Abstract
OBJECTIVE
Steatosis associates with insulin resistance and may even predict type 2 diabetes and cardiovascular complications. Because muscular insulin resistance relates to myocellular fat deposition and disturbed energy metabolism, we hypothesized that reduced hepatic ATP turnover (fATP) underlies insulin resistance and elevated hepatocellular lipid (HCL) contents.
RESEARCH DESIGN AND METHODS
We measured hepatic fATP using 31P magnetic resonance spectroscopy in patients with type 2 diabetes and age- and body mass–matched controls. Peripheral (M and M/I) and hepatic (suppression of endogenous glucose production) insulin sensitivity were assessed with euglycemic-hyperinsulinemic clamps.
RESULTS
Diabetic individuals had 29% and 28% lower peripheral and hepatic insulin sensitivity as well as 42% reduced fATP than controls. After adjusting for HCL, fATP correlated positively with peripheral and hepatic insulin sensitivity but negatively with waist circumference, BMI, and fasting plasma glucose. Multiple regression analysis identified waist circumference as an independent predictor of fATP and inorganic phosphate (PI) concentrations, explaining 65% (P = 0.001) and 56% (P = 0.003) of the variations. Hepatocellular PI primarily determined the alterations in fATP.
CONCLUSIONS
In patients with type 2 diabetes, insulin resistance relates to perturbed hepatic energy metabolism, which is at least partly accounted for by fat depots.
Alterations in liver function are frequently encountered in patients with type 2 diabetes (1,2). Nonalcoholic fatty liver (steatosis) and hepatic insulin resistance are typical for type 2 diabetes and may even precede or contribute to its development (2,3). It also relates to the development of cardiovascular diseases (4), besides the progression into steatohepatitis and cirrhosis (5). Steatosis likely results from excessive lipid overflow originating from augmented lipid stores in peripheral or visceral adipocytes, lipogenesis, or high dietary intake (2).
Magnetic resonance spectroscopy (MRS) allows noninvasive direct measurements of metabolite concentrations and tracing of metabolic fluxes in situ. Quantification of intramyocellular lipids and hepatocellular lipids (HCL) by 1H MRS revealed that both are linked to whole-body (peripheral) insulin resistance, abnormal endogenous glucose production (EGP), and liver glycogen storage in line with hepatic insulin resistance (2). According to one current paradigm, excessive ectopic lipid deposition precedes the development of type 2 diabetes (2). Yet, the underlying metabolic processes leading to lipid accumulation and insulin resistance in skeletal muscle and liver are not fully understood.
In skeletal muscle, insulin resistance relates not only to elevated lipids but also to impaired rates of phosphorylation (flux through ATP synthesis, fATP), as measured by 31P MRS in elderly (6) type 2 diabetic patients (7), their insulin-resistant first-degree relatives (8), and nondiabetic individuals with elevated plasma free fatty acids (FFAs) (9).
Although it has been suggested that nonalcoholic steatohepatitis is associated not only with insulin resistance but also with mitochondrial abnormalities (10), no data on the quantification of fATP in patients with type 2 diabetes have been reported so far in the human liver. Our previous studies showed that type 2 diabetic individuals have lower hepatic concentrations of PI and ATP (11). After developing a saturation transfer experiment for assessment of hepatocellular fATP (12), we tested the hypothesis that type 2 diabetic patients have lower hepatocellular fATP than nondiabetic controls, which should relate independently of HCL to hepatic insulin resistance.
RESEARCH DESIGN AND METHODS
The study protocol conformed with the Declaration of Helsinki (2008) and was approved by the appropriate institutional ethical board. Informed consent was obtained from each study participant after explanation of the purpose, nature, and potential complications of the study.
Volunteers
After recruitment by means of public advertisement, all participants underwent complete medical history, clinical examination, and laboratory tests to exclude cardiovascular, renal, and thyroid diseases, and viral hepatitis. All volunteers were white and had already participated in our previous study on absolute quantification of liver phosphorus metabolites (11). The study excluded patients taking insulin or thiazolidinediones, or presenting with islet cell antibodies and diabetes-related complications. Controls had similar body mass and age, but normal glucose tolerance during a 75-g oral glucose tolerance test and had neither a family history of diabetes nor regular drug treatment. None of the participants was physically trained or performed regular intense exercise. They had no evidence for liver diseases and consumed <20 g alcohol daily.
