Abstract
OBJECTIVE
Glucocorticoids (GCs) are regarded as diabetogenic because they impair insulin sensitivity and islet-cell function. This study assessed whether treatment with the glucagon-like peptide receptor agonist (GLP-1 RA) exenatide (EXE) could prevent GC-induced glucose intolerance.
RESEARCH DESIGN AND METHODS
A randomized, placebo-controlled, double-blind, crossover study in eight healthy men (age: 23.5 [20.0–28.3] years; BMI: 26.4 [24.3–28.0] kg/m2) was conducted. Participants received three therapeutic regimens for 2 consecutive days: 1) 80 mg of oral prednisolone (PRED) every day (q.d.) and intravenous (IV) EXE infusion (PRED+EXE); 2) 80 mg of oral PRED q.d. and IV saline infusion (PRED+SAL); and 3) oral placebo-PRED q.d. and intravenous saline infusion (PLB+SAL). On day 1, glucose tolerance was assessed during a meal challenge test. On day 2, participants underwent a clamp procedure to measure insulin secretion and insulin sensitivity.
RESULTS
PRED+SAL treatment increased postprandial glucose levels (vs. PLB+SAL, P = 0.012), which was prevented by concomitant EXE (vs. PLB+SAL, P = NS). EXE reduced PRED-induced hyperglucagonemia during the meal challenge (P = 0.018) and decreased gastric emptying (vs. PRED+SAL, P = 0.028; vs. PLB+SAL, P = 0.046). PRED+SAL decreased first-phase glucose- and arginine-stimulated C-peptide secretion (vs. PLB+SAL, P = 0.017 and P = 0.05, respectively), whereas PRED+EXE improved first- and second-phase glucose- and arginine-stimulated C-peptide secretion (vs. PLB+SAL; P = 0.017, 0.012, and 0.093, respectively).
CONCLUSIONS
The GLP-1 RA EXE prevented PRED-induced glucose intolerance and islet-cell dysfunction in healthy humans. Incretin-based therapies should be explored as a potential strategy to prevent steroid diabetes.
Glucocorticoids (GCs) are diabetogenic agents because they reduce insulin sensitivity (1), impair α-cell function (2), and, according to more recent findings, impair β-cell function (3,4). As such, chronic use of GCs was associated with odds ratios between 1.4 and 2.3 to develop diabetes (5–7). Loss of glycemic control during GC use is particularly due to impaired postprandial glucose metabolism, whereas fasting plasma glucose (FPG) levels are usually only mildly elevated (4,7). Although the exact prevalence of steroid-related diabetes is unknown, the widespread use of GCs indicates that it may represent a major clinical problem worldwide.
When initiating GC therapy in current clinical practice, preventive pharmacologic measures are taken to prevent some of the GC-related side effects, most notably osteoporosis and peptic ulcer disease (8,9). Despite the highly prevalent occurrence of steroid diabetes, to date, no strategies have been undertaken to prevent the adverse metabolic effects of GC treatment. Previous studies showed that metformin and the thiazolidinedione pioglitazone were unable to mitigate the effects of GCs on glucose tolerance (10), whereas the thiazolidinedione troglitazone prevented GC-induced hyperglycemia by enhancing GC clearance (10,11). Because of liver toxicity, however, troglitazone is no longer available for treatment in humans.
The gut hormone glucagon-like peptide (GLP)-1 and synthetic dipeptidyl-peptidase-4 resistant GLP-1 receptor agonists (GLP-1 RAs), such as exenatide (EXE), lower blood glucose by, glucose-dependently, enhancing insulin secretion and production and inhibiting glucagon secretion, and by slowing down gastric emptying (12). One year of EXE treatment was shown to improve clamp-measured β-cell function in patients with type 2 diabetes mellitus (T2DM) (13). The GLP-1 RA exendin-4 was shown to prevent GC-induced β-cell apoptosis in vitro (14). In a single patient with Cushing disease, GLP-1 infusion was as effective in lowering blood glucose levels compared with patients with “typical” T2DM (15). GLP-1 infusion effectively reduced stress hyperglycemia in patients undergoing coronary artery bypass grafting (16).
