.15.2.105. [DOI] [PubMed] [Google Scholar]
- Hackel D. B., Lebovitz H. E., Frohman L. A., Mikat E., Schmidt-Nielsen K. Effect of caloric restriction on the glucose tolerance and plasma insulin of the sand rat. Metabolism. 1967 Dec;16(12):1133–1139. doi: 10.1016/0026-0495(67)90059-5. [DOI] [PubMed] [Google Scholar]
- Letarte J., Fraser T. R. Stimulation by insulin of the incorporation of U-14C-glucose into lipids released by the liver. Diabetologia. 1969 Oct;5(5):358–359. doi: 10.1007/BF00452914. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The effect of a non-ionic detergent (Triton WR 1339) on the removal of triglyceride fatty acids from the blood of the rat. J Physiol. 1967 May;190(2):309–319. doi: 10.1113/jphysiol.1967.sp008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Lerner R. L., Stern M. P., Farquhar J. W. Role of insulin in endogenous hypertriglyceridemia. J Clin Invest. 1967 Nov;46(11):1756–1767. doi: 10.1172/JCI105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN K., HAINES H. B., HACKEL D. B. DIABETES MELLITUS IN THE SAND RAT INDUCED BY STANDARD LABORATORY DIETS. Science. 1964 Feb 14;143(3607):689–690. doi: 10.1126/science.143.3607.689. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Pfleger B. Overproduction of very low-density lipoproteins by livers of genetically obese rats. Am J Physiol. 1971 May;220(5):1178–1181. doi: 10.1152/ajplegacy.1971.220.5.1178. [DOI] [PubMed] [Google Scholar]
- Sims E. A., Goldman R. F., Gluck C. M., Horton E. S., Kelleher P. C., Rowe D. W. Experimental obesity in man. Trans Assoc Am Physicians. 1968;81:153–170. [PubMed] [Google Scholar]
- Stern J., Johnson P. R., Greenwood M. R., Zucker L. M., Hirsch J. Insulin resistance and pancreatic insulin release in the genetically obese Zucker rat. Proc Soc Exp Biol Med. 1972 Jan;139(1):66–69. doi: 10.3181/00379727-139-36078. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Hepatic metabolism of free fatty acids in experimental diabetes. Isr J Med Sci. 1972 Mar;8(3):309–316. [PubMed] [Google Scholar]
- York D. A., Steinke J., Bray G. A. Hyperinsulinemia and insulin resistance in genetically obese rats. Metabolism. 1972 Apr;21(4):277–284. doi: 10.1016/0026-0495(72)90070-4. [DOI] [PubMed] [Google Scholar]