Of 24 individuals recruited, 3 did not complete the experiment because they refused to continue the MRS session. Data sets of three participants could not be used due to low quality resulting from a poor local homogeneity of the static magnetic field. Data from one individual could not be used because of a technical problem with the scanner. Thus, complete MRS datasets for 17 individuals were available for analyses (Table 1).
Table 1.
Anthropometric and laboratory parameters
| Variable | Type 2 diabetes | Control |
|---|---|---|
| N (female) | 9 (3) | 8 (4) |
| Age (years) | 57.7 ± 6.1 | 60.1 ± 4.4 |
| Height (cm) | 174 ± 11 | 174 ± 10 |
| Body weight (kg) | 82 ± 14 | 78 ± 17 |
| BMI (kg/m2) | 26.9 ± 3.4 | 25.1 ± 3.9 |
| A1C (%) | 7.06 ± 0.98* | 5.57 ± 0.27 |
| Waist circumference (cm) | 100 ± 10 | 88 ± 19 |
| Fasting plasma glucose (mmol/L) | 8.8 ± 1.9* | 5.3 ± 0.6 |
| Fasting plasma insulin (mU/L) | 12.1 ± 3.9 | 8.5 ± 2.0 |
| Serum LDL cholesterol (mg/dL) | 119 ± 22 | 146 ± 33 |
| Serum HDL cholesterol (mg/dL) | 62 ± 9 | 59 ± 10 |
| Plasma triglyceride (mg/dL) | 163 ± 80 | 128 ± 79 |
| GGT (units/L) | 34 ± 17 | 27 ± 9 |
| AST (units/L) | 22 ± 5 | 24 ± 5 |
Data are expressed as mean ± SD, unless indicated otherwise.
*P < 0.001, significant differences between groups.
Type 2 diabetic patients showed acceptable glycemic (A1C: 7.06 ± 0.98% vs. 5.53 ± 0.27% in controls; P = 0.001) and lipid control on treatment with diet only (n = 1), sulfonylurea only (n = 3), or combined with metformin (n = 5) and statins (n = 7). Medications were withdrawn at least 3 days before the measurements. Measurements of fATP and insulin sensitivity were performed on different days under identical conditions of overnight fasting and withdrawal of medications.
Anthropometric measurements
Waist circumference was measured with a cloth tape with participants upright. Waist was defined as the midpoint between the highest point of the iliac crest and the lowest part of the costal margin in the midaxillary line (13).
Analytic procedures
Plasma glucose was measured by the glucose oxidase method (Glucose Analyzer II, Beckman Instruments Inc., Fullerton, CA) and plasma insulin by double antibody radio immunoassay (11). All other parameters were measured in the clinical chemistry laboratory with standardized quality-controlled assays.
Peripheral and hepatic insulin sensitivity
Whole-body glucose disposal (M, or M related to plasma insulin [M/I]) and EGP were measured in seven diabetic subjects (three women/four men) and in six controls (four women/two men) as described previously (7), who exhibited identical demographics as the whole group. Studies started at 6:30 a.m. with primed-continuous infusion (5 min: 3.6 mg ⋅ kg body wt−1 ⋅ fasting glucose [mg/dL]/90 [mg/dL]; 355 min: 0.036 mg ⋅ min−1 ⋅ kg body wt−1) of d-[6,6-2H2]glucose (98% enriched; CIL, Andover, MA). The euglycemic (∼5.5 mmol/L)-hyperinsulinemic (∼70 mU/L) clamp test was started at 10:00 a.m., with a primed continuous infusion of human regular insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark; 40 mU ⋅ [m body surface area]−2 ⋅ min−1) for 240 min. A 20% dextrose infusion labeled with d-[6,6-2H2]glucose (2% enriched) according to the hot-glucose infusion protocol was periodically adjusted to maintain euglycemia (7).
MRS
After overnight fasting, volunteers underwent a MRS protocol consisting of two consecutive sessions with a break of ∼30 min in a 3-T MR scanner (Bruker Biospin, Ettlingen, Germany) using a 10-cm linear polarized surface coil tuned to 1H and 31P frequencies.
