Abstract
OBJECTIVE
Ectopic lipid storage in muscle (intramyocellular lipids [IMCL]) and liver (hepatocellular lipids [HCL]) coexists with impaired myocellular flux through ATP synthase (fATPase) in certain cohorts with increased risk of type 2 diabetes. Because women with a history of gestational diabetes mellitus (pGDM) have elevated ectopic lipids and diabetes risk, we tested whether deteriorated energy metabolism contributes to these abnormalities.
RESEARCH DESIGN AND METHODS
A total of 23 glucose-tolerant nonobese pGDM and eight women with normal glucose metabolism during pregnancy with similar age, body mass, and physical activity underwent oral glucose tolerance tests (OGTT) and intravenous glucose tolerance tests at 4–5 years after delivery. OGTT values <463 mL ⋅ min−1 ⋅ m−2 were considered to indicate insulin resistance. pGDM were further stratified into insulin-resistant (pGDM-IR) and insulin-sensitive (pGDM-IS) groups. IMCL, HCL, and fATPase were measured with 1H/31P magnetic resonance spectroscopy.
RESULTS
pGDM had 36% higher fat mass and 12% lower insulin sensitivity. Log-transformed fATPase was lower in pGDM (10.6 ± 3.8 µmol ⋅ mL muscle−1 ⋅ min−1 vs. 12.1 ± 1.4 µmol ⋅ mL muscle−1 ⋅ min−1, P < 0.03) and related to plasma adiponectin after adjustment for body fat (r = 0.44, P < 0.04). IMCL were 61% and 69% higher in pGDM-IR (P < 0.05 vs. pGDM-IS) and insulin resistant women (P < 0.003 vs. insulin sensitive), respectively. HCL were doubled (P < 0.05) in pGDM and insulin resistant women, and correlated positively with body fat mass (r = 0.50, P < 0.01) and inversely with insulin sensitivity (r = −0.46, P < 0.05).
CONCLUSIONS
Glucose-tolerant pGDM show increased liver fat but only slightly lower muscular insulin sensitivity and ATP synthesis. This suggests that alteration of hepatic lipid storage represents an early and predominant abnormality in this cohort.
Insulin resistance tightly relates to abdominal obesity and ectopic fat deposition in skeletal muscle (intramyocellular lipids [IMCL]) and liver (hepatocellular lipids [HCL]) (1). Obesity-associated exposure of both tissues to elevated free fatty acids (FFAs) (1) or impaired secretion of the adipocytokine adiponectin, which regulates lipid metabolism and insulin action (2), contributes to insulin resistance. Impaired myocellular mitochondrial fitness (1) has also been linked to insulin resistance.
In skeletal muscle, elevation of IMCL is associated with diminished maximal oxidative capacity (3). Insulin-resistant humans with type 2 diabetes and their first-degree relatives can present with impaired flux through myocellular ATP synthase (fATPase), the final step of mitochondrial oxidative phosphorylation (4–6).
Women with a history of gestational diabetes mellitus (pGDM) are also frequently insulin resistant and more obese than women after normoglycemic pregnancy. Their markedly higher diabetes risk renders them as a suitable model of early metabolic alterations preceding type 2 diabetes (7). The strongest predictors for an early onset of diabetes include insulin requirement and early diagnosis of GDM during pregnancy and maternal BMI (7).
Increased IMCL identifies women who are more insulin resistant, diagnosed earlier, and prone to require insulin rather than diet only during pregnancy (8). Likewise, elevated HCL correlate tightly with insulin resistance in pGDM (9) who also exhibit lower plasma adiponectin, another predictor of glycemic deterioration (10). However, the role of myocellular energy metabolism for insulin resistance and accumulation of IMCL and HCL in this cohort is unknown.
We tested the primary hypothesis that pGDM exhibit lower myocellular fATPase than women with normal glucose tolerance during pregnancy. We further hypothesized that fATPase correlates inversely with IMCL and insulin resistance and that insulin resistant pGDM have higher HCL and/or lower plasma adiponectin.