Given these beneficial effects of GLP-1 RA treatment and the pathophysiologic defects underlying GC-induced glucose intolerance and diabetes, we aimed to assess whether intravenous (IV) infusion of the GLP-1 RA EXE could prevent the acute adverse effects of prednisolone (PRED) treatment on glucose metabolism, islet-cell function, and insulin sensitivity in healthy normoglycemic individuals.
RESEARCH DESIGN AND METHODS
Participants
Eight healthy men were recruited via local advertisements. Inclusion criteria included age = 18–35 years, BMI = 22.0–28.0 kg/m2, good physical health (determined by medical history, physical examination, and screening blood tests), and normoglycemia as defined by FPG <5.6 mmol/L and 2-h glucose <7.8 mmol/L after a 75 g of oral glucose tolerance test performed at the screening visit. Exclusion criteria were the presence of any disease, use of any medication, first-degree relative with type 2 diabetes, smoking, shift work, a history of GC use, and recent changes in weight or physical activity. The study was approved by an independent ethics committee, and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participation.
Experimental design
The study was a randomized, placebo-controlled, double-blind crossover study. After assessment of eligibility, participants received for 2 consecutive days, in random order 1) 80 mg of oral PRED every day (q.d.) and IV EXE infusion (PRED+EXE); 2) 80 mg oral PRED q.d. and IV saline infusion (PRED+SAL); and 3) oral placebo-PRED q.d. and IV saline infusion (PLB+SAL). On day 1, a standardized meal challenge was performed (Supplementary Fig. 1A). On day 2, participants underwent a combined clamp procedure, starting with a hyperinsulinemic-euglycemic clamp and followed by a hyperglycemic clamp with subsequent arginine stimulation (Supplementary Fig. 1B) (13). The three 2-day treatment blocks were separated by at least 4 weeks. Participants were instructed to refrain from intense physical activity 2 days before each treatment block.
The primary end point was the 4-h glucose area under the curve (AUCG) during the meal challenge test. From previous studies, we expected an increase in AUCG of 400 mmol/L ⋅ 240 min after PRED (4). A sample size of eight would provide 80% power to detect a significant reduction of 50% by EXE of PRED-induced augmentation of AUCG. Secondary end points included first- and second-phase C-peptide incremental area under the curve (iAUCCP) and arginine-stimulated C-peptide secretion (ASI-iAUCCP) during the hyperglycemic clamp.
Standardized meal challenge
On day 1 of each treatment block, participants underwent a standardized meal challenge test after an overnight fast of minimally 10 h. The meal contained 905 kcal (50 g fat, 75 g carbohydrates, 35 g protein) and 1 g liquid acetaminophen to estimate gastric emptying rates. Samples for determination of glucose, insulin, C-peptide, glucagon, acetaminophen, and EXE were obtained at times −120, −60, −30, 0, 10, 20, 30, 60, 90, 120, 150, 180, and 240 min, with the meal beginning immediately after the time 0 sample and consumed within 15 min. Eighty mg oral PRED or PLB was ingested 2 h before meal consumption and IV. EXE or SAL infusion started 60 min before the meal at an infusion rate of 40 ng/min for 30 min and was decreased to 20 ng/min for the remainder of the test (Supplementary Fig. 1A).
Hyperinsulinemic-euglycemic clamp and hyperglycemic clamp
On day 2 of each block, a combined hyperinsulinemic-euglycemic and hyperglycemic clamp procedure was done. After an overnight fast, an indwelling cannula was inserted into an antecubital vein for infusion of glucose and insulin. To obtain arterialized venous blood samples, a retrograde cannula was inserted in a contralateral wrist vein and maintained in a heated box at 50°C. Insulin was infused at a rate of 40 mU/m2 ⋅ min for 120 min; plasma glucose was kept at 5 mmol/L by a variable infusion of 20% glucose. The hyperglycemic clamp was started 90 min after cessation of exogenous insulin infusion. Plasma glucose concentration was then increased to 10 mmol/L by a body weight-adjusted IV bolus of 20% glucose, and a variable 20% glucose infusion was adjusted to maintain the targeted glucose level. After 80 min of hyperglycemia, an IV bolus of 5 g arginine (dissolved in 50 ml) was given over 45 s, and the glucose level was maintained at 10 mmol/L for 30 min. Oral PRED or PLB (80 mg) was administered 2 h before initiation of hyperinsulinemic-euglycemic clamp. IV EXE or SAL infusion was started 60 min before the start of the hyperglycemic clamp at an infusion rate of 40 ng/min for 30 min and was decreased to 20 ng/min for the rest of the hyperglycemic clamp (Supplementary Fig. 1B). Note that EXE was not infused during the hyperinsulinemic-euglycemic clamp, because in a pilot study, EXE strongly induced insulin secretion at a glucose level of 5 mmol/L. The hyperinsulinemic-euglycemic clamp was performed to be able to calculate the disposition index (DI) (see below).
