Abstract
OBJECTIVE
Insulin analogs are increasingly used in patients with type 2 diabetes. We compared the effect of basal, biphasic, prandial, and basal-bolus insulin regimens with insulin analogs to reach the hemoglobin A1c (HbA1c) target of <7% in people with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We conducted an electronic search for randomized controlled trials (RCTs) involving insulin analogs. RCTs were included if they lasted at least 12 weeks, reported the proportion of diabetic patients reaching the HbA1c target of <7% (primary outcome), and the number of patients in any arm was >30.
RESULTS
We found 16 RCTs, with 20 comparisons and 7,759 patients. A greater proportion of patients achieved the HbA1c goal of <7% with both biphasic (odds ratio 1.88 [95% CI 1.38–2.55]) and prandial (2.07 [1.16–3.69]) insulin compared with basal insulin; this was associated for biphasic insulin with greater hypoglycemia (event/patient/30 days, mean difference, 0.34 [range 0–0.69]) and weight gain in kg (1.0 kg [0.28–1.73]). Compared with biphasic insulin, the basal-bolus regimen was associated with a greater chance to reach the HbA1c goal (odds ratio 1.75 [95% CI 1.11–2.77]), with no greater hypoglycemia or weight gain. The effect of insulin analogs on long-term diabetes complications is still lacking.
CONCLUSIONS
A greater proportion of type 2 diabetic patients can achieve the HbA1c goal <7% with biphasic or prandial insulin compared with basal insulin; in absolute terms, the basal-bolus regimen was best for the attainment of the HbA1c goal.
Type 2 diabetes is a complex and progressive disease that has reached epidemic proportions worldwide (1). In the U.S., diabetes is the third cause of death for women and the fourth for men (2). Tight glycemic control, to maintain a hemoglobin A1c (HbA1c) concentration of <7%, is recommended for all nonpregnant adults with diabetes to minimize the risk of long-term microvascular (retinopathy, nephropathy, neuropathy) complications (3,4). The HbA1c goal is currently achieved in ∼50% of patients with type 2 diabetes, at least in selected populations (3).
Although traditionally used as a final treatment option, insulin has been recently recommended by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) as a third-line treatment after diet, exercise, and metformin fail to reach or maintain an HbA1c of <7% (5). According to the National Health Interview Survey, 28% of patients with type 2 diabetes are using insulin, alone (16%) or combined with oral antidiabetic drugs (12%) (6). The choice of an initial insulin regimen for people with type 2 diabetes, in whom oral drugs have failed, may be a difficult one because hypoglycemia and the fear of developing hypoglycemia remain substantial barriers to the initiation and optimal use of insulin (7).
Insulin analogs are modified human insulins developed to address the limitations of human insulin preparations to replicate the pattern of basal and postprandial endogenous secretion of insulin (8). Conversion of the insulin market to analogs, estimated to be 40% 45% in 2005, is projected to reach saturation within 2010 (9). Systematic reviews of insulin analogs have been published previously (10–13). Compared with human insulins, rapid, biphasic (premixed), and basal (long-acting) insulin analogs offer some benefit in better glycemic control and reduced hypoglycemia (10,12,13). However, we did not identify any reviews that evaluated the proportion of patients with type 2 diabetes achieving the HbA1c target <7% with insulin analogs. In this article, we performed a meta-analysis of the randomized controlled trials (RCTs) that compared the effect of different insulin regimens based on insulin analogs.
RESEARCH DESIGN AND METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for reporting systematic reviews and meta-analyses (14).
Data sources
We searched MEDLINE (1966 to January 2010), EMBASE (1980 to January 2010), the Cochrane Central Register of Controlled Trials, and CINAHL from inception to January 2010. The main search concepts were type 2 diabetes, hemoglobin A1c, long-acting insulin analogs, short-acting insulin analogs, biphasic insulin analogs, glargine, detemir, neutral protamine lispro, lispro, aspart, glulisine, randomized controlled trials, and clinical trials. We also reviewed reference lists of included articles, the US Food and Drug Administration and European Medicines Agency Web sites for the insulin analogs, as well as Web sites of public registries of clinical trials (ClinicalTrials.gov and ClinicalStudyResults.org).
