Abstract
OBJECTIVE
This study examined the association between objectively measured sedentary activity and metabolic syndrome among older adults.
RESEARCH DESIGN AND METHODS
Data were from 1,367 men and women, aged ≥60 years who participated in the 2003–2006 National Health and Nutrition Examination Survey (NHANES). Sedentary time during waking hours was measured by an accelerometer (<100 counts per minute). A sedentary bout was defined as a period of time >5 min. A sedentary break was defined as an interruption in sedentary time (≥100 counts per minute). Metabolic syndrome was defined according to the Adult Treatment Panel (ATP) III criteria.
RESULTS
On average, people spent 9.5 h (65% of wear time) as sedentary. Compared with people without metabolic syndrome, people with metabolic syndrome spent a greater percentage of time as sedentary (67.3 vs. 62.2%), had longer average sedentary bouts (17.7 vs. 16.7 min), had lower intensity during sedentary time (14.8 vs. 15.8 average counts per minute), and had fewer sedentary breaks (82.3 vs. 86.7), adjusted for age and sex (all P < 0.01). A higher percentage of time sedentary and fewer sedentary breaks were associated with a significantly greater likelihood of metabolic syndrome after adjustment for age, sex, ethnicity, education, alcohol consumption, smoking, BMI, diabetes, heart disease, and physical activity. The association between intensity during sedentary time and metabolic syndrome was borderline significant.
CONCLUSIONS
The proportion of sedentary time was strongly related to metabolic risk, independent of physical activity. Current results suggest older people may benefit from reducing total sedentary time and avoiding prolonged periods of sedentary time by increasing the number of breaks during sedentary time.
Metabolic syndrome has been established as a public health concern in the U.S. Thirty-four percent of adults in the U.S. meet the criteria for metabolic syndrome, with men and women over 60 years of age even more likely to have metabolic syndrome (1). Metabolic syndrome is most commonly defined according to the National Cholesterol Education Program’s Adult Treatment Panel (NCEP ATP) III criteria as three or more of the following conditions: abdominal obesity, high triglycerides, low HDL cholesterol, hypertension, and hyperglycemia (2). People with metabolic syndrome have an increased risk to develop health problems such as heart disease, diabetes, and stroke (3–5). Because of its high prevalence and significant health consequences, it is important to understand the risk factors of metabolic syndrome.
Physical inactivity (6,7), often defined as the lack of moderate-to-vigorous physical activity, and sedentary behavior (8,9) have been shown to be important risk factors of metabolic syndrome. These studies, however, are limited by the use of self-reported physical activity data. Previous studies with objective physical activity data by accelerometry have shown that physical inactivity is associated with metabolic risk factors in adults (10). However most studies focused on risk associated with lack of physical activity rather than sedentary time. Sedentary behavior is defined as engaging in activities at the resting level of energy expenditure and includes activities such as sleeping, sitting, lying down, playing on the computer, and watching television (11). Since sedentary behavior is not defined as the lack of moderate-to-vigorous physical activity, it is important to study it separately from light, moderate, or vigorous activity. Further, sedentary behavior is independently associated with health outcomes (11,12).
Few studies have used accelerometry to examine the role of sedentary activities on metabolic risk (13,14). These studies have shown that an increase in the duration and percentage of time spent in sedentary behavior are associated with an increase in metabolic risk in adults (13,14), while an increase in breaks taken from sedentary behavior is associated with a decrease in metabolic risk (13). It seems, therefore, that in addition to total time spent in sedentary behavior, the proportion and distribution of sedentary time within the day are independently associated with health outcomes. To our knowledge, there are no studies specifically among older adults that examined the association between objectively measured sedentary activity and metabolic syndrome. This is a particularly important research question as the prevalence of metabolic alterations is higher in older persons (1), and the amount of sedentary time increases with age; people aged 60 years and older spend more than 8 h per day in sedentary behaviors (15).