RESEARCH DESIGN AND METHODS
Volunteers
Twenty-three pGDM and eight women without any risk factors for type 2 diabetes serving as controls (CON) were recruited from the outpatient service of the Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna. GDM was diagnosed according to the criteria of the Fourth Workshop Conference of Gestational Diabetes. The women had no disease and were not taking medications. During their pregnancies, the women were continuously seen in the outpatient service so that all data on diagnosis and treatment were recorded and validated. Fifteen pGDM had been on insulin therapy (IT), and eight pGDM had been on diet treatment (DT) only. They were instructed to ingest an isocaloric diet (carbohydrate/protein/fat: 60/20/20%) and refrain from any physical exercise during the 3 days preceding the examinations. Metabolic tests were performed on different days during the first phase (days 5–8) of the menstrual cycle after 10–12-h overnight fasting. All participants gave written informed consent to the protocol, which had been approved by the institutional ethics board of the Medical University of Vienna.
Oral glucose tolerance test
A solution containing 75 g of glucose was ingested within 2 min, and venous blood samples were collected for measurements of glucose and hormones. During fasting, insulin sensitivity was assessed with the quantitative insulin-sensitivity check index (QUICKI) = 1/[log(fasting-glucose) + log(fasting-insulin)], which mostly indicates hepatic insulin sensitivity (11). Under dynamic conditions, the oral glucose insulin sensitivity (OGIS) index (12) was used, which quantifies glucose clearance per unit change of insulin and has been validated against the glucose clamp (12,13) and in terms of reproducibility and variability (14). The OGIS correlates more closely with total glucose disposal than with hepatic insulin sensitivity in a study directly comparing the glucose clamp with the oral glucose tolerance test (OGTT) (11). Thus, we used OGIS as a measure of whole-body insulin sensitivity during glucose ingestion. Insulin delivered into peripheral circulation was assessed with the total area under the insulin concentration curve (AUCINS), calculated with the trapezoidal rule. An integrated index of β-cell function (adaptation index), as its ability to compensate insulin resistance by increasing insulin release, was calculated as OGIS × ΔAUCCP, where ΔAUCCP is the suprabasal area under the C-peptide concentration curve. In addition, the frequent sampling intravenous glucose tolerance test was performed and analyzed for assessing SI as a measure of insulin sensitivity and disposition index, DI = SI × ΔAIRGLUC as a measure of combined effects of insulin secretion and sensitivity on glucose disposal as described previously (15).
In vivo magnetic resonance spectroscopy
Measurements were performed in participants lying supine inside a 3-T whole-body spectrometer (Bruker Biospin, Ettlingen, Germany). 1) 31P MRS (magnetic resonance spectroscopy): A 10-cm circular double-resonant surface coil was positioned over the medial head of the right gastrocnemius muscle. Intramyocellular concentrations of inorganic phosphate and ATP, as well as rate constant (kATP) and unidirectional fATPase, using the saturation transfer experiment, were measured as described (4,16). 2) 1H MRS: A 10-cm, circular, double-resonant surface coil was applied above the right soleus muscle to measure IMCL from localized 1H spectra using the series stimulated echo acquisition mode sequence within a volume of interest of 1.73 cm3 (5). HCL were quantified using the stimulated echo acquisition mode within a volume of interest of 27 cm3 (5).
Body fat mass and resting energy expenditure
Body fat mass (BFM) was assessed from bioimpedance analysis (Akern-RJL Systems, Florence, Italy). Prediction errors of body composition equations estimating percent fat free mass are based on empirically derived measurement errors associated with the reference method, hydrodensitometry. Resting energy expenditure (REE) was assessed from indirect calorimetry as described previously (8).
Laboratory tests
Plasma glucose was measured by the hexokinase method (Hitachi Ltd., Tokyo, Japan), HbA1c (A1C) was measured by high-performance liquid chromatography (VARIANT Hemoglobin Testing System, Hercules, CA), and ultrasensitive C-reactive protein was measured by particle-enhanced immunonephelometry (N High-Sensitivity-CRP Reagent, BN Systems; Dade Behring, Deerfield, IL). HDL- and LDL-cholesterol were quantified using standard laboratory procedures. Fasting plasma adiponectin was measured in duplicate using an enzyme-linked immunosorbent assay system (Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) with human recombinant adiponectin as a standard.