Study selection
We included RCTs if 1) patients aged >18 years with a diagnosis of type 2 diabetes, as defined by criteria current at the time of the trial (15) were included; 2) different insulin regimens (basal, biphasic, prandial or basal-bolus) using insulin analogs were evaluated; and 3) the proportion of diabetic patients reaching the HbA1c goal of ≤7% was reported. We included crossover trials with at least 12 weeks of follow-up before and after the crossover phase. The search had no language restriction; however, we excluded reviews, editorials, comments, letters, and abstracts. Trials were rejected if the intervention time was <3 months, the number of patients in any arm was <30 patients, or comparisons were between insulin analogs and conventional insulins within the same or different regimens.
Quality assessment
Two investigators (D.G. and K.E.) identified relevant publications and abstracted the data, and any disagreements were resolved by consensus. Methodologic quality was scored using criteria set out by Jadad et al. (16). This 5-point quality scale includes points for randomization (described as randomized, 1 point; table of random numbers or computer-generated randomization, additional 1 point), double-blind (described as double-blind, 1 point; use of masking such as identical placebo, additional 1 point), and follow-up (the numbers and reasons for withdrawal in each group are stated; 1 point) in the report of an RCT. We gave an additional point if the analysis was by intention-to-treat. We considered scores of ≤2 as low quality and a score of ≥3 as high quality because none of the studies were double-blinded, due to the visibly different properties of the comparators.
Insulin regimens
We compared four insulin regimens:
the biphasic regimen consisted of the biphasic (premixed) insulin analogs lispro 25/75, lispro 50/50, aspart 30/70, aspart 50/50, and aspart 70/30, with the numbers denoting the percentage of the rapid-acting/the long-acting component;
the basal regimen consisted of basal insulin analogs comprising the long-acting insulins glargine, detemir, and lispro/neutral protamine lispro;
the prandial regimen consisted of prandial insulin analogs, comprising short-acting insulins lispro, aspart, and glulisine; and
the basal-bolus regimen consisted of any combination of prandial and basal insulin analogs.
No studies comparing basal or prandial with basal-bolus regimens met the inclusion criteria.
Data synthesis and analysis
The proportion of patients with HbA1c <7% at the end of treatment was the primary outcome. Secondary outcomes were hypoglycemic events and weight gain. Statistically, binomial proportion lacks consensus over the calculation of the confidence intervals. We transformed the proportions into a quantity suitable for the usual fixed and random-effects summaries (the Freeman-Tukey variant of the arc sine square root transformed proportion) (17). The pooled proportion is calculated as a back-transformation of the weighted mean of the transformed proportions, using inverse arc sine variance weights for the fixed-effects model and DerSimonian-Laird weights (18) for the random-effects model.
Denominators used for calculating the response rate on each treatment group were those reported within original studies as eligible patients coming from randomization. The association between exposure (treatments) and outcome (proportions of patients with HbA1c <7%) was measured by the odds ratio (OR). When the OR and 95% CIs were available, we transformed them into logOR and calculated the corresponding variance and standard error using the formula proposed by Greenland (19). When the OR was not directly available from the article, we calculated it from tabular data and used the Woolf formula to evaluate the standard error of the logOR (20). If tabular data were not given and only response rate was available, the number of responses was calculated by multiplying the response rate by the number of randomized/eligible patients. Pooled OR with 95% CI was estimated, pooling the study-specific estimates by random-effects models fitted using SAS (PROC MIXED) software (SAS Institute) with maximum likelihood estimate. These models provided estimates adjusted for the heterogeneity between studies and the correlation within studies given by the randomized studies with more than two groups. That is a conservative approach because it raises the variability caused by the correlation within study, providing, as a result, wider and more reliable CIs.