The aim of this study was to examine the associations of specific sedentary activity parameters with metabolic syndrome using nationally representative National Health and Nutrition Examination Survey (NHANES) data (16) of adults 60 years of age and older living in the U.S.
RESEARCH DESIGN AND METHODS
This study used cross-sectional data from participants of the 2003–2004 and 2005–2006 NHANES study conducted in the U.S. NHANES provides a nationally representative sample with a stratified, multistage design. The three main aspects to the survey are an interview given by a trained interviewer in the participants’ home, a medical exam completed at a mobile examination center, and laboratory tests. The National Center for Health Statistics Ethics Review Board approved the survey protocols, and informed consent was obtained for all subjects.
There were 14,584 people aged 6 years and older who participated in the accelerometer study in NHANES. The 12,772 participants had at least 1 valid day of accelerometry data, which was defined as 10 or more hours of wear time (17). For the current study, we included participants 60 years of age and older (n = 2,656) and who had 4 or more valid days of accelerometer wear time (n = 2,401). Only participants scheduled for a morning blood draw had triglyceride and plasma glucose data because these samples were obtained after an overnight fast, bringing the final sample size to 1,367 (709 men and 658 women).
Measures
Accelerometry.
Activity was assessed using the ActiGraph AM-7164 accelerometer (ActiGraph, Fort Walton Beach, FL). The monitor was attached to a belt that strapped around the waist. Each participant was instructed to wear the accelerometer over their right hip. Participants were instructed to wear the monitor for seven consecutive days and to remove the monitor before going to bed and during showers, bathing, swimming, or other water activities. The monitors were returned by mail to the NHANES contractor where the data were downloaded, and the monitors were checked for calibration.
The uniaxial ActiGraph measures and records vertical acceleration as “counts” per minute, an arbitrary intensity indicator of the movements of each minute. Nonwear time was defined as intervals of at least 60 consecutive minutes of 0 counts with allowance for up to 2 min of counts between 0 and 100. Active minutes were defined as ≥100 counts per minute and sedentary minutes as counts <100 during wear time (18). A sedentary bout was defined as a period of time >5 min when count values fell into the sedentary range with only 1 allowable minute outside the sedentary range. A sedentary break was defined as an interruption in sedentary time when the count value exceeded 99 per minute. To identify specific sedentary activity parameters, five variables of interest were included in this study: 1) duration of valid sedentary time (hours); 2) proportion of total valid wear time that was sedentary time; 3) average duration of a sedentary bout; 4) average intensity during sedentary time, which is used as a quantitative indicator of stillness during sedentary time and is defined by the average count value during sedentary time; and 5) number of breaks in sedentary time. These five sedentary variables were averaged for one day and used as continuous variables. Sex-specific quartiles were also created for each variable.
Metabolic syndrome.
Metabolic syndrome was defined according to the ATP III guidelines as meeting three or more of the following criteria: 1) waist circumference ≥102 cm for men and ≥88 cm for women; 2) serum triglyceride level of ≥150 mg/dL; 3) HDL cholesterol level <40 mg/dL for men and <50 mg/dL for women; 4) fasting glucose level ≥100 mg/dL or use of antidiabetic medications (insulin or oral agents); or 5) systolic blood pressure ≥85 mmHg and/or diastolic blood pressure ≥130 mmHg, or use of antihypertensive medications (2). Up to four blood pressure reading were taken on participants. To determine blood pressure status, the last two blood pressure readings were averaged for participants that had three or four measurements. If participants only had two blood pressure readings, the last reading was used in the analysis. If participants had only one blood pressure reading, that reading was used in the analysis. Measurements with a systolic reading of zero were considered missing. According to the guidelines, medications for elevated triglycerides and reduced HDL cholesterol (fibrates and nicotinic acid) should be taken into account (2). This specific information, however, was not available in NHANES. People with one or two missing metabolic syndrome criteria who had three positive metabolic syndrome criteria were grouped in the metabolic syndrome group. Similarly, people with one or two missing metabolic syndrome criteria who had three negative metabolic syndrome criteria were grouped in the nonmetabolic syndrome group.