Study medication
PRED tablets were purchased from Pfizer AB (Sollentuna, Sweden), and PLB tablets were obtained from Xendo Drug Development (Groningen, the Netherlands). Tablets were capsulated to allow the treatment to be blinded (4). Commercially available EXE was purchased (Pharmacy VU University Medical Center) and diluted in saline containing 1% human serum albumin, forming a colorless solution. IV administration of EXE was chosen to be able to gain steady-state EXE levels within a short period of time. The plasma EXE target level was 100 pg/ml, which demonstrated good efficacy and tolerability during previous infusion experiments (17).
Analytic determinations
Blood glucose concentrations were measured using an YSI 2300 STAT Plus analyzer (YSI, Yellow Springs, OH). Insulin and C-peptide levels were determined using an immunometric assay (Advia Centaur; Siemens Medical Solutions Diagnostics, Deerfield, IL). Glucagon (Linco Research, St. Louis, MO) and acetaminophen (Abbott Laboratories, Abbott Park, IL) concentrations were determined by radioimmunoassay. EXE levels were determined by an immunoenzymetric assay as described previously (Amylin, San Diego, CA) (17). Body fat percentage was estimated by bioelectrical impedance analysis (BF-906; Maltron International, Rayleigh, Essex, UK).
Data analyses
Absolute area under the curves (AUCs) for glucose, insulin, C-peptide, glucagon, and acetaminophen were calculated during the 4-h meal challenge using the trapezoid method. The Matsuda whole-body insulin sensitivity index was calculated from the meal challenge. Whole-body insulin sensitivity as obtained from the hyperinsulinemic-euglycemic clamp was quantified by the M-value, calculated between min 90 and 120 during steady-state insulin concentrations. iAUCCP for the first-phase (min 0–10) and second-phase (min 10–80), and ASI-iAUCCP (min 80–110) were calculated during the hyperglycemic clamp using the trapezoid method. The DI from the clamp tests was calculated by the C-peptide iAUC multiplied by the M-value.
Statistical analyses
Data are presented as mean values ± SEM or, in case of skewed distribution, as median (interquartile range). Between-block differences were tested nonparametrically with the Friedman test and, in case of a significant result, further analyzed using the Wilcoxon signed-rank test. All statistical analyses were run on SPSS (SPSS Inc., Chicago, IL). A P < 0.05 was considered statistically significant.
RESULTS
Subject characteristics
Eight healthy Caucasian men were included, median (interquartile range): age = 23.5 (20.0–28.3) years; BMI = 25.8 (23.2–27.7) kg/m2; waist = 91 (82–95) cm; body fat = 21 (15–26) %; FPG = 5.0 (4.8–5.3) mmol/L; triglycerides = 1.1 (0.7–1.5) mmol/L; systolic blood pressure = 119 (117–125) mmHg; diastolic blood pressure = 77 (72–82) mmHg.
