Abstract
OBJECTIVE
To evaluate the frequency of normalization, the persistence of remission, and the impact on normalization of glycemic control and lipid profile, we analyzed data from a retrospective observational cohort study of type 1 diabetic children and adolescents with abnormal urinary albumin excretion (UAE).
RESEARCH DESIGN AND METHODS
All diabetic children and adolescents (n = 41) who had persistent abnormal UAE in the period of 1984 to 2008 and followed up until 2009 (follow-up duration = 13.1 ± 6.2 years) were included in the study. Nine patients progressed to macroalbuminuria; 24 patients were administered ACE inhibitor treatment.
RESULTS
The cumulative prevalence of abnormal UAE was 9%. During follow-up, 14 of 17 untreated and 19 of 24 treated patients reverted to normoalbuminuria. In the remission group compared with the nonremission group, A1C levels during follow-up decreased (7.5 ± 1.0 vs. 9.4 ± 1.2%, P < 0.0001) and serum HDL cholesterol increased (52.7 ± 11.3 vs. 42.7 ± 8.6 mg/dL, P < 0.05). The micro-macroalbuminuric patients had lower HDL cholesterol (51.0 ± 11.4 vs. 62.4 ± 13.6 mg/dL, P < 0.0001) than 134 normoalbuminuric diabetic patients.
CONCLUSIONS
Microalbuminuria and macroalbuminuria were not permanent in most of our diabetic children and adolescents. If abnormal UAE values are high and persist for >1 year, only long-lasting treatment with ACE inhibitors seems able to induce persistent remission, especially when associated with good metabolic control and high HDL cholesterol levels.
In the early 1980s, it was widely accepted that microalbuminuria (MA) inexorably predicted progressive nephropathy (1,2). In the 1990s, three studies on pediatric patients (3–5) challenged the predictive value of MA for renal disease. Several further studies in adults confirmed that MA may be transient so that now MA is considered a surrogate marker for underlying renal structural damage (6) and a less precise predictor of diabetic nephropathy risk than described originally. In fact, <20% of patients (7,8) with type 1 diabetes and MA progressed to overt nephropathy, whereas approximately 50–60% reverted to normal (7,8). Furthermore, it is noteworthy that approximately one-third of the patients with >50 years of diabetes (the Golden Years Cohort), despite showing MA or macroalbuminuria, were not affected by renal deterioration (9). It is well known that the chances of reversal to normoalbuminuria are increased by blockers of the renin-angiotensin system (6,10,11), despite the fact that others denied that assumption (7). It has recently been demonstrated that the blockade of the renin-angiotensin system is not effective in primary prevention of diabetic nephropathy (12). Furthermore, there is little evidence on the effectiveness of ACE inhibitors within the pediatric population, and in the few studies published, the sample size was small and the follow-up was short (13,14).
The present longitudinal nonrandomized study presents the long-term outcome of our diabetic children and adolescents who in the last 25 years developed MA and were treated or not treated with ACE inhibitors in an uncontrolled way. The preliminary results of this cohort of patients were published in 1990 (15).
The aims of the study were as follows: 1) to study the percentage of patients reverting from MA to normoalbuminuria with and without therapy; 2) to observe the persistence with time of normalization; and 3) to calculate the ability of metabolic control and lipid profile to predict the risk of nephropathy.