Covariates.
Covariates included age, sex, self-reported race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), education (less than high school, high school or general equivalency diploma, more than high school), alcohol consumption (never, former, current drinker, missing), smoking (never, former, current), BMI (kg/m2), self-reported diabetes and heart disease, and physical activity (moderate-to-vigorous physical activity [minutes >2,020 counts] and average accelerometer count during active minutes).
Statistical analysis
Data were analyzed using SAS 9.2 Software (SAS Institute, Cary, NC) and Sudaan 10.0 (RTI International, Research Triangle Park, NC) to account for the complex survey design and incorporate the sample weights. Sample weights were recalculated from the NHANES mobile examination center weights to reflect the “nonresponse” related to not having complete accelerometer data. Reweighting was conducted within age (60–69 years, 70–79 years, and 80+ years), race/ethnicity (non-Hispanic whites, non-Hispanic blacks, and Mexican Americans), and gender strata. Resulting sample weights were used for all analyses. The t tests for continuous variables and χ2 tests for categorical variables were used to examine the differences in characteristics between people with and without metabolic syndrome. Analysis of variance was conducted to examine the association between sedentary variables and the metabolic syndrome components. Adjusted means and standard errors adjusted for sex and age are presented. Logistic regression analyses were performed to examine the associations between the sedentary variables (in quartiles) and metabolic syndrome. Four models were fitted; model 1 adjusted for sex and age. Model 2 was additionally adjusted for education, race/ethnicity, alcohol consumption, smoking status, and BMI. Model 3 additionally adjusted for diabetes, heart disease, and physical activity by the average counts during active time was added to model 4. Interactions between sex, age, race/ethnicity, and all sedentary variables were tested but were found to be nonsignificant at α = 0.05.
RESULTS
Out of the 1,367 people in the total analyzed sample, 665 (48.6%) participants met the criteria for having metabolic syndrome. As shown in Table 1, participants with metabolic syndrome were more often women (P < 0.01), had a lower educational level (P < 0.01), were more often nondrinkers (P < 0.01), had a higher BMI (P < 0.001), had a higher prevalence of diabetes and heart disease, spent less time in the moderate-to-vigorous activity range, and had a lower average accelerometry count during active minutes in the day (P < 0.01).
Table 1.
Baseline characteristics of the study population
| Metabolic syndrome |
P |
||
|---|---|---|---|
| No | Yes | ||
| n | 702 | 665 | |
| Characteristic |
|||
| Sex, % | |||
| Men | 49.3 | 38.4 | <0.01 |
| Women | 50.7 | 61.6 | |
| Age (years), mean (SD)* | 71.0 (8.0) | 71.0 (7.4) | 0.94 |
| Ethnicity, % | |||
| Non-Hispanic white | 80.9 | 84.3 | 0.14 |
| Non-Hispanic black | 9.1 | 8.1 | |
| Hispanic | 3.3 | 3.8 | |
| Other | 6.7 | 3.8 | |
| Education, % | |||
| <High school | 23.7 | 26.5 | <0.01 |
| High school | 25.2 | 32.6 | |
| >High school | 51.1 | 40.9 | |
| Smoking, % | |||
| Never | 39.6 | 45.7 | 0.14 |
| Former | 47.9 | 40.6 | |
| Current | 12.5 | 13.7 | |
| Alcohol, % | |||
| Never | 11.2 | 20.2 | <0.01 |
| Former | 30.1 | 30.4 | |
| Current | 55.6 | 46.4 | |
| Missing | 3.1 | 2.9 | |
| BMI (kg/m2), mean (SD) | 25.7 (4.7) | 31.0 (5.5) | <0.01 |
| Waist circumference (cm), mean (SD) | 93.3 (12.4) | 107.3 (12.2) | <0.01 |
| HDL cholesterol (mg/dL), mean (SD) | 63.3 (16.7) | 47.8 (13.4) | <0.01 |