Heterogeneity of the effect across studies was assessed by Q2 statistics, which is distributed as χ2 statistics. A value of P < 0.10 was used to indicate lack of homogeneity among effects. I2 statistics were provided to quantify the percentage of total variation across studies that was attributable to heterogeneity rather than to chance (21). A value >50% represented substantial variability. The method of Macaskill et al. (22) was used for assessing publication bias. It consists of a funnel-plot regression of log(RR) or log(OR) on the sample size, weighted by the inverse of the pooled variance.
For hypoglycemic events and weight gain, we recorded the mean difference between groups, along with its measure of dispersion. If no measure of dispersion was reported for the between-group difference, we conservatively estimated it by the worst ratio between mean and standard deviation among the available studies. If a trial reported the number of episodes in each group or reported an event rate in a form other than episodes per patient per 30 days, we converted this information into episodes per patients per 30 days.
RESULTS
We identified 2,650 citations, of which we reviewed 126; of these, 16 (23–38) performed comparisons between different insulin regimens and were included in the meta-analysis (Supplementary Fig. 1). Most trials were multinational and sponsored by industry. All studies were RCTs (Table 1): among these, 13 were parallel group and 3 (23,24,27) were crossover. All studies were of open-label design. Trial duration was 3 to 36 months. The trials enrolled a total of 7,759 patients (range, 60–2,091). Patients were a mean age of 58.2 years, with slight prevalence of male sex (55.6%). The study population had a median HbA1c level of 8.6% (interquartile range 8.4–9.1%).
Table 1.
Characteristics of the randomized controlled trials included in the meta-analysis
| Trials (first author, year) | N | Mo | Insulin regimen | Design analog (shot/day) | Age(years) | Male (%) | OHAs | < /≤ 7% | Naïve | Start HbA1c | Titration | Statistics | Quality score | Insulin dose (units/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malone, 2004 (23) | 105 | 4 | Biphasic | CO Lispro25 (×2) | 55.3 | 62.3 | 8.7% | 0.62 | ||||||
| Basal | Glargine | 55.3 | 62.3 | Met | ≤ | Yes | 8.7% | No | No ITT | 3 | 0.57 | |||
| Malone, 2005 (24) | 97 | 4 | Biphasic | CO Lispro25 (×2) | 59.3 | 42 | 8.5% | 0.42 | ||||||
| Basal | Glargine | 59.3 | 42 | Met | ≤ | No | 8.5% | No | ITT | 2 | 0.28 | |||
| Raskin, 2005 (25) | 233 | 6 | Biphasic | P Aspart30 (×2) | 52.6 | 53 | 9.7% | 0.82 | ||||||
| Basal | Glargine | 52.3 | 57 | Met + Pio | < | Yes | 9.8% | Yes | ITT | 2 | 0.55 | |||
| Kann, 2006 (26) | 255 | 6 | Biphasic | P Aspart30 (×2) | 61.5 | 53.9 | 9.2% | 0.39 | ||||||
| Basal | Glargine | 61 | 48.8 | Glimepiride | < | Mix | 8.9% | No | ITT | 2 | 0.39 | |||
| Jacober, 2006 (27) | 60 | 4 | Biphasic | CO Lispro50 (×2) + Lispro25 | 54.9 | 56.7 | 9.2% | 0.35 | ||||||