Standardized meal challenge
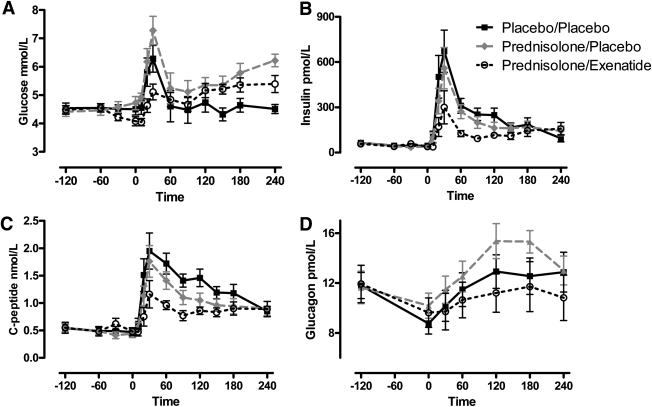
PRED+SAL treatment increased AUCG compared with PLB+SAL (P = 0.012), which was prevented by concomitant EXE administration (Table 1, Fig. 1A). AUC for insulin was not decreased by PRED+SAL, although AUC for C-peptide (AUCCP) tended to be lower (P = 0.07). PRED+EXE significantly decreased both AUC for insulin and AUCCP (Table 1, Fig. 1B and C). PRED+SAL nonsignificantly increased glucagon secretion compared with PLB+SAL (P = 0.09), which was mitigated by EXE treatment (Fig. 1D). EXE significantly decreased AUC for acetaminophen compared with both PLB and PRED, compatible with its gastric-emptying slowing effects. The Matsuda whole-body insulin sensitivity index increased during EXE treatment compared with PLB and PRED (Table 1).
Table 1.
Results from the standardized meal challenge
|
P value |
||||||
|---|---|---|---|---|---|---|
| PLB+SAL (N = 8) | PRED+SAL (N = 8) | PRED+EXE (N = 8) | PLB+SAL vs. PRED+SAL | PLB+SAL vs. PRED+EXE | PRED+SAL vs. PRED+EXE | |
| AUCG (mmol/L ⋅ 240 min) | 1,199 (1,043–1,248) | 1,335 (1,254–1,501) | 1,247 (1,156–1,260) | 0.012 | 0.263 | 0.025 |
| AUCI (nmol/L ⋅ 240 min) | 66.2 (35.2–81.0) | 52.5 (27.2–70.2) | 32.2 (22.8–40.1) | 0.263 | 0.017 | 0.017 |
| AUCCP (nmol/L ⋅ 240 min) | 354 (212–382) | 270 (191–328) | 203 (184–221) | 0.069 | 0.017 | 0.017 |
| AUCGCG (pmol/L ⋅ 240 min) | 2,845 (1,941–2,965) | 3,085 (2,859–3,633) | 2,232 (1,850–3,149) | 0.091 | 0.499 | 0.018 |
| AUCACET (mg/L ⋅ 240 min) | 1,272 (1,002–1,485) | 1,449 (1,243–1,542) | 940 (801–1,039) | 0.6 | 0.028 | 0.046 |
| Matsuda index (no dimension) | 22.6 (13.0–39.4) | 20.4 (16.1–41.2) | 36.8 (26.9–50.0) | 0.575 | 0.025 | 0.012 |
AUCACET, acetaminophen area under the curve; AUCGCG, glucagon area under the curve; AUCI, insulin area under the curve.
Figure 1.
The effect of PRED with or without concomitant EXE infusion on plasma glucose (A), insulin (B), C-peptide (C), and glucagon (D) levels during the meal challenge. PRED increased AUCG, which was prevented by EXE (A), despite lower insulin and C-peptide levels (B,C). EXE infusion reduced postprandial glucagon levels compared with PRED (D). Mean ± SEM shown. Black solid line with closed squares: PLB+SAL; gray intersected line with closed circles: PRED+SAL; black dotted line with open circles: PRED+EXE.
Combined clamp procedure
C-peptide secretion, hyperglycemic clamp.
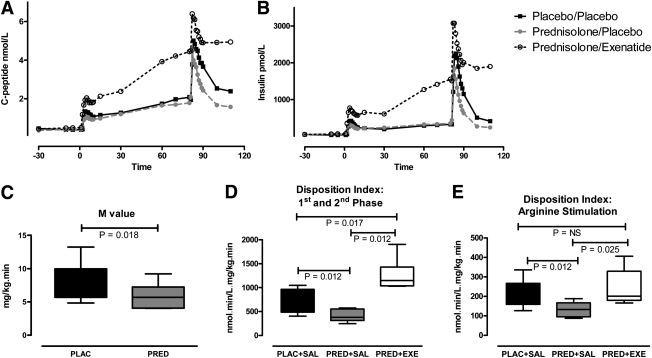
PRED decreased first-phase iAUCCP and ASI-iAUCCP (vs. PLB+SAL; P = 0.017 and P = 0.05, respectively) but did not affect second-phase iAUCCP (Table 2). EXE restored PRED-induced reductions in first-phase iAUCCP and ASI-iAUCCP, and significantly improved C-peptide secretion during the entire clamp compared with PRED+SAL and PLB+SAL (Table 2, Fig. 2A). Insulin iAUC results were not different from C-peptide iAUC results (Fig. 2B).