| Triglycerides (mg/dL), median (interquartile range) | 104.7 (79.8–128.0) | 178.3 (133.5–231.5) | <0.01 |
| Glucose (mg/dL), median (interquartile range) | 97.0 (92.2–105.7) | 109.9 (101.4–123.9) | <0.01 |
| Systolic blood pressure (mmHg), mean (SD) | 125.3 (19.7) | 140.4 (19.3) | <0.01 |
| Diastolic blood pressure (mmHg), mean (SD) | 65.0 (12.0) | 66.9 (14.2) | 0.05 |
| Diabetes, % | 9.6 | 24.8 | <0.01 |
| Heart disease, % | 10.0 | 14.2 | 0.01 |
| Moderate-to-vigorous physical activity (min), median (interquartile range) | 6.9 (1.9–17.9) | 3.2 (1.2–8.3) | <0.01 |
| Average count value during active minutes, median (interquartile range) | 533.9 (408.5–662.0) | 462.7 (380.1–568.6) | <0.01 |
*Average age not representative of study population; NHANES groups all people ≥85 years of age and older as 85-years-old.
On average, people spent 9.5 h (65% of wear time) as sedentary. The correlation between total sedentary time and percent sedentary time was high (correlation coefficient 0.75); the correlation between total sedentary time with average length of a sedentary bout, intensity during sedentary time, and number of sedentary breaks was lower (correlation coefficients 0.55, −0.53, and 0.09, respectively). Table 2 shows the age- and sex-adjusted means of the five sedentary variables according to each metabolic syndrome component and metabolic syndrome. Participants with a large waist circumference, low HDL cholesterol, high triglycerides, and metabolic syndrome had a higher percentage of sedentary time out of total wear time, a longer average sedentary bout, a lower average intensity during sedentary time (not statistically significant for HDL), and fewer breaks in sedentary time (all P < 0.05) after adjusting for age and sex.
Table 2.
Adjusted means* (SE) of five sedentary variables according to metabolic syndrome
| Sedentary variables |
||||||
|---|---|---|---|---|---|---|
| Duration of sedentary time (hours) | % of sedentary time of total wear time | Average length of sedentary bout (min) | Intensity during sedentary time (counts) | Number of sedentary breaks | ||
| Large waist circumference | No, mean (SE) | 9.27 (0.13) | 62.63 (0.49) | 16.31 (0.21) | 15.76 (0.24) | 88.38 (0.73) |
| Yes, mean (SE) | 9.60 (0.11) | 66.63 (0.60) | 17.73 (0.18) | 15.03 (0.18) | 82.36 (0.85) | |
| P value | 0.04 | <0.01 | <0.01 | 0.02 | <0.01 | |
| Low HDL | No, mean (SE) | 9.39 (0.10) | 64.22 (0.52) | 16.95 (0.15) | 15.49 (0.17) | 85.46 (0.50) |
| Yes, mean (SE) | 9.71 (0.13) | 67.71 (0.65) | 17.86 (0.34) | 14.78 (0.44) | 81.97 (1.47) | |
| P value | 0.06 | <0.01 | 0.03 | 0.19 | 0.03 | |
| High triglycerides | No, mean (SE) | 9.29 (0.10) | 63.84 (0.56) | 16.99 (0.17) | 15.82 (0.17) | 85.34 (0.73) |
| Yes, mean (SE) | 9.62 (0.15) | 67.03 (0.62) | 17.55 (0.17) | 14.62 (0.22) | 82.42 (0.94) | |
| P value | 0.05 | <0.01 | 0.01 | <0.01 | 0.02 | |
| Low glucose | No, mean (SE) | 9.28 (0.13) | 63.63 (0.52) | 16.96 (0.24) | 15.63 (0.20) | 85.55 (0.84) |
| Yes, mean (SE) | 9.51 (0.12) | 66.07 (0.66) | 17.37 (0.16) | 15.18 (0.21) | 83.36 (0.75) | |
| P value | 0.12 | <0.01 | 0.17 | 0.14 | 0.06 | |
| High blood pressure | No, mean (SE) | 9.44 (0.12) | 64.59 (0.57) | 16.81 (0.21) | 15.54 (0.21) | 86.03 (0.64) |
| Yes, mean (SE) | 9.50 (0.12) | 65.65 (0.58) | 17.49 (0.21) | 15.13 (0.22) | 83.37 (0.80) | |
| P value | 0.76 | 0.15 | 0.05 | 0.20 | 0.01 | |
| Metabolic syndrome | No, mean (SE) | 9.26 (0.10) | 63.15 (0.52) | 16.68 (0.18) | 15.80 (0.20) | 86.69 (0.65) |
| Yes, mean (SE) | 9.70 (0.12) | 67.33 (0.57) | 17.74 (0.20) | 14.78 (0.22) | 82.27 (1.03) | |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
*Adjusted for age and sex.