RESEARCH DESIGN AND METHODS
From 1984 to 2008, all diabetic patients (n = 490) followed up in our center underwent repeated evaluations of daily urinary albumin excretion (UAE) (15). Forty-four patients (9%) were identified as having abnormal UAE on the basis of two or all three 24-h urine collections >30 mg/day according to the International Society for Pediatric and Adolescent Diabetes criteria (16), with no urinary tract infection. Only the patients (41/44 [93%]; 20 male and 21 female) with a follow-up to 2009 were included in the study. The age at onset of diabetes was 8.6 ± 3.6 years (range 1.33–14.25). At first abnormal UAE detection, age was 12.9 ± 3.8 years (range 4.8–23.3), 12 patients were prepubertal, 22 patients were pubescent, and 7 patients were pubertal; the duration of diabetes was 4.2 ± 4.7 years (range 0–16.7). Mean duration of follow-up from MA onset was 13.1 ± 6.2 years (range 1.5–24.8). Only 5% were followed up for <5 years and 41% had >15 years of follow-up. A total of 134 diabetic patients matched for age with normal UAE were used as controls to evaluate lipid parameters. In the control group, 15% were overweight (BMI >75th percentile) and 1% were obese (BMI >95th percentile), whereas in the 41 patients with abnormal UAE, at the time of first detection, 12% were overweight and 5% were obese. The difference was not statistically significant. ACE inhibitors (enalapril) were administered to 24 of 41 patients (59%) for some period of time, whereas 17 patients were never treated. Therapy started at a mean age of 15.5 ± 4.5 years (minimum 8.8 years) and lasted for 6.0 ± 4.3 years. The choice of the patients to be treated was influenced by the time MA was discovered. Between 1984 and 1989, we did not treat any patients because there was insufficient evidence in the literature in regard to the efficacy and safety of therapy in normotensive diabetic patients with MA; from 1990 to 1995, after new data reporting favorable results of ACE inhibitor use were published (10), we treated all patients with abnormal UAE; since 1996, because of new data indicating a spontaneous normalization of MA (3,4), which took place, according to our data (5), after the end of puberty, we treated only selected patients with MA. The selection criteria were the presence of macroalbuminuria or persistence of values of UAE >100 mg/day for >12 months in pubescent patients and for >6 months in postpubertal patients. The initial dose was 10 mg once per day in the morning for all patients and was decreased to 5 mg/day because of the appearance of side effects in seven patients (hypotension; cough in one patient) and increased after a mean period of 4.8 ± 2.6 years to 20 mg/day because of lack of UAE value improvement (increase or decrease of <10% of the initial value) in five patients. When UAE values remained normal for >18 months, therapy was stopped.
Methods
UAE was evaluated every 3–12 months in all patients. Each patient was trained on the two separate urine collections of the daytime (h 8–20) and night-time (h 20–8). Albuminuria was determined by radioimmunoassay with double antibody (Sclavo, Milan, Italy). The upper limit of the normal range was 30 mg/day (15). MA was defined as the presence of UAE between 30 and 300 mg/24 h in two or all three 24-h urine samples over a period of 3–6 months (16), with no urinary tract infection, and macroalbuminuria as the presence of UAE >300 mg/day. Urine culture was performed in all patients. Creatinine was evaluated once per year with the Jaffè method. Glomerular filtration rate (GFR) was calculated with the Schwartz and Counahan-Barratt methods until age 18 years and with the estimated Modification of Diet in Renal Disease (MDRD)afterward. Because the values obtained in the transition phase by means of the two methods were statistically different (P < 0.0001), the analysis of the possible GFR variations was performed separately by comparing the first and last value of each time period (<18 years/>18 years). BMI was expressed according to the Italian percentiles for age and sex. HbA1c levels were measured by HPLC with the Auto A1c TM Analyzer HA 8110 (Kyoto Daiichi, Kagaku, Japan). The normal range was 4.3–5.9%. Serum cholesterol, HDL cholesterol, and triglycerides were measured with standard laboratory techniques; LDL cholesterol was calculated with the Friedewald equation. Detailed Tanner staging of puberty was available in all patients. Arterial blood pressure was measured with the patients seated using a standard mercury sphygmomanometer at each examination and calculated as the mean of 2 measurements. Patients with a blood pressure >95th percentile for age were considered hypertensive (17).