Calculations and statistics
Data were compared between pGDM and CON. Relationships among fATPase, IMCL, HCL, insulin sensitivity, and metabolic and inflammatory parameters were further analyzed in pGDM and CON, insulin-sensitive (IS) subjects (n = 15), insulin-resistant (IR) subjects (n = 16), insulin-sensitive (pGDM-IS, n = 9), and insulin-resistant pGDM (pGDM-IR, n = 14). The cutoff value (462.8 mL · min−1 · m−2) for insulin resistance was derived from OGIS values in control women of this study and other studies. The lowest quantile of the distribution gave the value defined as cutoff point between normal and impaired (lower) OGIS. The identical approach has been applied in previous studies (8,10). Further, all women and pGDM were divided into three groups according to OGIS tertiles.
Statistical analyses were performed using SAS Software 9.1.3 (SAS Institute, Inc., Cary, NC). Data are presented as means ± SD in text and tables or box-and-whisker plots in figures. Data exhibiting skewed distribution were log-transformed before statistical analysis. Comparisons between groups were performed using ANOVA for metric parameters. The Tukey test was applied for post hoc testing, and the Wilcoxon rank-sum test was applied for nonparametric parameters. Pearson correlation coefficients were computed for normally distributed data. Spearman correlation coefficients were computed in case of non-normal distribution. Bivariate correlation analyses were performed by applying partial correlations adjusting for BFM. P < 0.05 was considered statistically significant.
RESULTS
Study population
All women had normal glucose tolerance based on the 75-g OGTT and comparable habitual physical activities according to Baecke’s questionnaire (Table 1). pGDM had greater BFM and waist circumference than CON (Table 1). This also held true for pGDM-IR, but not for pGDM-IS. Adjustment for BFM abolished the difference of HDL-cholesterol between pGDM and CON.
Table 1.
Clinical characteristics and metabolic parameters (means ± SD) of women with pGDM and their insulin-resistant (pGDM-IR) and insulin-sensitive (pGDM-IS) subgroups compared with control subjects (CON)
| pGDM | pGDM-IR | pGDM-IS | CON | P | ||||
|---|---|---|---|---|---|---|---|---|
|
N |
23 |
14 |
9 |
8 |
||||
| Age (years) |
37 ± 5 |
37 ± 5.9 |
39 ± 3 |
35 ± 4 |
NS* |
NS† |
NS‡ |
0.06§ |
| Time after delivery (m) |
57 ± 11 |
56 ± 14 |
59 ± 6 |
45 ± 15 |
0.02* |
NS† |
NS‡ |
NS§ |
| BMI (kg/m2) |
25.5 ± 3.6 |
26.5 ± 3.0 |
24.2 ± 4.1 |
25.0 ± 2.9 |
NS* |
NS† |
NS‡ |
NS§ |
| BFM (kg) |
24.5 ± 6 |
26.1 ± 5.1 |
22.2 ± 6.8 |
18.0 ± 3.3 |
<0.03* |
NS† |
<0.02‡ |
NS§ |
| Waist circumference (cm) |
85.5 ± 9.4 |
87.9 ± 7.3 |
82.2 ± 11.2 |
76.0 ± 8.2 |
0.03* |
NS† |
0.02‡ |
NS§ |
| Triglycerides (mg/dL) |
85.2 ± 38.6 |
94.1 ± 42.4 |
71.3 ± 28.7 |
97.0 ± 37.5 |
NS* |
NS† |
NS‡ |
NS§ |
| HDL-cholesterol (mg/dL) |
55.6 ± 12.5 |
52.8 ± 13.3 |
59.9 ± 10.4 |
64.5 ± 10.1 |
<0.05* |
NS† |
0.02‡ |
NS§ |
| A1C (%) |
5.4 ± 0.4 |
5.3 ± 0.5 |
5.4 ± 0.3 |
5.2 ± 0.2 |
NS* |
NS† |
NS‡ |
NS§ |
| GGT (units/L) |
18 ± 7 |
20 ± 7 |
15 ± 6 |
23 ± 24 |
NS* |
0.03† |
NS‡ |
NS§ |
| Adiponectin (µg/mL) |
7.9 ± 2.5 |
7.3 ± 2.2 |
8.9 ± 2.7 |
9.1 ± 2.3 |
NS* |
NS† |
NS‡ |
NS§ |
| us-CRP (mg/dL) |
0.19 ± 1.67 |
0.20 ± 0.17 |
0.17 ± 0.18 |
0.25 ± 0.24 |
NS* |
NS† |
NS‡ |
NS§ |
| Physical activity score |
2.68 ± 0.51 |
2.58 ± 0.5 |
2.85 ± 0.53 |
2.75 ± 0.12 |
NS* |
NS† |
NS‡ |
NS§ |
| Resting energy expenditure (kcal/24 h) | 1541 ± 255 | 1587 ± 250 | 1449 ± 270 | 1440 ± 215 | NS* | NS† | NS‡ | NS§ |
IR was defined by means of OGIS <462.8 mL ⋅ min−1 ⋅ m−2.