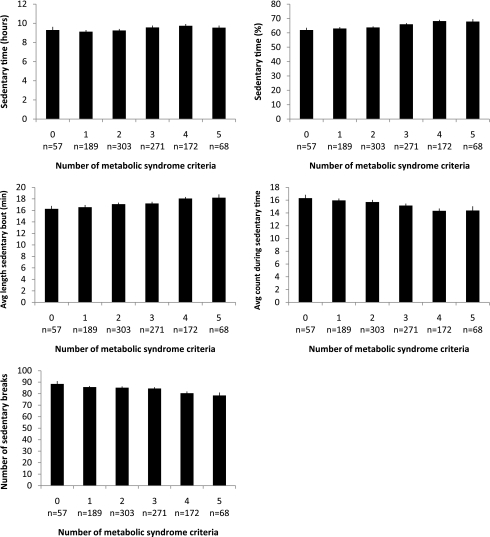
Figure 1 shows the age- and sex-adjusted mean values of the five sedentary variables according to the number of metabolic syndrome criteria. A significant positive trend with the number of metabolic syndrome criteria was found for the duration and percentage of sedentary time and the average length of a sedentary bout (all P < 0.01). The intensity during sedentary time and the number of breaks in sedentary time had a significant negative trend with the number of metabolic syndrome criteria (all P < 0.01).
Figure 1.
Adjusted mean values* and standard errors of the five sedentary variables according to the number of metabolic syndrome criteria†. *Adjusted for age and sex. †All P < 0.01. P value is based on test for trend using linear regression model.
The relationship between each sedentary variable and metabolic syndrome is presented in Table 3. Model 1, adjusted for age and sex, shows the greater likelihood of having metabolic syndrome in highest quartiles of sedentary time duration, percent sedentary time, and average length of a sedentary bout. Further, compared with the highest quartile, the lowest quartile of the intensity during sedentary time and the number of sedentary breaks was associated with an increased likelihood of metabolic syndrome. Model 2 was additionally adjusted for ethnicity, alcohol consumption, smoking, and BMI, and model 3 was additionally adjusted for diabetes and heart disease. In model 3, a higher percentage of sedentary time out of total wear time (quartile 2: odds ratio [OR] 1.52 [95% CI 1.04–2.21]; quartile 3: 1.59 [1.02–2.49]; quartile 4: 1.61 [1.05–2.48]) and fewer sedentary breaks (quartile 3: 1.50 [1.02–2.21]) were related to a significantly increased likelihood of metabolic syndrome. Percentage sedentary time and sedentary breaks remained statistically significant after additional adjustment for physical activity in model 4. A higher intensity during sedentary time was related to an increased likelihood of metabolic syndrome in model 2 (quartile 4: 1.66 [1.03–2.67]) but was no longer statistically significant in models 3 and 4 (1.59 [0.96–2.62]), although the test for trend over the intensity categories remained statistically significant (P = 0.05). In additional analysis we adjusted for minutes of moderate-to-vigorous physical activity in model 4 instead of average counts per minute during active time, which did not change our results. Further, the relation between the intensity during sedentary time and sedentary breaks with metabolic syndrome did not change after adjustment for total sedentary time (data not shown).