Table 2.
Results from the hyperglycemic clamp
| P value | ||||||
|---|---|---|---|---|---|---|
| PLB+SAL (N = 8) | PRED+SAL (N = 8) | PRED+EXE (N = 8) | PLB+SAL vs. PRED+SAL | PLB+SAL vs. PRED+EXE | PRED+SAL vs. PRED+EXE | |
| 1st iAUCCP | 6 (4–8) | 4 (2–6) | 10 (8–13) | 0.017 | 0.017 | 0.012 |
| (nmol ⋅ min/L) | ||||||
| 2nd iAUCCP | 30 (17–48) | 26 (18–53) | 111 (63–117) | 0.779 | 0.012 | 0.012 |
| (nmol ⋅ min/L) | ||||||
| 1st+2nd iAUCCP | 83 (79–107) | 71 (41–100) | 201 (160–249) | 0.208 | 0.012 | 0.012 |
| (nmol ⋅ min/L) | ||||||
| ASI iAUCCP | 26 (24–34) | 18 (17–29) | 37 (28–43) | 0.05 | 0.093 | 0.012 |
| (nmol ⋅ min/L) |
Figure 2.
The effect of PRED with or without concomitant EXE infusion on C-peptide (A) and insulin (B) concentrations during the hyperglycemic clamp. PRED+SAL decreased first-phase (min 0–10) and arginine-stimulated C-peptide secretion (min 80–110), which were completely restored and improved by concomitant EXE administration (black solid line with closed squares: PLB+SAL; gray intersected line with closed circles: PRED+SAL; black dotted line with open circles: PRED+EXE). C: PRED-induced changes in M-value during the euglycemic clamp. PRED reduced combined first- and second-phase (min 0–80) DI (D) and arginine-stimulated (min 80–110) DI (E), which was restored and improved by EXE (box-and-whisker plots [min-max] are shown).
Insulin sensitivity, euglycemic clamp.
Insulin levels reached steady-state during min 90–120 of the euglycemic clamp, averaging 431 ± 62 pmol/l (PLB+SAL), 418 ± 73 pmol/l (PRED+SAL), and 422 ± 57 pmol/l (PRED+EXE). PRED acutely decreased the M-value obtained from the euglycemic clamp by 20% (P = 0.018) (Fig. 2C). Adjustment of the M-value by insulin levels during the steady-state part of the clamp (M/I) did not affect the results (data not shown). Note that the effects of EXE on whole-body insulin sensitivity were not assessed during the euglycemic clamp; EXE was administered during the hyperglycemic clamp only.
Disposition index
PRED+SAL decreased the DI from T = 0–80 min of the hyperglycemic clamp (combined first- and second-phase; P = 0.012) and the DI from T = 80–110 min of the hyperglycemic clamp (arginine stimulation; P = 0.012). The PRED-induced decrease in DI was fully restored by concomitant EXE infusion, and EXE significantly improved the DI compared with PLB+SAL from T = 0–80 min (Fig. 2D and E).
EXE plasma levels/adverse effects
Mean EXE plasma levels equaled 65 ± 4 pg/ml between T = 0 and T = 240 of the meal challenge (Supplementary Fig. 2A) and 80 ± 4 pg/ml between T = −30 and T = 110 of the hyperglycemic clamp (Supplementary Fig. 2B). No adverse effects of either PRED or EXE treatment were experienced by the participants during the meal or clamp procedure.