Statistical analysis
In each patient with abnormal UAE values, in addition to the first abnormal value, all measurements performed during the entire follow-up of pathologic UAE values were used to calculate mean UAE values. The remaining parameters, HbA1c and lipid profiles, were evaluated both at the onset of abnormal UAE and during the whole abnormal UAE period or the possible subsequent normalization period. All calculations were performed with the commercially available program SPSS 14.0.1 (Statistical Package for Social Science, Chicago, IL). Data distribution was analyzed by means of skewness and kurtosis coefficients and Kolmogorov–Smirnov test. In normally distributed variables, comparison between groups was performed by unpaired Student t test, and comparison within groups was performed by a paired t test. Pearson correlation coefficients were used to calculate correlations between normally distributed values. In non-normally distributed variables, a Mann–Whitney U test, a Wilcoxon test, and Spearman rank correlation coefficients were used. Categoric variables were analyzed using a χ2 test. P < 0.05 was considered as significant for each test.
RESULTS
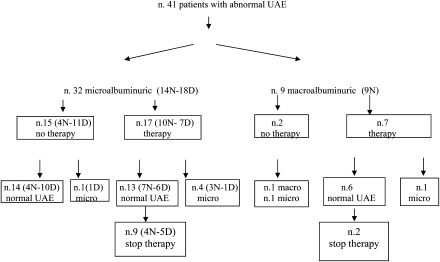
Of the 490 patients followed up, 44 were identified as having MA, and 10 (23%) of these progressed to macroalbuminuria. Therefore, the cumulative prevalence of abnormal UAE was 9% (95% CI 6.5–11.5): 7% (34 patients) with MA and 2% (10 patients) with macroalbuminuria. Of the 41 patients with MA included in the study, 9 progressed to macroalbuminuria (Fig. 1) after 1–3.5 years of MA. No patients with macroalbuminuria were overweight.
Figure 1.
Patient flowchart (N, nighttime MA; D, only daytime MA).
Eight of these 41 patients showed abnormal UAE values since diabetic onset. These eight patients were all pubescent and showed an older age at onset (12.4 ± 1.4 vs. 7.7 ± 3.4 years, P < 0.0001) than the patients with later onset of MA (5.2 ± 4.7 years after diabetes diagnosis).
During follow-up, 14 of 17 patients (82%) who had never received ACE inhibitors went into spontaneous remission; the duration of abnormal UAE had been 2.1 ± 1.7 years, and the remission was sustained for 9.6 ± 6.3 years (Table 1). In the remaining three untreated patients, one remained with MA and two progressed to macroalbuminuria, although one of these two patients then regressed to MA (Fig. 1). Of the 24 patients who were treated with ACE inhibitors, 19 achieved normal UAE levels on treatment (79%); 11 of these 19 patients stopped therapy according to the protocol, and all were able to maintain normal levels even after medication withdrawal. Maintenance of normal levels off treatment was achievable in 9 of 17 patients who had only MA when ACE inhibitors were started and in two of the seven patients who presented macroalbuminuria when ACE inhibitors were started. In those achieving normal levels off treatment who had only MA, ACE inhibitor treatment was carried out for a mean of 2.4 ± 1.1 years, and the remission off treatment has lasted for 11.3 ± 5.3 years. In those achieving normal levels off treatment who had macroalbuminuria, ACE inhibitor treatment was carried out for a mean of 7.7 ± 2.3 years and the remission off treatment has lasted for 9.0 ± 3.9 years. Of the 18 patients with only daytime abnormal UAE values, 11 did not receive ACE inhibitors and 10 spontaneously regressed to normoalbuminuria. No patients with only daytime abnormal UAE values developed macroalbuminuria.
Table 1.