Table 3.
ORs and 95% CI of metabolic syndrome according to quartiles of sedentary behavior
| Model 1* |
Model 2† |
Model 3‡ |
Model 4§ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
| Duration of sedentary time | ||||||||||||
| 1 (lowest) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.11 | 0.80 | 1.55 | 0.95 | 0.63 | 1.45 | 0.90 | 0.59 | 1.39 | 0.89 | 0.58 | 1.37 |
| 3 | 1.75 | 1.32 | 2.32 | 1.25 | 0.84 | 1.87 | 1.15 | 0.78 | 1.70 | 1.13 | 0.76 | 1.68 |
| 4 (highest) | 1.66 | 1.11 | 2.48 | 1.26 | 0.89 | 1.79 | 1.18 | 0.81 | 1.73 | 1.16 | 0.77 | 1.74 |
| P value for trend | <0.01 | 0.06 | 0.17 | 0.25 | ||||||||
| % of sedentary time of total wear time | ||||||||||||
| 1 (lowest) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.62 | 1.15 | 2.28 | 1.62 | 1.11 | 2.38 | 1.52 | 1.04 | 2.21 | 1.52 | 1.03 | 2.24 |
| 3 | 2.32 | 1.53 | 3.52 | 1.76 | 1.14 | 2.71 | 1.59 | 1.02 | 2.49 | 1.59 | 1.00 | 2.52 |
| 4 (highest) | 3.10 | 2.08 | 4.62 | 1.90 | 1.25 | 2.88 | 1.61 | 1.05 | 2.48 | 1.61 | 0.97 | 2.67 |
| P value for trend | <0.01 | <0.01 | 0.02 | 0.04 | ||||||||
| Average length of sedentary bout | ||||||||||||
| 1 (lowest) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.14 | 0.77 | 1.68 | 1.02 | 0.66 | 1.58 | 0.98 | 0.63 | 1.52 | 0.98 | 0.63 | 1.52 |
| 3 | 1.51 | 1.00 | 2.28 | 1.17 | 0.74 | 1.87 | 1.12 | 0.73 | 1.72 | 1.11 | 0.72 | 1.71 |
| 4 (highest) | 2.16 | 1.44 | 3.23 | 1.37 | 0.99 | 1.90 | 1.23 | 0.86 | 1.76 | 1.21 | 0.85 | 1.73 |
| P value for trend | <0.01 | 0.03 | 0.16 | 0.18 | ||||||||
| Intensity during sedentary time | ||||||||||||
| 1 (highest) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 1.06 | 0.73 | 1.54 | 1.10 | 0.71 | 1.71 | 1.11 | 0.72 | 1.71 | 1.11 | 0.72 | 1.72 |
| 3 | 1.44 | 0.90 | 2.30 | 1.44 | 0.90 | 2.30 | 1.37 | 0.85 | 2.20 | 1.37 | 0.85 | 2.20 |
| 4 (lowest) | 2.01 | 1.36 | 2.99 | 1.66 | 1.03 | 2.67 | 1.59 | 0.96 | 2.62 | 1.59 | 0.96 | 2.62 |
| P value for trend | <0.01 | 0.02 | 0.05 | 0.05 | ||||||||
| Number of sedentary breaks | ||||||||||||
| 1 (highest) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 2 | 0.97 | 0.64 | 1.47 | 1.18 | 0.77 | 1.80 | 1.15 | 0.74 | 1.78 | 1.16 | 0.75 | 1.79 |
| 3 | 1.59 | 1.13 | 2.24 | 1.49 | 1.03 | 2.15 | 1.50 | 1.02 | 2.21 | 1.53 | 1.05 | 2.23 |
| 4 (lowest) | 2.09 | 1.31 | 3.35 | 1.36 | 0.83 | 2.21 | 1.27 | 0.75 | 2.14 | 1.28 | 0.76 | 2.17 |
| P value for trend | <0.01 | 0.12 | 0.21 | 0.20 | ||||||||
*Adjusted for age and sex.