| Basal | Glargine | 54.9 | 56.7 | Met/Su/Tzd | ≤ | Yes | 9.2% | No | No ITT | 2 | 0.28 | |||
| Kazda, 2006 (28) | 107 | 6 | Biphasic | P Lispro50 (×3) | 59.4 | 54 | 8.1% | 0.59 | ||||||
| Basal | Glargine | 59.4 | 54 | No | < | Yes | 8.1% | No | ITT | 2 | 0.43 | |||
| Kazda, 2006 (28) | 106 | 6 | Biphasic | P Lispro50 (×3) | 59.4 | 54 | 8.1% | 0.59 | ||||||
| Prandial | Lispro (×3) | 59.4 | 54 | No | < | Yes | 8.2% | No | ITT | 2 | 0.50 | |||
| Kazda, 2006 (28) | 105 | 6 | Prandial | P Lispro (×3) | 59.4 | 54 | 8.2% | 0.50 | ||||||
| Basal | Glargine | 59.4 | 54 | No | < | Yes | 8.1% | No | ITT | 2 | 0.43 | |||
| Holman, 2007 (29) | 469 | 12 | Biphasic | P Aspart30 (×2) | 61.7 | 64.1 | 8.6% | 0.53 | ||||||
| Basal | Detemir | 61.7 | 64.1 | Met + Su | ≤ | Yes | 8.4% | Yes | ITT | 4 | 0.49 | |||
| Holman, 2007 (29) | 474 | 12 | Biphasic | P Aspart30 (×2) | 61.7 | 64.1 | 8.6% | 0.53 | ||||||
| Prandial | Aspart (×3) | 61.7 | 64.1 | Met + Su | ≤ | Yes | 8.6% | Yes | ITT | 4 | 0.61 | |||
| Holman, 2007 (29) | 472 | 12 | Prandial | P Aspart (×3) | 61.7 | 64.1 | 8.6% | 0.61 | ||||||
| Basal | Detemir | 61.7 | 64.1 | Met + Su | ≤ | Yes | 8.4% | Yes | ITT | 4 | 0.49 | |||
| Robbins, 2007 (30) | 315 | 6 | Biphasic | P Lispro50 (×3) | 57.7 | 49.8 | 7.8% | 0.7 | ||||||
| Basal | Glargine | 57.7 | 49.8 | Met | ≤ | No | 7.8% | Yes | ITT | 4 | 0.6 | |||
| Bretzel, 2008 (31) | 418 | 10.5 | Prandial | P Lispro (×3) | 59.7 | 59 | 8.7% | 0.53 | ||||||
| Basal | Glargine | 60 | 52 | Met, Su | ≤ | Yes | 8.7% | Yes | ITT | 4 | 0.50 | |||
| Hirao, 2008 (32) | 117 | 6 | Biphasic | P Aspart30 (×2) | 58.5 | 58.7 | 10.6% | — | ||||||
| Prandial | Lispro (×3) | 57.9 | 61.2 | No | < | Yes | 10.6% | No | No ITT | 2 | — | |||
| Rosenstock, 2008 (33) | 374 | 6 | Biphasic | P Lispro50 (×3) | 55.4 | 53 | 8.8% | 1.2 | ||||||
| Basal-bolus | Lispro (×3) + Glargine | 54 | 52 | No Su | < | No | 8.9% | Yes | Complete | 3 | 1.4 | |||
| Liebl, 2009 (34) | 715 | 4 | Biphasic | P Aspart30 (×2) | 61.7 | 63 | 8.4% | 0.62 | ||||||
| Basal-bolus | Aspart (×3) + Detemir | 60.3 | 63 | No | ≤ | No | 8.5% | Yes | ITT | 2 | 0.88 | |||
| Buse, 2009 (35) | 2091 | 6 | Biphasic | P Lispro25 (×2) | 57 | 52.8 | 9.1% | 0.47 | ||||||
| Basal | Glargine | 57 | 52.8 | Met/Su/Tzd | ≤ | Yes | 9.0% | Yes | ITT | 4 | 0.4 | |||
| Raz, 2009 (36) | 1115 | 31 | Basal | P Glargine | 60 | 63.3 | 8.3% | 0.52 | ||||||
| Prandial | Lispro (×3) | 60 | 63.3 | No | < | No | 8.4% | Yes | ITT | 4 | 0.60 | |||
| Strojek, 2009 (37) | 480 | 6 | Basal | P Glargine | 56.1 | 41.2 | Met + | 8.5% | 0.29 | |||||
| Biphasic | Aspart30 (×1) | 55.9 | 46.8 | Glimep | < | Yes | 8.5% | Yes | ITT | 2 | 0.32 | |||
| Holman, 2009 (38) | 698 | 36 | Biphasic | P Aspart30 (×2) + Aspart | 61.7 | 64.1 | 8.6% | 0.78 | ||||||
| Basal-bolus | Aspart (×3) + Detemir | 61.7 | 64.1 | No | ≤ | No | 8.6% | Yes | ITT | 4 | 0.97 |
CO, crossover group; P, parallel group; OHAs, oral hypoglycemic agents; Met, metformin; Su, sulfonylurea; Tzd, thiazolidinedione; ITT, intention to treat.