GGT, gamma glutamyl transferase; us-CRP, ultra-sensitive C-reactive protein.
*pGDM vs. CON.
†pGDM-IS vs. pGDM-IR.
‡pGDM-IR vs. CON.
§pGDM-IS vs. CON.
Insulin sensitivity and secretion
pGDM had marginally higher postload glycemia (Table 2) and lower OGIS than CON (Fig. 1B). Within pGDM, OGIS was 21% lower (P < 0.007) in the insulin resistant than in the insulin sensitive subgroup. After adjustment for BFM, the difference in OGIS between pGDM-IR and pGDM-IS remained (adjusted means: 408 mL ⋅ min−1 ⋅ m−2 vs. 513 mL ⋅ min−1 ⋅ m−2; P < 0.0001) and tended to be lower even in pGDM-IS compared with CON (adjusted means: 412 mL ⋅ min−1 ⋅ m−2 vs. 514 mL ⋅ min−1 ⋅ m−2; P = 0.06). The intergroup difference for postload glycemia disappeared. Insulin delivery (AUCINS) was higher in pGDM-IR compared with pGDM-IS (Table 2). The adaptation index was not different between pGDM and CON, reflecting normal glucose tolerance due to adequate β-cell compensation for a minor degree of insulin resistance in pGDM (data not shown). Fasting C-peptide reflecting basal insulin secretion rates and AUCGLUC were higher in pGDM-IR than in pGDM-IS (P < 0.05 and P = 0.0007; Table 2), although these differences disappeared after adjustment for BFM.
Table 2.
Glucose metabolism, insulin sensitivity, and secretion (all expressed as means ± SD) in women with pGDM and their insulin-resistant (pGDM-IR) and insulin-sensitive (pGDM-IS) subgroups compared with control subjects (CON)
| pGDM | pGDM-IR | pGDM-IS | CON | P | ||||
|---|---|---|---|---|---|---|---|---|
|
N |
23 |
14 |
9 |
8 |
||||
| Fasting glucose (mg/dL) |
90 ± 9 |
92.3 ± 11 |
86.3 ± 3 |
84 ± 9 |
NS* |
NS† |
NS‡ |
NS§ |
| Fasting insulin (µU/mL) |
9.8 ± 5.7 |
11.6 ± 6.5 |
7.2 ± 2.5 |
7.6 ± 1.6 |
NS* |
NS† |
||
| Fasting C-peptide (ng/mL) |
1.89 ± 0.98 |
2.26 ± 1.12 |
1.37 ± 0.42 |
2.00 ± 0.43 |
NS* |
<0.05† |
||
| QUICKI |
0.348 ± 0.027 |
0.337 ± 0.024 |
0.364 ± 0.024 |
0.357 ± 0.018 |
NS* |
<0.05† |
||
| 1-h glucose (mg/dL)OGTT |
144 ± 42 |
155 ± 44 |
126 ± 32 |
106 ± 47 |
NS* |
NS† |
0.04‡ |
NS§ |
| 2-h glucose (mg/dL)OGTT |
109 ± 32 |
117 ± 39 |
98 ± 12 |
85 ± 22 |
0.05* |
NS† |
NS‡ |
NS§ |
| Fasting FFA (µmol/L)OGTT |
551 ± 206 |
522 ± 150 |
597 ± 277 |
659 ± 256 |
NS* |
NS† |
||
| 2-h FFA (mmol/L)OGTT |
33 ± 18 |
39 ± 19 |
23 ± 12 |
42 ± 25 |
NS* |
<0.04† |
NS‡ |
NS§ |
| AUCGLUC (mol/L min) |
1.21 ± 0.13 |
1.30 ± 0.08 |
1.10 ± 0.07 |
1.07 ± 0.16 |
NS* |
<0.05† |
||
| AUCINS (nmol/L min) | 61.75 ± 41.0 | 74.76 ± 47.8 | 41.52 ± 12.4 | 50.39 ± 41.6 | NS* | <0.05† | ||
IR was defined by means of OGIS <462.8 mL ⋅ min−1 ⋅ m−2.