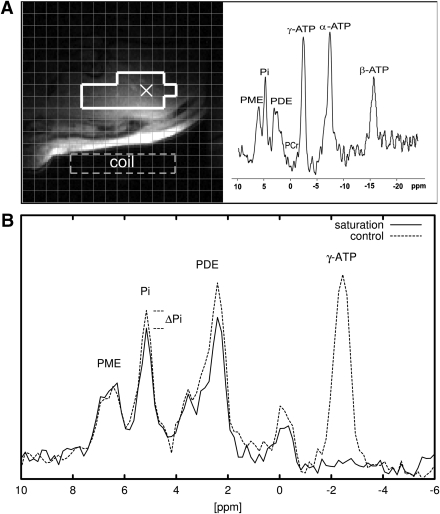
In session 1, subjects were supine in the scanner with the coil positioned over the lateral aspect of the liver. Axial and oblique gradient echo images were acquired during one breath-hold each. HCL was measured using localized 1H MRS (STEAM, 20 × 20 × 20 mm; 5 scans: echo time, 15, 20, 30, 50, 70 ms; repetition time, 2.5 s) (Fig. 1A). Fat and water peaks were integrated after phasing and baseline adjustment using XWIN-NMR software (Bruker Biospin, Ettlingen, Germany). A 30-mm slab within the liver was selected using 1D-ISIS encoding for 31P acquisitions (Fig. 1A). After a baseline scan, alternated spectra with saturation of the γ-ATP resonance frequency and the frequency mirrored relative to the PI resonance were acquired (Fig. 1B). In addition, a combined saturation transfer inversion recovery experiment was performed in each individual to determine the effective longitudinal relaxation time (T1*). Spectra were phased and baseline corrected. The peak amplitude was used for quantification because only peak ratios between consecutive scans were used as described (12).
Figure 1.
Liver MR data of one patient with type 2 diabetes. A: Axial slice through the liver (gradient echo, prone position). The grid corresponds to the chemical shift imaging matrix. The voxels surrounded by the bold white line were selected for quantification. The MR spectrum corresponding to the “×” in the image is shown on the right panel. B: MR spectra from the saturation transfer experiment (solid line). The ATP resonance is completely suppressed, and the PI signal is lower than in the baseline control experiment (dashed line).
In session 2, lasting ∼50 min, the subjects were prone in the scanner on top of the surface coil with the lateral aspect of the liver over the coil center. After shimming, gradient echo-based images were acquired during one breath-hold. A 3-dimensional k-space weighted chemical shift imaging sequence (field of view: 20 × 20 × 20 cm, repetition time: 1 s, bandwidth: 5 kHz, 2,048 acquisitions in 34 min) was applied, reconstructed, and quantified offline using custom-made software in combination with jMRUI/AMARES, as described (12,14).
Calculations and analyses
Water and lipid signals were nonlinearly fitted as a single exponential for T2 correction. HCL were calculated as lipids/(water + lipids) × 100. The chemical exchange constant, k of the saturation transfer experiment is assessed from the PI signal before (M0) and after saturation of γ-ATP (Ms). Then, k is calculated as k = 1/T1* × (1 − Ms/M0), and fATP as fATP = k × PI.
Rates of glucose appearance (Ra) were calculated by dividing the tracer (d-[6,6-2H2]glucose) infusion rate multiplied by tracer enrichment by the percent of tracer enrichment in plasma and subtracting the tracer infusion rate. During the clamp, Ra was calculated using Steele's nonsteady state equations. EGP was calculated from the difference between Ra and mean glucose infusion rates. Insulin-mediated EGP represents EGP during the last 30 min of the clamp. EGP suppression was calculated as 100 × [(1 − EGP insulin-mediated)/(EGP baseline)].
Data are presented as means ± SD and compared between the groups using the two-tailed Student t test. Relationships between variables were analyzed with Pearson correlations for normally distributed data and with Spearman correlations for the scattered HCL values. Regression analyses for the dependent variables fATP and PI were done using the independent variables of age, BMI, waist circumference, HCL, alanine aminotransferase, A1C, EGP, and FFA suppression, M/I, serum triglycerides, and low-density lipoprotein.