Nine trials enrolled insulin-naïve patients (23,25,27–29,31,32,35,37), six enrolled insulin-treated patients (24,30,33,34,36,38), and one enrolled mixed patients (26). The median score of methodologic quality was 2.5 (interquartile range 2–4). Ten trials (25,29–31,33–38) used a predefined titration step of insulin doses. Eleven trials had a clear intention-to-treat analysis (24–26,28–31,34–38). Eleven trials (23–27,29–31,33,35,37) adopted combined treatment regimens of oral drugs plus insulin (whichever regimen); the most-represented oral drugs were metformin, a sulfonylurea, or a glitazone.
Biphasic versus basal
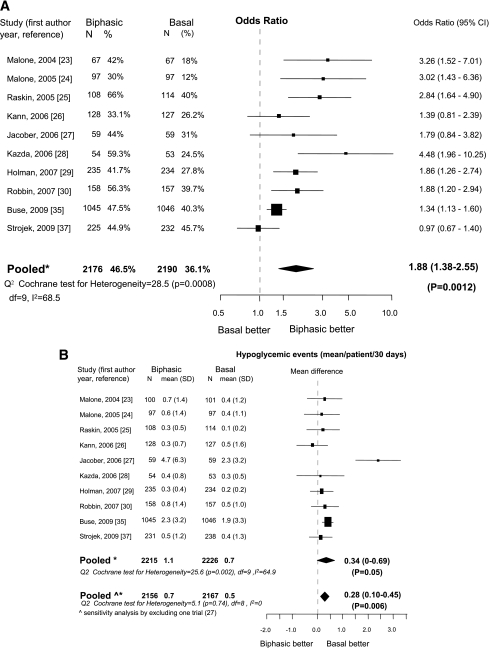
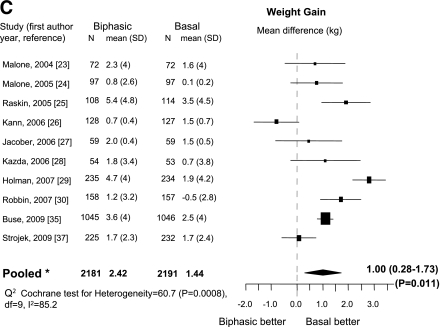
Ten trials (23–30,35,37) were eligible. Most included insulin-naïve patients (23,25,27–30,35,37) and adopted combined treatment regimens of oral drugs plus insulin (23–27,29,30,35,37). Compared with basal insulin, patients treated with biphasic insulin had a greater chance to reach the HbA1c goal of <7% (OR 1.88 [95% CI 1.38–2.55]; Fig. 1A). There was no evidence of publication bias (P = 0.15). Heterogeneity was high (I2 = 68%) but diffuse. Compared with basal insulin, biphasic insulin was associated with a significant increase in hypoglycemic events (0.34 mean events/patient/30 days), but significant heterogeneity was observed (I2 = 64.9%). Exclusion of one trial (27) with the highest hypoglycemic rate completely eliminated heterogeneity (I2 = 0%; Fig. 1B). Biphasic insulin was also associated with greater weight gain (mean difference, 1 kg [95% CI 0.28–1.73]) with high heterogeneity (I2 = 85.2%; Fig. 1C). Final median (interquartile range) insulin dose was 0.5 units/kg (0.39–0.62) for biphasic and 0.41 units/kg (0.29–0.55) for basal insulin (P = 0.16).
Figure 1.
Biphasic versus basal: A: Outcome, proportion (%) of patients with HbA1c <7%. B: Change in incidence of hypoglycemia. C: Change in weight. N = number of patients in each arm. *Mixed-effects model: estimates adjusted for the correlation within studies and heterogeneity between studies; ^sensitivity analysis.