Clinical and laboratory data in 41 diabetic children and adolescents with abnormal UAE, showing values in the whole group of patients and in those with MA or macroalbuminuria, treated or untreated
| All patients (n = 41) | Microalbuminuric patients (n = 32) | Macroalbuminuric patients (n = 9) | Untreated (n = 17) | Treated (n = 24) | |
|---|---|---|---|---|---|
| Mean UAE values during the whole abnormal UAE period (mg/24 h) | 166.2 ± 127.9 | 114.9 ± 59.4* | 356.9 ± 124.5 | 157.7 ± 122.7 | 187.3 ± 134.7 |
| HbA1c during the whole abnormal UAE period (%) | 7.8 ± 1.3 | 7.7 ± 1.3 | 8.1 ± 1.3 | 7.6 ± 1.5 | 8.0 ± 1.3 |
| Duration of abnormal UAE before the normalization (years) | 5.6 ± 4.6 (n = 33) | 4.7 ± 4.4† (n = 27) | 10.3 ± 2.5 (n = 6) | 2.1 ± 1.7* (n = 14) | 8.7 ± 4.2 (n = 19) |
| Duration of normalization after abnormal UAE (years) | 8.1 ± 5.9 (n = 33) | 8.3 ± 5.9 (n = 27) | 5.3 ± 4.5 (n = 6) | 9.6 ± 6.3 (n = 14) | 6.8 ± 5.8 (n = 19) |
| Duration of therapy before the normalization (years) | — | 4.6 ± 3.7‡ (n = 13) | 9.2 ± 2.6 (n = 6) | — | 6.0 ± 3.8 (n = 19) |
| Duration of therapy before stop therapy (years) | — | 2.4 ± 1.1* (n = 9) | 7.7 ± 2.3 (n = 2) | — | 3.4 ± 2.5 (n = 11) |
| Duration of remission off treatment (years) | — | 11.3 ± 5.3 (n = 9) | 9.0 ± 3.9 (n = 2) | — | 10.9 ± 5.0 (n = 11) |
*P < 0.0001;
†P < 0.01;
‡P < 0.025 vs. the respective group.
Arterial blood pressure was elevated in eight patients: persistently in two patients (one macroalbuminuric patient and one microalbuminuric patient) and fluctuating in six patients (two macroalbuminuric patients and four microalbuminuric patients). The distribution of hypertensive patients was not different between the groups with MA or macroalbuminuria and between normalized and non-normalized patients.
Creatinine values remained within the normal range (<1.20 mg/dL) in all patients throughout follow-up. Estimated GFR mean values were not statistically different between start and end of both pediatric and adult periods. However, in two patients who had developed macroalbuminuria (the first untreated and the second treated and improved from macroalbuminuria to MA), we found a progressive loss of filtration function up to 79 and 62 mL/min/1.73 m2, respectively.
In the remission group compared with the nonremission group, mean UAE values during the whole abnormal UAE period were lower (137.0 ± 107.5 vs. 286.7 ± 142.8 mg/24 h; P = 0.002), HbA1c levels during the whole follow-up period decreased (7.5 ± 1.0 vs. 9.4 ± 1.2%; P < 0.0001), and serum HDL cholesterol at first abnormal UAE detection was higher (P = 0.04) (Table 2). HDL cholesterol levels after treatment with ACE inhibitors compared with levels at entry were higher (P < 0.001 paired t test in the 21 patients with available data) in 18 normalized patients and 3 patients with levels of abnormal, although decreased, UAE values. Furthermore, lower HDL cholesterol levels and higher LDL cholesterol levels characterized the whole group of micro-macroalbuminuric patients compared with the control group of normoalbuminuric diabetic patients (P < 0.0001 and P = 0.004, respectively) (Table 2). In the micro-macroalbuminuric group, 9 of 41 patients (22%) showed lower HDL cholesterol levels than cut point by age/sex of the general population (18), whereas in the control group of normoalbuminuric patients, these abnormal values were found in 5.2% of the patients (7/134) (χ2 = 8.66, P = 0.003). The percentage of overweight patients was not different between those with normal HDL values and those with low HDL values. Moreover, we observed a positive relationship between UAE levels and HbA1c (P = 0.03) and triglycerides (P = 0.024), and a negative correlation with HDL cholesterol levels (P = 0.017). HDL cholesterol values were not correlated with BMI or HbA1c. Total cholesterol values measured in the whole period were highly significantly correlated (P < 0.0001) with both triglycerides (r = 0.62) and LDL cholesterol (r = 0.69), whereas they were never correlated with HDL cholesterol (r = 0.09, P = not significant).