RESULTS
Diabetic participants had 67% higher fasting plasma glucose concentrations, 27% higher A1C, and similar body weight, but tended to have greater waist circumference (P = 0.167) than controls (Table 1). Insulin sensitivity was 29% (M) and 45% (M/I) lower in diabetic volunteers than in controls, confirming peripheral insulin resistance (Table 2).
Table 2.
Liver fat, energy metabolism, and hepatic and peripheral insulin sensitivity
| Variable | Type 2 diabetes | Control |
|---|---|---|
| HCL (% signal) | 10.6 ± 9.3 | 8.2 ± 10.9 |
| Absolute PI (mmol/L) | 0.96 ± 0.19* | 1.38 ± 0.38 |
| k (s−1) | 0.28 ± 0.07 | 0.33 ± 0.12 |
| fATP (mmol ⋅ L−1 ⋅ min−1) | 16.2 ± 5.2† | 28.0 ± 13.0 |
| EGP baseline (mg ⋅ kg−1 ⋅ min−1) | 1.6 ± 0.3 | 1.8 ± 0.4 |
| EGP suppression (%) | 72 ± 15‡ | 100 ± 17 |
| FFA baseline (µmol/L) | 569 ± 122 | 430 ± 260 |
| FFA suppression (%) | 95 ± 2* | 97 ± 2 |
| M (mg ⋅ kg−1 ⋅ min−1) | 5.3 ± 1.3§ | 7.5 ± 1.9 |
| M/I (mg ⋅ kg−1 ⋅ min−1 ⋅ mU−1 ⋅ L) | 0.06 ± 0.03|| | 0.11 ± 0.02 |
Data are shown as mean ± SD. Significant differences between groups: *P = 0.011;
†P = 0.025;
‡P = 0.009;
§P = 0.003;
||P = 0.005.
Mean HCL content was not different between groups (Table 2). The distribution of the individual HCL values covered a broad range from <1 to 33% across the groups, with values >5.5% in six diabetic and five nondiabetic participants. Fasting EGP was similar in both groups, whereas EGP was completely suppressed during insulin stimulation only in controls but not in diabetic participants, indicating hepatic insulin resistance. Fasting plasma FFAs were comparable, but FFA suppression was lower in diabetic patients. Hepatocellular fATP was 42% lower and k, the chemical exchange rate constant, tended to be lower in patients compared with controls (Table 2). Previous medical treatment with or without metformin did not affect fATP (16.5 ± 6.0 vs. 16.0 ± 5.0 mmol/L/min).
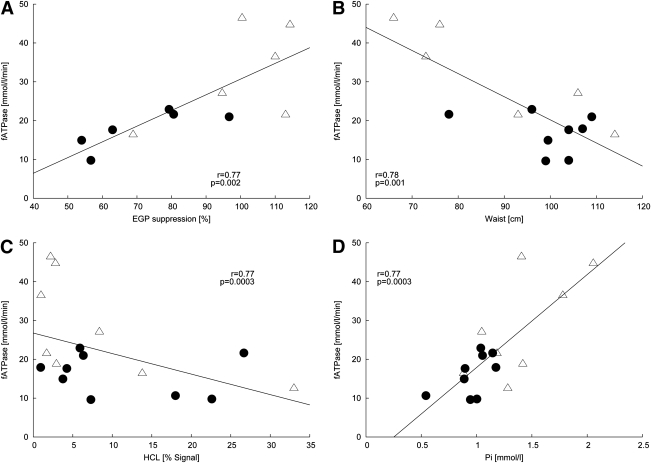
fATP positively correlated with both peripheral (M: r = 0.67, P = 0.01; M/I: r = 0.61, P = 0.03) and hepatic insulin resistance (suppression of EGP: r = 0.77, P = 0.002), even after adjustment for plasma insulin during the clamp (r = 0.84, P = 0.001) across all participants (Fig. 2A). Also, k values related to M (r = 0.67, P = 0.01) but not to M/I (r = 0.47, P = 0.11). Furthermore, fATP correlated negatively with waist circumference (r = −0.78 P = 0.001; Fig. 2B), fasting plasma glucose (r = −0.58, P = 0.01), BMI (r = −0.55, P = 0.02), HCL (r = −0.51, P = 0.04; Fig. 2C), and A1C (r = −0.50, P = 0.04). Hepatocellular PI (r = 0.77, P = 0.0003) related positively to fATP (Fig. 2D). After adjusting for HCL, the relationships of fATP with waist circumference (r = −0.81 P = 0.001), M (r = 0.66, P = 0.02), M/I (r = 0.64, P = 0.03), EGP suppression (r = 0.72, P = 0.009), BMI (−0.54, P = 0.03), and fasting plasma glucose (r = −0.52, P = 0.04) remained significant. fATP did not correlate significantly with fasting FFA (r = 0.203, P = 0.507) or FFA suppression (r = 0.51, P = 0.07).