Biphasic versus prandial
Three trials (28,29,32) were eligible. All trials included insulin-naïve patients. No difference between treatments was shown for achievement of HbA1c goal (OR 1.04 [95% CI 0.37–2.92]; Supplementary Fig. 2). There was no evidence of publication bias (P = 0.39). There was no mean (95% CI) difference in the incidence of hypoglycemia events/patient/30 days (−0.15 [−0.57 to 0.27]) or weight gain (0.1 kg [−0.71 to 1.9]) between the two regimens. One trial did not report insulin dose (32). For the remaining two trials, no difference in daily insulin dose was observed between the two regimens.
Prandial versus basal
Four studies (28,29,31,36) were eligible. Compared with basal insulin, prandial insulin was associated with a nonsignificant chance of achievement the HbA1c goal (OR 1.58 [95% CI 0.77–3.23]; Supplementary Fig. 3). An evidence of publication was detected (P = 0.07). A sensitivity analysis excluding one trial (36) gave significant results (OR 2.07 [95% CI 1.16–3.69]) with no evidence of heterogeneity (I2 = 0%) or publication bias (P = 0.37). Compared with basal insulin, prandial insulin was associated with a nonsignificant increase in the incidence of hypoglycemia, with high heterogeneity (I2 = 99.7%), which persisted elevated (I2 = 97.7%) after exclusion of one trial. Prandial insulin was also associated with a greater weight increase of borderline significance. Final median (interquartile range) insulin dose was 0.56 units/kg (0.51–0.60) for prandial and 0.49 units/kg (0.46–0.51) for basal insulin (P = 0.06).
Biphasic versus basal-bolus
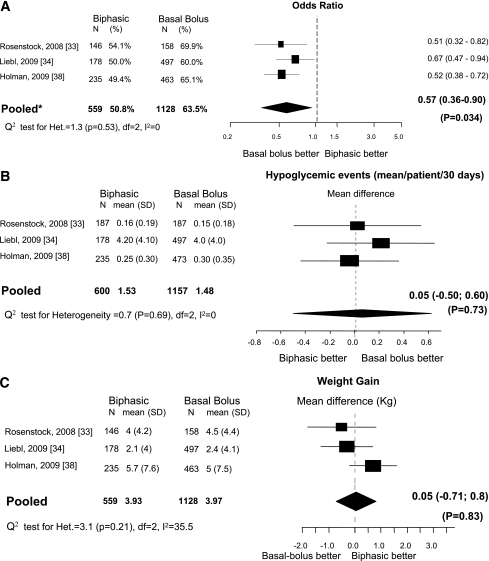
Three studies (33,34,38) were eligible. All trials included patients who were not insulin-naïve. The inclusion of the 3-year extension of the Treating to Target in Type 2 Diabetes (4-T) trial (38) in the present comparison was justified by the switching of 73.7% of the former original prandial and 81.5% of the basal regimen to a basal-bolus regimen. Compared with biphasic insulin, patients treated with a basal-bolus regimen had a higher chance to reach the HbA1c goal (OR, 1.75 [95% CI 1.11–2.77]), with no evidence of heterogeneity (I2 = 0%; Fig. 2). There was no evidence of publication bias (P = 0.78). There was no difference in incidence of hypoglycemia and weight gain between the two regimens. Final median (interquartile range) insulin dose was 0.78 units/kg (0.66–1.09) for biphasic insulin and 0.97 units/kg (0.90–1.29) for the basal-bolus regimen (P = 0.40).
Figure 2.
Biphasic versus basal-bolus. A: Outcome, proportion (%) of patients with HbA1c <7%. B: Change in incidence of hypoglycemia. C: Change in weight. N = number of patients in each arm. *Mixed-effects model: estimates adjusted for the correlation within studies and heterogeneity between studies.
CONCLUSIONS
The ultimate goal of treatment of diabetes is improvement in microvascular and macrovascular complications, and death. Most trials included in this analysis were not specifically designed to evaluate these clinical outcomes. Although not ideal, HbA1c is the most-used intermediate outcome to optimize glycemic control in a clinical setting. The ADA position statement on standards of medical care in diabetes (4) insists on the optimal HbA1c target of <7% for most nonpregnant adults, stressing the evidence that microvascular complications are better mitigated with this goal with respect to macrovascular complications (level of evidence A vs. B, respectively).