Fasting FFA, basal free fatty acid; 2-h FFA, free fatty acid at 120 min; QUICKI, quantitative insulin-sensitivity check index; AUCGLUC, area under the glucose concentration curve; AUCINS, area under the insulin concentration curve.
*pGDM vs. CON.
†pGDM-IR vs. pGDM-IS.
‡pGDM-IR vs. CON.
§pGDM-IS vs. CON.
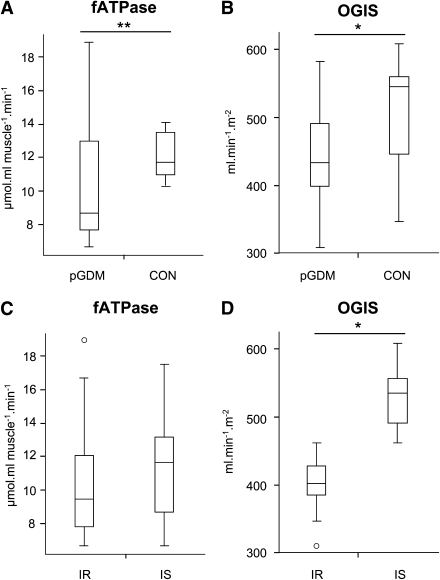
Figure 1.
Myocellular fATPase (A and C) and insulin sensitivity (B and D) as assessed from the OGTT (OGIS) in pGDM compared with CON (A and B) and in IR and IS subgroups of the total cohort according to their insulin sensitivity (OGIS) (C and D). Data are presented as box-and-whisker plots. Boxes delineate lower and upper quartile, whiskers represent minima and maxima, medians are indicated by solid line within boxes, and small circles/asterisks represent experimental outliers. fATPase: pGDM vs. CON (10.6 ± 3.8 µmol ⋅ mL muscle−1 ⋅ min−1 vs. 12.1 ± 1.4 µmol ⋅ mL muscle−1 ⋅ min−1; P < 0.12, **after log transformation: P < 0.03), OGIS (glucose clearance): pGDM vs. CON (447 ± 67 mL ⋅ min−1 ⋅ m−2 vs. 508 ± 87 mL ⋅ min−1 ⋅ m−2; P < 0.05), fATPase: IR vs. IS (10.7 ± 3.7 µmol ⋅ mL muscle−1 ⋅ min−1 vs. 11.4 ± 3.0 µmol ⋅ mL muscle−1 ⋅ min−1; P = 0.6 NS), OGIS (glucose clearance): IR vs. IS (402 ± 38 mL ⋅ min−1 ⋅ m−2 vs. 528 ± 46 mL ⋅ min−1 ⋅ m−2; P < 0.0001).
Myocellular energy metabolism
pGDM had 12% lower fATPase than CON (Fig. 1A) only on log-transformation of the data (P < 0.03), which had to be performed because of outliers. Adjusting for BFM diminished the difference (P = 0.37). Interestingly, fATPase did not differ between the IR and IS subgroups of pGDM and the total population (P = 0.30; Fig. 1C). Further, fATPase did not differ between lowest and highest OGIS tertiles, both in all women (P > 0.2) and in pGDM alone (P > 0.4). kATP was not different between pGDM and CON (0.06 ± 0.02 1/s vs. 0.07 ± 0.01 1/s; P = 0.1) or between subgroups (pGDM-IR vs. pGDM-IS: 0.06 ± 0.02 1/s vs. 0.06 ± 0.02 1/s; P = 0.6). fATPase was also comparable between pGDM with and without insulin therapy (IT) during their pregnancies.