Figure 2.
Correlations with hepatocellular ATP production (fATP) with (A) hepatic insulin resistance (EGP suppression; all: r = 0.77, P = 0.002; type 2 diabetic subjects: r = 0.79, P = 0.01; controls: r = 0.54, P = 0.17), (B) waist circumference (all: r = −0.78, P = 0.001; type 2 diabetic subjects: r = 0.57, P = 0.11; controls: r = 0.68, P = 0.064), (C) HCL contents (all: r = −0.51, P = 0.038; type 2 diabetic subjects r = −0.20, P = 0.61; controls: r = 0.62, P = 0.10), (D) hepatic concentrations of PI (all: r = 0.77, P = 0.0003; type 2 diabetic subjects: r = 0.57, P = 0.11; controls: r = 0.68, P = 0.064) across all participants (type 2 diabetic subjects: ●, controls: △).
Multiple regression analysis for the dependent variable fATP identified waist circumference as the independent predictor of fATP (r2 = 0.65, adjusted r2 = 0.62, P = 0.001). Likewise, multiple regression analysis for the dependent variable PI identified waist circumference as the independent predictor of PI (r2 = 0.56, adjusted r2 = 0.52, P = 0.003).
CONCLUSIONS
This study found that patients with type 2 diabetes have markedly lower hepatic ATP production than nondiabetic individuals of comparable age and body mass. Of note, this alteration of fATP occurred without evidence for clinical liver disease, as indicated by the lack of a history of hepatitis, alcohol consumption, or significant elevation of liver enzymes (11).
Across all subjects, hepatocellular fATP correlated negatively with HCL, as recently reported for the relationships between hepatic absolute PI and ATP concentrations and HCL (11). However, we detected a clear difference in fATP between diabetic and nondiabetic participants, despite comparable HCL values and equal distribution of steatosis (HCL >5.5%) in both groups (67% of diabetic and 63% of nondiabetic participants). Thus, other factors than HCL more likely explain the lower fATP in patients. After adjustment for HCL, hepatic and whole-body insulin sensitivity remained tightly and negatively related to fATP.
Absolute hepatic concentrations of PI and ATP are also associated with hepatic but not with peripheral insulin sensitivity (11). Under similar conditions of overnight fasting, myocellular fATP correlates negatively to peripheral insulin sensitivity, which is mostly accounted for by skeletal muscle, but not to hepatic insulin sensitivity (7). This suggests that hepatocellular fATP could serve as a better indicator of overall insulin resistance than myocellular fATP.
In type 2 diabetes, insulin resistance develops as a result of inherited factors as well as from acquired factors that result in glucolipotoxicity due to chronic elevation of FFAs, triglycerides, or glucose (2,3). In rats, a high-fat diet for 7 weeks induced obesity, insulin resistance, and hepatic steatosis and also reduced respiratory capacity and increased oxidative stress in liver mitochondria (15). Further, mitochondria from fatty rat liver have lower amounts of ATP synthase protein (16). A short-term high-fat diet also leads to insulin resistance in liver, but not in skeletal muscle (17), whereas a hypocaloric diet decreases both hepatic fat content and insulin resistance within 48 h (18). In conclusion, impaired energy metabolism in the liver could be an early defect in the pathogenesis of type 2 diabetes.