We found that biphasic and prandial insulin were both associated with a greater proportion of type 2 diabetic patients achieving the HbA1c goal of <7% compared with basal insulin. This effect was consistent across pooled trials. The basal-bolus regimen was better than biphasic, and in absolute terms, was the best regimen for the attainment of the HbA1c goal. Ultimately, the proportion of patients with a HbA1c goal of <7% using the basal-bolus regimen was 63.5%. The effect of the different insulin regimens on hypoglycemia was quite similar across different comparisons, with the exception of a greater event rate with the biphasic compared with basal insulin. Biphasic and prandial insulin were both associated with greater weight gain compared with basal insulin, and this effect was also consistent across pooled trials.
Biphasic insulin is less effective than basal insulin in decreasing fasting glucose levels but is more effective in decreasing postprandial glucose levels (10,11). Our analyses showed biphasic insulin was associated with more hypoglycemia than basal insulin (∼0.34 mean events/patient/30 days). These data are consistent with previous analyses (9,10) showing that biphasic insulin is more likely to cause hypoglycemia than basal insulin, although in one study (10) the effect of the different insulin regimens could not be pooled in a meta-analysis. The incidence of hypoglycemia was similar between the other comparisons. The overall median incidence of hypoglycemia across all the comparisons was generally low, at 0.4 events/patient/30 days (range, 0–4.71 [interquartile range 0.3–1.0]). Biphasic and prandial insulin both caused more weight gain than basal insulin; however, weight gain was limited, at 1 and 1.94 kg, respectively. No difference in weight gain was found between biphasic versus prandial insulin or biphasic insulin versus the basal-bolus regimen.
The reduced frequency of insulin injections and glucose monitoring, associated with its inherent simplicity, has led to the basal insulin regimen becoming the first option in initiating insulin therapy in recent guidelines (5). Although successful for many patients, at least 60% of patients taking basil insulin were not able to reach the HbA1c target. When the HbA1c goal is not attained despite successful basal insulin dose titration, or when titration is limited by hypoglycemia, treatment is generally intensified by addition of prandial or biphasic insulin. According to our analysis, this escalation in insulin regimens from the simple basal to the complex basal-bolus may result in a further gain in the proportion of type 2 diabetic patients attaining the HbA1c goal (63.5% with the basal-bolus regimen), which still leaves ∼33% of patients not at the target goal.
This study has limitations. The degree of heterogeneity was high for some comparisons, although results in most cases were qualitatively similar across studies in directions of the results. Moreover, sensitivity analysis eliminated heterogeneity in most cases. In addition, there was no evidence of publications bias.
Most studies had a short follow-up, and firm conclusions cannot be draw about the long-term comparative effectiveness of the various regimens. Any improvements seen in HbA1c in these short-term trials may not be sustained over a longer period, although studies with longer follow-up gave similar results. Only one long-term study (mean patient participation after randomization was 963 days) was specifically designed for assessing cardiovascular outcomes in survivors to a first myocardial infarction comparing basal with biphasic insulin, but the study was stopped for lack of efficacy (36). Other long-term trials reported clinical outcomes as adverse events.
In conclusion, our analysis of 7,759 type 2 diabetic patients using insulin analogs indicates that the HbA1c target <7% can be achieved in 35 to 63.5%, depending on the particular insulin regimen. Basal insulin is associated with a lower proportion of diabetic patients at target compared with prandial or biphasic insulin, but with less hypoglycemia and weight gain compared with biphasic insulin. The best achievement rate is obtained with a basal-bolus regimen compared with biphasic insulin, without further risk of hypoglycemia or weight gain. More studies are needed to understand better the effect of insulin analogs on long-term diabetes complications.
Acknowledgments
The study was partly supported by the Second University of Naples. The funding sources had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation or review of the manuscript; or the decision to submit the manuscript for publication.
No potential conflicts of interest relevant to this article were reported.