Table 2.
Lipid profile in microalbuminuric patients in the whole group and in subgroups subdivided according to remission or enalapril treatment (values referred to first abnormal UAE detection)
| All microalbuminuric patients (n = 41) | Normoalbuminuric patients (n = 134) | Persistent (n = 8) | Normalized (n = 33) | Pre-therapy (n = 21) | Post-therapy (n = 21)° | |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 76.9 ± 37.7 | 73.8 ± 39.4 | 102.8 ± 47.2 | 71.6 ± 34.0* | 80.8 ± 45.4 | 85.4 ± 35.3 |
| Total cholesterol (mg/dL) | 171.7 ± 32.9 | 166.7 ± 31.1 | 168.0 ± 32.0 | 172.4 ± 33.6 | 179.7 ± 34.6 | 180.8 ± 28.1 |
| LDL cholesterol (mg/dL) | 104.1 ± 30.2† | 88.2 ± 23.9 | 128.0 ± 44.5 | 99.1 ± 24.6 | 106.8 ± 27.6 | 107.9 ± 26.6 |
| HDL cholesterol (mg/dL) | 51.0 ± 11.4‡ | 62.4 ± 13.6 | 42.7 ± 8.6 | 52.7 ± 11.3* | 49.3 ± 10.9§ | 58.6 ± 10.0 |
°Paired t test.
*P < 0.05;
†P < 0.005;
‡P < 0.0001;
§P < 0.001 vs. the respective group.
CONCLUSIONS
Our results showed that in an unselected cohort of young patients with type 1 diabetes and abnormal UAE, reverting to normal is not a rare event in untreated patients (82%) and ACE inhibitor–treated patients (79%). The explanation for the similar percentage in the two groups depends mainly on the fact that since 1996 we treated the patients who were more severely affected, selected because they showed high UAE levels (>100 mg/day) and had not spontaneously regressed after >1 year. It is difficult to compare our data with other published data, reporting normalization in 50–60% of adults, because many variables were not considered homogeneously in the various studies. For example, the method of collecting urine samples (24 h or morning sample) was not uniform, which may influence the definition of abnormal UAE. If we had used only the morning sample, we would not have included and labeled as microalbuminuric a significant number of patients (18). Also, the duration of follow-up was different, and this is fundamental to establish the exact number of regressors. Finally, transient cases (i.e., with MA disappearing within 1 year) were included in some studies (19). Despite the reported differences, it has long been known that MA can frequently normalize in adolescents, and as early as 1996 Rudberg and Dahlquist (4) reported that only 35% of patients progressed during a 3-year follow-up. However, our study may contain some bias to justify the high percentage of normalizations compared with the literature, that is, the inclusion of near-normal patients, with low-grade abnormal UAE, present only during daytime, and of short duration, or the inclusion of pubertal patients. During this period, important hormonal changes could act as an “exercise test” for the kidneys of predisposed patients, which would be temporary and would finish at the end of puberty by itself (5). Supporting this hypothesis is the fact that in many of our patients in whom diabetes started at mid-puberty, MA was already present at the diagnosis of diabetes. On the other hand, if this bias exists when considering the whole group of patients, the same confounding factors cannot be advocated when dealing with the most pathologic cases, that is, those with persistent nocturnal high-grade abnormal UAE and those with macroalbuminuria, in whom a high percentage of remissions is confirmed. In our patients with macroalbuminuria, it continued in the two untreated patients, whereas regression occurred in almost all treated patients (6/7 treated). In these cases, the regression was probably due to long-lasting ACE inhibitor therapy, which had to be continued for several years (mean 9 years) to be effective. We should not forget that approximately 30 years ago, dealing with similar patients, Viberti et al. (1) reported “proteinuria of this degree (≥0.5 g/24 h) is the earliest indicator of a progressive and relentless decline of glomerular function leading a few years later to end-stage renal failure.” The macroalbuminuria regressed in our patients, but once regression was reached, it remained stable without relapse for a mean period of 9 years, even after therapy withdrawal in two patients. We are aware that the number of patients is too low to be able to draw definite conclusions, but, if confirmed, this should be considered clinically remarkable. However, we cannot be certain that the regression to normoalbuminuria corresponds