Ectopic lipids
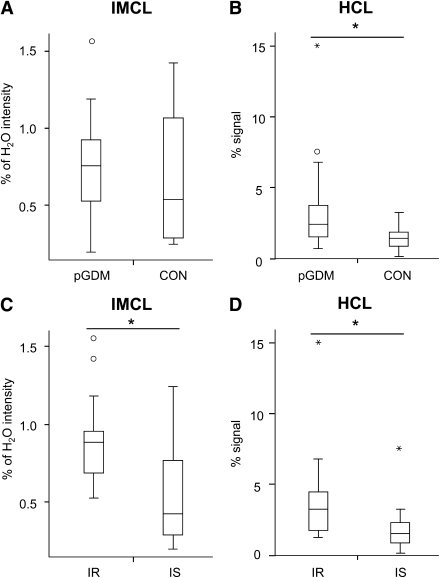
IMCL were not different between pGDM and CON (Fig. 2A), but 61% higher in pGDM-IR than in pGDM-IS (P < 0.05). Among all women, IR women had 69% higher IMCL than IS women (P < 0.007; Fig. 2C). pGDM on IT during their pregnancies had higher IMCL than those on diet treatment (DT) (IT vs. DT: 0.86 ± 0.29% vs. 0.51 ± 0.25%, P = 0.01).
Figure 2.
IMCL and HCL. Ectopic lipids in skeletal muscle (IMCL; A and C) and liver (HCL; B and D) of pGDM compared with CON (A and B) and in IR and IS subgroups of the total cohort according to their insulin sensitivity (OGIS) (C and D). Data presented as box-and-whisker plots. Boxes delineate lower and upper quartile, whiskers represent minima and maxima, respectively, medians are indicated by solid line within boxes, and small circles/asterisks represent experimental outliers. IMCL: pGDM vs. CON (0.73 ± 0.32% H2O vs. 0.69 ± 0.5% H2O; P = 0.08 NS) HCL: pGDM vs. CON (3.7 ± 3.5% vs. 1.5 ± 0.9% signal; P < 0.05) IMCL: IR vs. IS (0.90 ± 0.3% H2O vs. 0.54 ± 0.32% H2O; P < 0.003) HCL: IR vs. IS (4.0 ± 3.3% vs. 2.0 ± 1.8% signal; P < 0.05).
pGDM had 2.5-fold higher HCL (P < 0.05) than CON (Fig. 2B). Likewise, HCL were twice as high in IR than in IS subjects (P < 0.05; Fig. 2D), but not different among pGDM subgroups. Adjusting for BFM diminished these differences. HCL were not different between pGDM with or without IT during pregnancy (DT vs. IT: HCL 4.8 ± 5.0% signal vs. 3.1 ± 2.4% signal).
Correlation analyses for fATPase and HCL
Partial correlation analysis, controlled for BFM, revealed a relationship between fATPase and plasma adiponectin across all women (P < 0.04, r = 0.44). fATPase did not relate to measures of insulin sensitivity (OGIS: r2 = 0.002, SI: r2 = 0.07) or glucose tolerance (AUCGLUC: r2 = 0.005) in any subgroup. However, the disposition index related to HCL (r = 0.59, P = 0.002) and fATPase (r = 0.37, P = 0.04) in all women. Fasting plasma FFA did not relate to fATPase, whereas 2-h postload plasma FFA related to HCL (r = 0.59, P = 0.01) and insulin sensitivity as assessed from OGIS (r = −0.44, P = 0.04) or SI (r = −0.42, P = 0.04) in pGDM.
HCL related to insulin sensitivity (r = −0.47, P < 0.02) across all women and to IMCL (r = −0.59, P = 0.05) and A1C across IR (r = 0.64, P < 0.03). After adjustment for BFM, HCL still related to A1C (r = 0.57, P < 0.02) in all women. Within pGDM, the correlation of HCL with hip circumference (r = 0.59, P = 0.04) and A1C (r = 0.62, P = 0.03) remained after adjustment for BFM.
CONCLUSIONS
We found that myocellular fATPase is lower in pGDM than in CON and does not relate to insulin sensitivity or IMCL, but correlates positively with plasma adiponectin across all women, suggesting a yet unknown relationship between energy metabolism and adipocyte function. HCL were greater in pGDM, and HCL and postload FFA were negatively related to insulin sensitivity.