†Adjusted for age, sex, ethnicity, education, alcohol consumption, smoking, and BMI.
‡Adjusted for age, sex, ethnicity, education, alcohol consumption, smoking, BMI, diabetes, and heart disease.
§Adjusted for age, sex, ethnicity, education, alcohol consumption, smoking, BMI, diabetes, heart disease, and physical activity.
CONCLUSIONS
Using NHANES data, the relationship between objectively measured sedentary time and metabolic syndrome was examined in a representative sample of older adults living in the U.S. Compared with people without metabolic syndrome, people with metabolic syndrome spent more hours and a greater percentage of their wear time as sedentary. Further, people with metabolic syndrome had a longer average sedentary bout, a lower intensity during sedentary time, and fewer breaks in sedentary time. A higher percentage of total wear time spent as sedentary time was associated with a significantly greater likelihood of metabolic syndrome even after adjustment for physical activity. Additionally, fewer sedentary breaks were associated with a higher likelihood of metabolic syndrome, and a significant trend was found for intensity during sedentary time where the likelihood of metabolic syndrome increased with a decrease in intensity.
Previous research with self-reported data is consistent with the findings in this study on the association between time spent in sedentary behaviors and metabolic syndrome. Self-reported sedentary behavior, such as television viewing time, has been positively associated with metabolic risk factors and metabolic syndrome (8,9,19,20). Several studies with objectively measured sedentary data have shown that the number of hours of sedentary time is related to metabolic risk factors (13,14,21). A study among Australian adults found that sedentary time was positively associated with metabolic syndrome (14). Other studies found that physical activity, but not sedentary time, predicts metabolic risk (21) and insulin resistance (22).
Using a large nationally representative sample of older adults, our study was the first to explore different parameters of objectively measured sedentary behavior such as the average length of a sedentary bout; the intensity during sedentary time, which is a quantitative indicator of stillness; and sedentary breaks with metabolic risk in older adults. One previous study of adults (mean age 53.4 years) has been conducted on breaks in sedentary time and metabolic syndrome and corroborates our findings that fewer breaks in sedentary time were significantly associated with a larger waist, higher triglyceride levels, and higher 2-h glucose levels (13). The results of our study suggest that not only is total sedentary time an important health risk factor, but patterns (i.e., the way sedentary time is accumulated) of sedentary behaviors might be important risk factors as well.
An important strength of our study is that sedentary behavior was measured objectively by accelerometry, but accelerometers are not sensitive to detect all activities such as biking, standing, and upper-body movement. The cross-sectional design of this study limits inference about the direction of causality between the sedentary variables and metabolic risk. Future studies should prospectively study the association between sedentary behavior and metabolic syndrome.
Current findings suggest that not only is it important to reduce sedentary time among older adults, but minimizing prolonged periods of uninterrupted sedentary time and increasing sporadic movements during sedentary time may also be protective. In practice, this may be achieved by taking brief activity breaks to disrupt prolonged periods of sitting or by increasing movements while sitting. These minimal changes may result in increases in daily energy expenditure (23) or could provide direct effects on metabolic regulation (24).