to a normalization of renal structure, as already hypothesized by others (6,20). In fact, the progressive decrease of estimated GFR found in our treated patient with abnormal UAE, which improved from macroalbuminuria to a mild MA, seems consistent with that hypothesis. We cannot exclude that the treatment was started too late to stop the progression of renal disease in this patient. Few studies are available in the literature on children and adolescents with macroalbuminuria (14), and no comparable data exist for children with a similar follow-up. Several studies agree in conferring on glycemic control a determinant role in abnormal UAE progression (4,7,8). Our findings are consistent with these data. Normalized patients had significantly lower HbA1c levels than patients with persistently abnormal UAE. A further modifiable factor, recently identified as a predictor of renal disease progression, is the lipid profile (19,21), which, according to some authors, has a pathogenic role in progressive glomerular injury (7). On the other hand, patients with type 1 diabetes and nephropathy have a 10-fold greater risk of cardiovascular diseases than those without nephropathy (8,22), and multiple lipid abnormalities, such as higher total cholesterol, LDL cholesterol (19,21), and triglycerides (7), and lower HDL cholesterol levels (21,23), have been described. We found some of these abnormalities in patients with abnormal UAE: higher LDL cholesterol in the whole group versus control group and lower triglycerides in the normalized versus non-normalized patients. However, the changes in these lipids may not be reliable, because they are secondary to worse glycemic control (19). In confirmation of the data by Molitch et al. (23) in adult diabetic patients with albuminuria, the most significant and persistent abnormality found in our study was the presence of lower HDL cholesterol levels in the microalbuminuric patients, found in the whole group versus normoalbuminuric patients, in normalized versus non-normalized patients, and finally post- versus pre-ACE inhibition (Table 2). This finding is not surprising, because HDL cholesterol, unrelated to glycemic control (19) and the remaining lipids, is basically constant over time and more closely represents the genetically determined part of a certain patient. This is supported by sibling-pair studies of candidate genes (24). The central role of HDL cholesterol in characterizing the diabetic patients with long-term good prognosis also was confirmed by the Golden Years Cohort (type 1 diabetes of >50 years duration), and the authors who reported these data concluded that the high HDL levels could be used as an early clinical marker of good prognosis in type 1 diabetes (9) and that “these features can be tied together in terms of the antithesis of the metabolic syndrome X.” Other studies have reported no significant changes in HDL cholesterol, but this could have been because this parameter was measured at different times. In fact, HDL cholesterol in our study was measured at the first detection of abnormal UAE, at a much younger age, when the confounding factors such as antihypertensive therapy or the complication itself did not interfere with the basal lipid profile of the patient. We have no explanation for the enalapril effect, not only on MA but also on HDL cholesterol, which was the only lipid to be influenced by the treatment. The effect on HDL cholesterol is more difficult to explain because it was found in both normalized and persistent micro-macroalbuminuric patients. In the latter patients, UAE values tended to decrease during treatment; thus, the lack of complete regression may simply be due to time. Furthermore, it is known that the antiproteinuric effect of ACE inhibitors cannot entirely be explained by changes in blood pressure (6).
Our study has some limitations. The small sample size, the nonstandardized criteria of randomized controlled trials in the choice of patients to be treated, the method of collecting urine samples, and the variable duration of follow-up may limit the precision of our findings. Other factors, including genetic factors, should be examined. Finally, high HDL cholesterol values may be associated with high levels of physical activity and excessive alcohol intake, but none of our patients, subsequently interviewed on the subject, reported these situations.