CONCLUSIONS
GCs are known to impair glucose metabolism by inducing insulin resistance and, more recently, β-cell dysfunction (1,4). This study is the first to demonstrate that treatment with the GLP-1RA EXE prevents PRED-induced glucose intolerance as assessed by a standardized meal challenge test. During the hyperglycemic clamp, EXE infusion restored PRED-induced impairment of β-cell function variables and even significantly improved a number of these variables relative to the control situation. In contrast with the findings observed during the clamp procedure, EXE treatment given during the meal challenge improved glucose tolerance but resulted in decreased insulin plasma levels. This observation is in line with a previous study in healthy individuals in whom subcutaneous EXE treatment reduced postprandial glucose excursions despite significantly lower insulin levels (18). The glucose-lowering effects of EXE were attributed to decreased glucagon secretion and gastric emptying, and because of its glucose-dependent mode of action, EXE did not further stimulate insulin secretion in the presence of normoglycemia (18). Our study similarly found reduced postprandial glucagon secretion and gastric emptying after EXE treatment. Studies using stable isotope techniques have demonstrated that EXE may also reduce hepatic glucose output and increase whole-body glucose disposal in the postprandial state, independently of its more established effects on islet hormone secretion and gastric emptying (19,20). In our study, EXE improved whole-body insulin sensitivity during the meal challenge as estimated by the Matsuda index; however, reduced glucose appearance resulting from decreased gastric emptying seemed primarily responsible for improving glucose tolerance. We did not assess the effects of EXE on whole-body insulin sensitivity during the hyperinsulinemic-euglycemic clamp for previous mentioned reasons.
In this proof-of-principle study, both treatment regimens were administered for a short period of time, that is, for 2 consecutive days per study block. Although the acute metabolic effects of both PRED and EXE may to some extent differ from their effects after prolonged administration, it provides a good model to study the effects of each of both drugs and their interaction. IV EXE infusion was able to prevent the acute adverse effects of PRED on glucose tolerance, and additional benefits from EXE treatment may be expected when both compounds are administered for a more prolonged time period. Chronic GC use is associated with increased appetite, significant weight gain, increased visceral fat mass, altered secretion of adipocytokines, and dyslipidemia (1), all of which contribute to the adverse effects of GCs on glucose metabolism. In clinical studies in patients with T2DM, chronic EXE treatment was shown to reduce appetite, resulting in substantial weight loss, decreased truncal fat mass, and increased secretion of adiponectin (13,21,22). Also, EXE improved postprandial dyslipidemia (23). The strong reduction of postprandial glucose levels by EXE, rather than a pronounced effect on FPG (13), matches the profile of GC-induced hyperglycemia, which is predominantly present during the day (7).
During the hyperglycemic clamp experiments, pharmacologic concentrations of EXE were able to restore PRED-induced changes in β-cell function, including first-phase and ASI C-peptide secretion and DI calculated for the entire hyperglycemic clamp. GC exposure was demonstrated to impair various pathways in the β-cell in vitro. These include both steps in the uptake and metabolism of glucose, but GCs also affected distal pathways in the insulin exocytosis process, resulting in impaired insulin secretion in response to different secretagogues (3,4). Because EXE was able to restore insulin secretion, one may speculate that GCs do not block the pathways mediating GLP-1 action on β-cells. However, it was recently reported that a 2-week treatment with oral PRED reduced the insulinotropic effects of endogenous GLP-1 and glucose-dependent insulinotropic polypeptide (24).
A limitation of our study is that we treated healthy subjects with GCs. GCs are prescribed to treat acute and chronic inflammatory diseases, as well as autoimmune diseases. Chronic inflammation is also associated with whole-body insulin resistance and β-cell dysfunction, as recently reported in patients with rheumatoid arthritis (25). Therefore, the complex interrelationship among inflammation, GC, and GLP-1RA treatment needs to be studied prospectively in relevant patient populations.
The plasma levels of EXE reached with our infusion protocol were lower than those usually obtained after subcutaneous injection of EXE 10 μg b.i.d. (the recommended dose for the current treatment in T2DM). Although good efficacy was demonstrated by current plasma EXE levels, the full potential of GLP-1 RA treatment to prevent PRED-induced glucose intolerance may be fully unveiled in clinical studies administering EXE at the usual dose.
This study provides evidence that the GLP-1 RA EXE may prevent PRED-induced glucose intolerance and restore islet-cell functional balance. Long-term studies in relevant populations should explore the potential of GLP-1 RA treatment as a novel strategy to prevent steroid diabetes.