The lack of a correlation of fATP with circulating plasma triglycerides or FFAs suggests that increased lipid availability cannot explain the abnormality of hepatic fATP. However, hepatocellular fATP related negatively to BMI and even more tightly to waist circumference, which explained most of the variation of fATP. Of note, waist circumference is the best anthropometric marker of abdominal visceral fat (19), which suggests, but does not prove, a role of visceral fat for hepatic energy metabolism.
Hepatocellular fATP further related to short- and long-term glycemia, as measured by fasting plasma glucose and A1C, so that defective hepatic energy metabolism could also reflect deterioration of glucose metabolism in the progression of type 2 diabetes. Abnormalities in fATP have been found in skeletal muscle not only of patients with overt type 2 diabetes (7,20) but also of their nondiabetic first-degree relatives, who are at increased risk for type 2 diabetes (8). Impairment of ATP synthesis in skeletal muscle has been further related to insulin resistance, myocellular lipid deposition, and genetic susceptibility to the onset of type 2 diabetes (21). To our knowledge, however, no data on a similar association between lipid contents, insulin sensitivity, and ATP production, which also includes ATP generation from glycolytic flux, have been reported in the livers of patients with type 2 diabetes. Although our results suggest that abnormal phosphorus metabolism relates to hepatic insulin resistance and HCL, we cannot rule out that reduced ATP turnover is rather a consequence of reduced energy demand or altered energy homeostasis that may relate to increased lipid availability and hyperglycemia. In this case, impaired fATP would result from chronic hyperglycemia and/or insulin resistance due to glucose toxicity. Of note, there is evidence that insulin stimulates uptake of PI by way of a sodium-dependent transporter, at least in cultured rat hepatocytes, so that desensitization of this system could lead to lower hepatocellular PI levels (22).
Our MRS protocol is time-consuming, which also explains the dropout rate in this study. Alternatively, the tight relationship between hepatocellular fATP and PI, which is a direct consequence of a comparable rate constant, k, suggests that quantification of the absolute PI concentration could be sufficient for assessing hepatic energy metabolism. Altered tissue morphology could affect the MR relaxation rates, which in turn could affect the absolute quantification of metabolites and fATP. This simplification should, therefore, only be considered when there is strong evidence against alterations in MR relaxation times. The tight correlation between ATP and PI (11) renders errors due to altered MR relaxation times highly unlikely. Participants in the current study did not show clinical signs of cholestasis or impaired liver function, as expressed by normal results on liver enzyme tests and function parameters, although we cannot be completely certain because we had no histologic confirmation. Thus, absolute quantification of hepatic 31P metabolites (11,12), together with k from this study, appears to be a clinically applicable measure of hepatic energy metabolism. Even more, it appears that waist circumference could also serve as a viable predictor of hepatic ATP production.
Nevertheless, some limitations of the current study need to be considered. We studied elderly volunteers who had a mean age of ~60 years, which might result in lower fATP analogous to data on myocellular fATP (8). The ages of diabetic and nondiabetic participants in this study were similar, however, and fATP in these elderly controls was almost identical to that previously reported for young, lean, healthy volunteers (12).
Finally, the saturation transfer experiment provides a measure of total ATP turnover, which includes cycling at the levels of glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase of the glycolytic and gluconeogenic pathways (23). Because fasting EGP was comparable between both groups, despite different degrees of glycemia, a variable contribution of cycling is rather unlikely. Finally, some spectra of the saturation transfer experiment contained residual phosphocreatine resulting from a minor compromise between optimizing localization and signal-to-noise ratio. Of note, the phosphocreatine is dominating skeletal muscle spectra, implying the contamination in PI, which determines fATP, is lower by an order of magnitude. Moreover, chemical shift displacement shifts the PI signal further inward into the liver. The phosphodiester signals appear affected by the saturation pulse (Fig. 1A); this could, besides off-resonance effects, indicate chemical exchange such as ATP used in phosphodiesterase formation (24).
In conclusion, patients with type 2 diabetes without clinical liver disease exhibit lower ATP production than nondiabetic otherwise comparable individuals. The reduction in flux through ATP synthesis depends primarily on the lower hepatic PI and ATP concentrations. Insulin resistance relates to the perturbed hepatic energy metabolism, which can be at least be partly explained by fat mass as assessed from waist circumference.