Our pGDM featured slightly greater fat mass, postload glycemia, and insulin resistance than CON. Except for HDL-cholesterol, they did not differ from CON in other variables, including physical activity and resting energy expenditure. This held true even for pGDM-IR, suggesting that this cohort is at lower risk for type 2 diabetes than other mostly obese and glucose-intolerant pGDM, but already exhibits distinct metabolic features.
We found moderately lower myocellular fATPase in pGDM compared with CON, which was significant only on log-transformation. Our cohort was carefully matched to CON, except for insulin sensitivity, and probably bears a low risk for rapid progression to diabetes. The observed small differences could also be due to this specific cohort maintaining normal glucose tolerance for up to 4 years postpartum. The difference in fATPase between pGDM and CON, however, vanished on correction for BFM and was not found when comparing pGDM-IR with pGDM-IS. Although one might speculate that the reduction of fATPase resulted from greater body fat content as reported for obese insulin-resistant and overweight type 2 diabetes cohorts (17), our data do not allow such a conclusion. Basal fATPase, the final step of mitochondrial oxidative phosphorylation, is impaired only in some cohorts presenting with severe insulin resistance and elevated IMCL, such as elderly and lean relatives of patients with type 2 diabetes (4). The overall higher insulin sensitivity compared with other populations at risk for or having overt type 2 diabetes (5,6,18) suggests that muscle metabolism was almost normal in our pGDM.
The finding that IMCL were not different between pGDM and CON, but ∼69% higher in IR than IS, underlines the contention that IMCL generally correlate with insulin resistance (8,19) and can be elevated in patients with type 2 diabetes and their insulin-resistant first-degree relatives (4). Further, the pGDM of our previous study exhibited higher IMCL along with greater insulin resistance and BFM (8). Of note, baseline fATPase is not necessarily different in patients with overt type 2 diabetes (5,6), indicating that mitochondrial dysfunction is not a uniform feature of type 2 diabetes (5). The increase in IMCL relates to not only diminished mitochondrial capacity for oxidative phosphorylation but also lipid availability. Plasma FFA elevation decreases insulin-stimulated fATPase and insulin sensitivity before changes in IMCL (16). Of note, postchallenge plasma FFAs were not different between pGDM and CON and only slightly higher in IR-pGDM than in IS-pGDM. The increase in insulin secretion further argues against mitochondrial dysfunction at the level of the β-cell, which has been observed in GDM and some pGDM (20).
Despite the only discrete alterations in muscular metabolism, liver fat content was more than double in all pGDM and even three times more in IR-pGDM compared with that in CON, which was still below the detection limits of clinical routine ultrasound. Higher HCL have been reported in obese pGDM (9) and type 2 diabetic patients, in whom HCL tightly correlate with whole-body and hepatic insulin resistance (5). Steatosis even predicts the development of insulin resistance, type 2 diabetes, and cardiovascular end points (1,21). Of note, similar to skeletal muscle, impaired mitochondrial function has been recently detected in livers of patients with type 2 diabetes (1,22). It has been proposed that primary muscular insulin resistance promotes shifting of ingested carbohydrates away from skeletal muscle to hepatic de novo lipogenesis, thereby increasing HCL independently of obesity (18). Alternatively, steatosis and/or impaired hepatic mitochondrial function could lead to muscular insulin resistance (1,22). We cannot address this issue directly, because liver biopsies are not permitted in healthy humans and noninvasive assessment with in vivo 31P MRS was not available.
In pGDM, the 2-h post-glucose load plasma FFA further correlated positively with HCL and negatively with whole-body insulin sensitivity. Even after correction for BFM, pGDM and their IR subgroup also exhibited positive relationships of HCL with hip circumference and A1C. Of note, despite the greater BFM, none of the anthropometric parameters reached the cutoff values of the metabolic syndrome. From this it seems that impaired insulin action in these pGDM is primarily located at the level of the liver and already present in the glucose-tolerant state.