Acknowledgments
D.H.v.R. and M.M.L.L. are supported by the Dutch Top Institute Pharma (TIP) grant T1-106. R.E.v.G. is supported by the EFSD/MSD Clinical Research Programme 2008. D.M.O. is supported by the Ministry of Innovation, Science, Research and Technology of the German state of North-Rhine Westphalia, and the EU European Cooperation in the field of Scientific and Technical Research (COST) Action BM0602.
D.H.v.R., R.E.v.G., M.M.L.L., and D.M.O. report no conflict of interest in relation to this article. Through M.D., the VU University Medical Center received research grants from Amylin, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, sanofi-aventis, and Takeda; consultancy fee from Eli Lilly, Merck, Novo Nordisk, and sanofi-aventis; and speaker fee from Eli Lilly, Merck, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
D.H.v.R. designed and conducted the study and wrote the article. R.E.v.G. and M.M.L.L. assisted with conduction of the study. D.M.O. and M.D. designed the study and reviewed the article.
Parts of this study were presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank Mark Fineman (Amylin Pharmaceuticals) for the determination of EXE plasma levels.
Footnotes
Clinical trial reg. no. NCT00744224, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1677/-/DC1.
D.H.v.R. and R.E.v.G. contributed equally to this work.
References
- 1.van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 2009;39:81–93 [DOI] [PubMed] [Google Scholar]
- 2.Wise JK, Hendler R, Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest 1973;52:2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 1997;99:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Raalte DH, Nofrate V, Bunck MC, et al. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol 2010;162:729–735 [DOI] [PubMed] [Google Scholar]
- 5.Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care 2006;29:2728–2729 [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 1994;154:97–101 [PubMed] [Google Scholar]
- 7.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract 2009;15:469–474 [DOI] [PubMed] [Google Scholar]
- 8.Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 2010;6:82–88 [DOI] [PubMed] [Google Scholar]
- 9.Trikudanathan S, McMahon GT. Optimum management of glucocorticoid-treated patients. Nat Clin Pract Endocrinol Metab 2008;4:262–271 [DOI] [PubMed] [Google Scholar]
- 10.Morita H, Oki Y, Ito T, Ohishi H, Suzuki S, Nakamura H. Administration of troglitazone, but not pioglitazone, reduces insulin resistance caused by short-term dexamethasone (DXM) treatment by accelerating the metabolism of DXM. Diabetes Care 2001;24:788–789 [DOI] [PubMed] [Google Scholar]
- 11.Willi SM, Kennedy A, Wallace P, Ganaway E, Rogers NL, Garvey WT. Troglitazone antagonizes metabolic effects of glucocorticoids in humans: effects on glucose tolerance, insulin sensitivity, suppression of free fatty acids, and leptin. Diabetes 2002;51:2895–2902 [DOI] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 13.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranta F, Avram D, Berchtold S, et al. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 2006;55:1380–1390 [DOI] [PubMed] [Google Scholar]
- 15.Ritzel RA, Kleine N, Holst JJ, Willms B, Schmiegel W, Nauck MA. Preserved GLP-1 effects in a diabetic patient with Cushing’s disease. Exp Clin Endocrinol Diabetes 2007;115:146–150 [DOI] [PubMed] [Google Scholar]
- 16.Sokos GG, Bolukoglu H, German J, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol 2007;100:824–829 [DOI] [PubMed] [Google Scholar]
- 17.Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes 2004;53:2397–2403 [DOI] [PubMed] [Google Scholar]
- 18.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003;88:3082–3089 [DOI] [PubMed] [Google Scholar]
- 19.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008;294:E846–E852 [DOI] [PubMed] [Google Scholar]
- 20.Zheng D, Ionut V, Mooradian V, Stefanovski D, Bergman RN. Exenatide sensitizes insulin-mediated whole-body glucose disposal and promotes uptake of exogenous glucose by the liver. Diabetes 2009;58:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunck MC, Diamant M, Eliasson B, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 23.Bunck MC, Corner A, Eliasson B, et al. One-year treatment with exenatide vs. insulin glargine: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis; 2010;212:223–229 [DOI] [PubMed] [Google Scholar]
- 24.Hansen KB, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab 2010;95:3309–3317 [DOI] [PubMed] [Google Scholar]
- 25.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum 2006;54:2765–2775 [DOI] [PubMed] [Google Scholar]