Acknowledgments
This work was supported by grants from the Austrian Science Foundation (P15656), Austrian National Bank (OENB 11459), European Foundation for the Study of Diabetes (GlaxoSmithKline and Lilly grants) and German Research Foundation (SFB575, project 13) to M.R. No other potential conflicts of interest relevant to this article were reported.
A.I.S., J.S., and M.C. acquired and analyzed data. A.I.S. and M.R. wrote the manuscript. M.K., E.M., and J.S. contributed to the discussion and reviewed the manuscript.
Parts of the data were presented as abstracts at the following meetings: 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 2009; 45th EASD Annual Meeting, Vienna, Austria, 2009; 17th European Congress of Obesity, Amsterdam, 2009; 24th Federation of International Danube Symposia on Diabetes Mellitus, Salzburg, Austria, 2009; Keystone Symposium, Banff, Alberta, Canada, 2009; and the 11th Meeting of the Austrian Diabetes Society, Linz, Austria, 2006.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–1231 [DOI] [PubMed] [Google Scholar]
- 2.Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2006;2:335–348 [DOI] [PubMed] [Google Scholar]
- 3.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463–478 [DOI] [PubMed] [Google Scholar]
- 4.Loria P, Lonardo A, Targher G. Is liver fat detrimental to vessels? Intersections in the pathogenesis of NAFLD and atherosclerosis. Clin Sci (Lond) 2008;115:1–12 [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen A. Update on nonalcoholic fatty liver disease: genes involved in nonalcoholic fatty liver disease and associated inflammation. Curr Opin Clin Nutr Metab Care 2010;13:391–396 [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm A, Krššák M, Schmid AI, Nowotny P, Waldhäusl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006;55:136–140 [PubMed] [Google Scholar]
- 10.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–1192 [DOI] [PubMed] [Google Scholar]
- 11.Szendroedi J, Chmelik M, Schmid AI, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology, 2009;58:1333–1341 [DOI] [PubMed] [Google Scholar]
- 12.Schmid AI, Chmelík M, Szendroedi J, et al. Quantitative ATP synthesis in human liver measured by localized 31P spectroscopy using the magnetization transfer experiment. NMR Biomed 2008;21:437–443 [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research Group Relationship of body size and shape to the development of diabetes in the diabetes prevention program. Obesity (Silver Spring) 2006;14:2107–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chmelík M, Schmid AI, Gruber S, et al. Three-dimensional high-resolution magnetic resonance spectroscopic imaging for absolute quantification of 31P metabolites in human liver. Magn Reson Med 2008;60:796–802 [DOI] [PubMed] [Google Scholar]
- 15.Raffaella C, Francesca B, Italia F, Marina P, Giovanna L, Susanna I. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity (Silver Spring) 2008;16:958–964 [DOI] [PubMed] [Google Scholar]
- 16.Vendemiale G, Grattagliano I, Caraceni P, et al. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology 2001;33:808–815 [DOI] [PubMed] [Google Scholar]
- 17.Brøns C, Vaag A. Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes. J Physiol 2009;587:3977–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirk E, Reeds DN, Finck BN, Mayurranjan MS, Patterson BW, Klein BS, et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552–1560 [Erratum: Gastroenterology 2009;137:393. Mayurranjan MS corrected to Mayurranjan SM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankinen T, Kim SY, Pérusse L, Després JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord 1999;23:801–809 [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 21.Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 2009;58:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterworth PJ, Younus MJ. Uptake of phosphate by rat hepatocytes in primary culture: a sodium-dependent system that is stimulated by insulin. Biochim Biophys Acta 1993;1148:117–122 [DOI] [PubMed] [Google Scholar]
- 23.Thoma WJ, Uğurbil K. Saturation-transfer studies of ATP-Pi exchange in isolated perfused rat liver. Biochim Biophys Acta 1987;893:225–231 [DOI] [PubMed] [Google Scholar]
- 24.Ryan J, Rogers GN, Toscano DG, Storm DR. Formation of adenosine 5′-phosphoroglycerol from ATP and glycerol by rat liver plasma membranes. J Biol Chem 1977;252:1719–1722 [PubMed] [Google Scholar]