Steatosis may result not only from abnormal fat metabolism but also from disturbed cytokine release. Of note, pGDM exhibit higher HCL and lower plasma adiponectin, which is a sensitive predictor for future deterioration of glucose metabolism (10). Adiponectin has been linked to improved muscular and hepatic insulin sensitivity, which might result from anti-inflammatory and antiatherogenic activity, decreased triglyceride synthesis, and stimulated β-oxidation (2). Adiponectin may also increase muscular mitochondrial number and function, and exert antidiabetic effects (23). In support of this contention, the current study showed that plasma adiponectin relates positively to fATPase in all women after adjustment for BFM. Moreover, prevention of the development of type 2 diabetes in pGDM by thiazolidinediones (24,25) could be mediated by increased plasma adiponectin with subsequent reduction of HCL and hepatic and peripheral insulin resistance. In addition, primary abnormal hepatic mitochondrial function could lead to steatosis, because patients with type 2 diabetes have reduced hepatocellular ATP levels that correlate with hepatic insulin sensitivity and HCL (22).
The limitations of the study are the small sample size and the specific selection of pGDM, which restrict the extrapolation of the results to all pGDM. On the other hand, the selected group underwent intensive phenotyping, including tissue-specific metabolic assessment.
In summary, glucose-tolerant nonobese pGDM do not show major alterations of muscle glucose and energy metabolism, but already exhibit a subclinical increase of liver fat content suggesting early abnormalities or adaptations of hepatic metabolism.
Acknowledgments
This study was supported by the Austrian Science Foundation (P15656), Austrian National Bank (OENB 11459), European Foundation for the Study of Diabetes (EFSD-GSK and Lilly grants), Schmutzler-Stiftung, Skröder-Stiftung, German Research Foundation (DFG, SFB 512) to M.R., and German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
No other potential conflicts of interest relevant to this article were reported.
T.P. researched data, wrote the article, and contributed to discussion. C.W. researched data, wrote the article, and contributed to discussion. A.I.S. researched data. J.S. contributed to discussion and reviewed the article. M.C. researched data and contributed to discussion. G.P. performed insulin sensitivity calculations and contributed to discussion. M.K. researched data and contributed to discussion. E.M. contributed to discussion. T.F. researched data. W.W. contributed to discussion. A.K.-W. contributed to discussion. M.R. planned the study, wrote the article, contributed to discussion, and reviewed and edited the article.
References
- 1.Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol 2009;20:50–56 [DOI] [PubMed] [Google Scholar]
- 2.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 2006;27:762–778 [DOI] [PubMed] [Google Scholar]
- 3.Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 2003;88:1785–1791 [DOI] [PubMed] [Google Scholar]
- 4.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 2007;50:113–120 [DOI] [PubMed] [Google Scholar]
- 7.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 8.Kautzky-Willer A, Krssak M, Winzer C, et al. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 2003;52:244–251 [DOI] [PubMed] [Google Scholar]
- 9.Tiikkainen M, Tamminen M, Häkkinen AM, et al. Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Obes Res 2002;10:859–867 [DOI] [PubMed] [Google Scholar]
- 10.Winzer C, Wagner O, Festa A, et al. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care 2004;27:1721–1727 [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 12.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 [DOI] [PubMed] [Google Scholar]
- 13.Mari A, Pacini G, Brazzale AR, Ahrén B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia 2005;48:748–751 [DOI] [PubMed] [Google Scholar]
- 14.Utzschneider KM, Prigeon RL, Tong J, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 2007;50:2516–2525 [DOI] [PubMed] [Google Scholar]
- 15.Pacini G, Tonolo G, Sambataro M, et al. Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am J Physiol 1998;274:E592–E599 [DOI] [PubMed] [Google Scholar]
- 16.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhäusl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006;55:136–140 [PubMed] [Google Scholar]
- 17.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54:8–14 [DOI] [PubMed] [Google Scholar]
- 18.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 2007;104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 1999;42:113–116 [DOI] [PubMed] [Google Scholar]
- 20.Buchanan TA, Xiang AH, Peters RK, et al. Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 2000;49:782–788 [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein E, Lavine JE, Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis 2008;28:380–385 [DOI] [PubMed] [Google Scholar]
- 22.Szendroedi J, Chmelik M, Schmid AI, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 2009;50:1079–1086 [DOI] [PubMed] [Google Scholar]
- 23.Civitarese AE, Ukropcova B, Carling S, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 2006;4:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 25.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]