In summary, the proportion of sedentary time was strongly related to metabolic risk, independent of physical activity. Time spent in sedentary behavior increases with age (15). Older adults may be at a higher risk of developing metabolic syndrome based on this age-related increase of time spent in sedentary behaviors. This was the first study to examine multiple sedentary parameters including sedentary intensity and sedentary breaks in relation to metabolic syndrome, and future studies should focus on these specific variables to corroborate our results. Current results, though cross-sectional in nature, suggest that potential behavioral interventions for the prevention of metabolic syndrome in older adults might include the promotion of physical activity as well as reduction in total sedentary time and disruption of prolonged sedentary bouts.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
A.B. analyzed and interpreted data and wrote the first draft of the article. T.B.H. contributed to the conceptualization of the study idea, interpreted data, and contributed to the drafts of the article. J.J.M. contributed to the data analyses, interpreted data, and reviewed the drafts of the article. R.J.B., P.C., K.Y.C., D.B., and R.P.T. contributed to the interpretation of data and reviewed drafts of the paper. A.K. conceptualized the study idea, guided the process of data analysis and interpretation, and contributed to the drafts of the article.
We acknowledge the assistance of Danita D. Byrd-Holt, Social & Scientific Systems, Inc., for help with the statistical analysis.
References
- 1.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006 National Health Statistics Reports, No. 13, Hyattsville, MD, National Center for Health Statistics, May 5, 2009 (DHHS publ. no. 2009-1250) [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 2005;13:322–327 [PubMed] [Google Scholar]
- 3.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 2002;156:1070–1077 [DOI] [PubMed] [Google Scholar]
- 4.McNeill AM, Katz R, Girman CJ, et al. Metabolic syndrome and cardiovascular disease in older people: the cardiovascular health study. J Am Geriatr Soc 2006;54:1317–1324 [DOI] [PubMed] [Google Scholar]
- 5.Zhang WW, Liu CY, Wang YJ, Xu ZQ, Chen Y, Zhou HD. Metabolic syndrome increases the risk of stroke: a 5-year follow-up study in a Chinese population. J Neurol 2009;256:1493–1499 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi G, Rossi V, Muscari A, Magalotti D, Zoli M, Pianoro Study Group Physical activity is negatively associated with the metabolic syndrome in the elderly. QJM 2008;101:713–721 [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002;25:1612–1618 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Kohl HW, 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res 2005;13:608–614 [DOI] [PubMed] [Google Scholar]
- 9.Wijndaele K, Duvigneaud N, Matton L, et al. Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults. Eur J Clin Nutr 2009;63:421–429 [DOI] [PubMed] [Google Scholar]
- 10.Park S, Park H, Togo F, et al. Year-long physical activity and metabolic syndrome in older Japanese adults: cross-sectional data from the Nakanojo Study. J Gerontol A Biol Sci Med Sci 2008;63:1119–1123 [DOI] [PubMed] [Google Scholar]
- 11.Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary”. Exerc Sport Sci Rev 2008;36:173–178 [DOI] [PubMed] [Google Scholar]
- 12.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab 2007;32:76–88 [DOI] [PubMed] [Google Scholar]
- 13.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666 [DOI] [PubMed] [Google Scholar]
- 14.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008;31:369–371 [DOI] [PubMed] [Google Scholar]
- 15.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol 2008;167:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 2005–2006 public data general release file documentation. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/general_data_release_doc_05_06.pdf Accessed 16 August 2007
- 17.National Cancer Institute. Risk factor monitoring and methods: SAS programs for analyzing NHANES 2003-2004 accelerometer data. Available from http://riskfactor.cancer.gov/tools/nhanes_pam/ Accessed 11 August 2010
- 18.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–188 [DOI] [PubMed] [Google Scholar]
- 19.Dunstan DW, Salmon J, Healy GN, et al. AusDiab Steering Committee Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care 2007;30:516–522 [DOI] [PubMed] [Google Scholar]
- 20.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc 2008;40:639–645 [DOI] [PubMed] [Google Scholar]
- 21.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care 2007;30:337–342 [DOI] [PubMed] [Google Scholar]
- 22.Ekelund U, Brage S, Griffin SJ, Wareham NJ, ProActive UK Research Group Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care 2009;32:1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine JA. Non-exercise activity thermogenesis (NEAT). Nutr Rev 2004;62:S82–S97 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007;56:2655–2667 [DOI] [PubMed] [Google Scholar]