D.G. and K.E. participated in conception and design. D.G, A.C, K.E, and C.P. analyzed and interpreted the data. K.E and D.G. drafted the article. D.G, K.E., M.I.M., P.C, G.B, and A.C. critically revised the article for important intellectual content. D.G., K.E., M.I.M., G.B., A.C., and P.C. had final approval of the article. M.I.M. and G.B. provided study materials or patients. P.C. and D.G. provided statistical expertise. K.E. and D.G. obtained funding. M.I.M., G.B., and D.G. provided administrative, technical, or logistic support. D.G., K.E., M.I.M., G.B., P.C, and A.C. collected and assembled the data.
The authors thank Dr. Veikko Koivisto, Lilly Diabetes Europe, for helpful advice and suggestions in reading the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1710/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:e21–e181 [DOI] [PubMed] [Google Scholar]
- 3.Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes ; Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, Department of Health and Human Services, Centers for Disease Control and Prevention, 2005 [Google Scholar]
- 7.Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15 [DOI] [PubMed] [Google Scholar]
- 8.Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174–183 [DOI] [PubMed] [Google Scholar]
- 9.Hauber A, Gale EAM. The market in diabetes. Diabetologia 2006;49:247–252 [DOI] [PubMed] [Google Scholar]
- 10.Qayyum R, Bolen S, Maruthur N, et al. Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes. Ann Intern Med 2008;149:549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasserson DS, Glasziou P, Perera R, Holman RR, Farmer AJ. Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta-analyses. Diabetologia 2009;52:1990–2000 [DOI] [PubMed] [Google Scholar]
- 12.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ 2009;180:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008;25:924–932 [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W-65–W-94 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1999;17:1–12 [DOI] [PubMed] [Google Scholar]
- 17.Stuart A, Ord JK. Kendall's Advanced Theory of Statistics. 6th ed. London, Edward Arnold, 1994 [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 19.Greenland S. Quantitative methods in the review of epidemiology literature. Epidemiol Rev 1987;9:1–30 [DOI] [PubMed]
- 20.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet 1955;19:251–253 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 22.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med 2001;20:641–654 [DOI] [PubMed] [Google Scholar]
- 23.Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH, Lispro Mixture-Glargine Study Group Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther 2004;26:2034–2044 [DOI] [PubMed] [Google Scholar]
- 24.Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with Type 2 diabetes. Diabet Med 2005;22:374–381 [DOI] [PubMed] [Google Scholar]
- 25.Raskin P, Allen E, Hollander P, et al. INITIATE Study Group Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260–265 [DOI] [PubMed] [Google Scholar]
- 26.Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006;114:527–532 [DOI] [PubMed] [Google Scholar]
- 27.Jacober SJ, Scism-Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin-naïve patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes Obes Metab 2006;8:448–455 [DOI] [PubMed] [Google Scholar]
- 28.Kazda C, Hülstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006;20:145–152 [DOI] [PubMed] [Google Scholar]
- 29.Holman RR, Thorne KI, Farmer AJ.et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 30.Robbins DC, Beisswenger PJ, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2007;29:2349–2364 [DOI] [PubMed] [Google Scholar]
- 31.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 32.Hirao K, Arai K, Yamauchi M, Takagi H, Kobayashi M, Japan Diabetes Clinical Data Management Study Group Six-month multicentric, open-label, randomized trial of twice-daily injections of biphasic insulin aspart 30 versus multiple daily injections of insulin aspart in Japanese type 2 diabetic patients (JDDM 11). Diabetes Res Clin Pract 2008;79:171–176 [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008;31:20–25 [DOI] [PubMed] [Google Scholar]
- 34.Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B, PREFER Study Group Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab 2009;11:45–52 [DOI] [PubMed] [Google Scholar]
- 35.Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009;32:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strojek K, Bebakar WM, Khutsoane DT, et al. Once-daily initiation with biphasic insulin aspart 30 versus insulin glargine in patients with type 2 diabetes inadequately controlled with oral drugs: an open-label, multinational RCT. Curr Med Res Opin 2009;25:2887–2894 [DOI] [PubMed] [Google Scholar]
- 38.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]