In summary, our findings suggest that if abnormal and nocturnal elevated UAE does not spontaneously decrease in 1–2 years in diabetic children and adolescents, ACE inhibition should be used. Its long-lasting use also seemed effective in our small cohort of patients with macroalbuminuria, especially when associated with better glycemic control and higher HDL cholesterol levels. This provides a rationale for future studies on administration of products able to directly increase HDL cholesterol, such as n-3 fatty acids (25), before MA onset in patients at risk, that is, those with low HDL cholesterol.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.S. wrote the article; C.B. researched data; S.Z. contributed to discussion and edited the article; G.M., M.S., A.R., and S.G. researched data; and A.C. reviewed the article.
Footnotes
Received 3 July 2010 and accepted 21 October 2010. DOI: 10.2337/dc10-1177
References
- 1.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982;1:1430–1432 [DOI] [PubMed] [Google Scholar]
- 2.Mathiesen ER, Oxenbøll B, Johansen K, Svendsen PA, Deckert T. Incipient nephropathy in type 1 (insulin-dependent) diabetes. Diabetologia 1984;26:406–410 [DOI] [PubMed] [Google Scholar]
- 3.Shield JPH, Hunt LP, Karachaliou F, Karavanaki K, Baum JD. Is microalbuminuria progressive? Arch Dis Child 1995;73:512–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudberg S, Dahlquist G. Determinants of progression of microalbuminuria in adolescents with IDDM. Diabetes Care 1996;19:369–371 [DOI] [PubMed] [Google Scholar]
- 5.Salardi S, Cacciari E. Is microalbuminuria progressive? Arch Dis Child 1996;75:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACE Inhibitors in Diabetic Nephropathy Trialist Group Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001;134:370–379 [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 8.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 2004;47:1020–1028 [DOI] [PubMed] [Google Scholar]
- 9.Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 11.Strippoli GFM, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004;329:828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudberg S, Østerby R, Bangstad H-J, Dahlquist G, Persson B. Effect of angiotensin converting enzyme inhibitor or beta blocker on glomerular structural changes in young microalbuminuric patients with type I (insulin-dependent) diabetes mellitus. Diabetologia 1999;42:589–595 [DOI] [PubMed] [Google Scholar]
- 14.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 2008;336:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salardi S, Cacciari E, Pascucci MG, et al. Microalbuminuria in diabetic children and adolescents. Relationship with puberty and growth hormone. Acta Paediatr Scand 1990;79:437–443 [DOI] [PubMed] [Google Scholar]
- 16.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K, International Society for Pediatric and Adolescent Diabetes ISPAD Clinical Practice Consensus Guidelines 2006–2007. Microvascular and macrovascular complications. Pediatr Diabetes 2007;8:163–170 [DOI] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(2 Suppl 4th Report):555–576 [PubMed] [Google Scholar]
- 18.Cook S, Auinger P, Huang TTK. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr 2009;155:S6.e15–e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcovecchio ML, Dalton RN, Prevost AT, et al. Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care 2009;32:658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group. Development and progression of renal insufficiency in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolonen N, Forsblom C, Thorn L, et al. FinnDiane Study Group Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia 2009;52:2522–2530 [DOI] [PubMed] [Google Scholar]
- 22.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 1998;41:784–790 [DOI] [PubMed] [Google Scholar]
- 23.Molitch ME, Rupp D, Carnethon M. Higher levels of HDL cholesterol are associated with a decreased likelihood of albuminuria in patients with long-standing type 1 diabetes. Diabetes Care 2006;29:78–82 [DOI] [PubMed] [Google Scholar]
- 24.Bu X, Warden CH, Xia YR, et al. Linkage analysis of the genetic determinants of high density lipoprotein concentrations and composition: evidence for involvement of the apolipoprotein A-II and cholesteryl ester transfer protein loci. Hum Genet 1994;93:639–648 [DOI] [PubMed] [Google Scholar]
- 25.Belalcazar LM, Reboussin DM, Haffner SM, et al. Look AHEAD (Action for Health in Diabetes) Obesity, Inflammation, and Thrombosis Research Group Marine ω-3 fatty acid intake: associations with cardiometabolic risk and response to weight loss intervention in the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010;33:197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]