Abstract
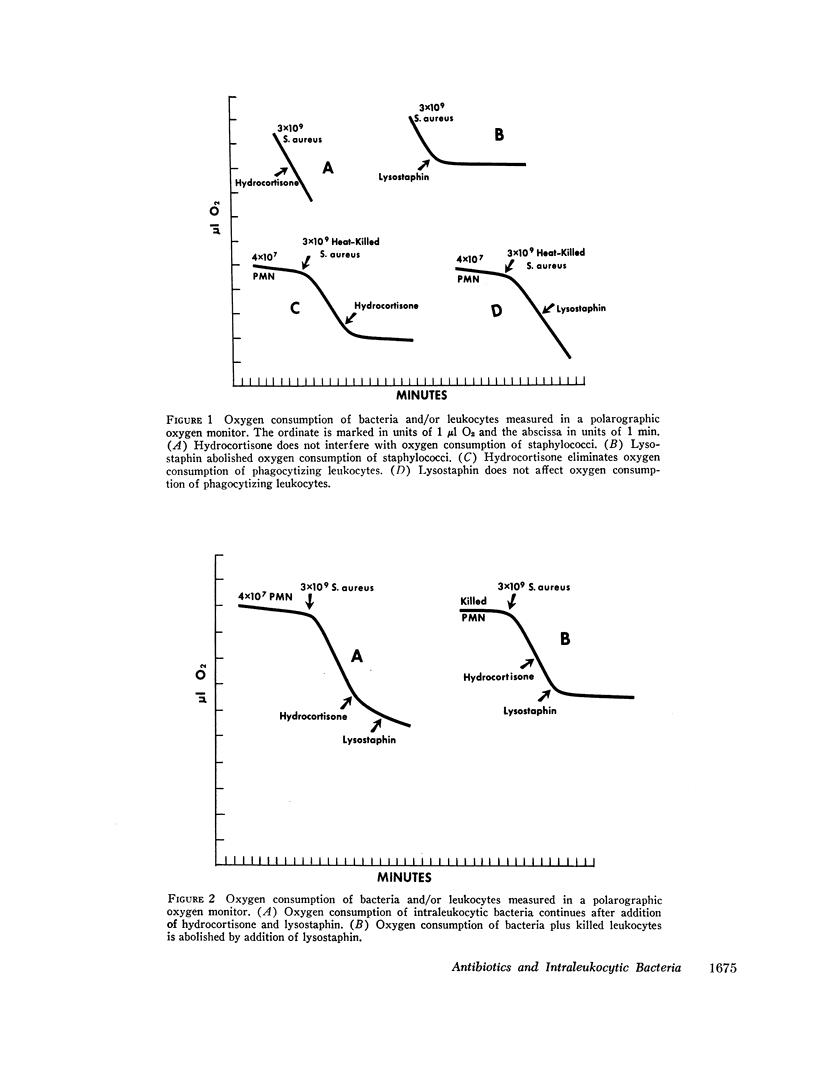
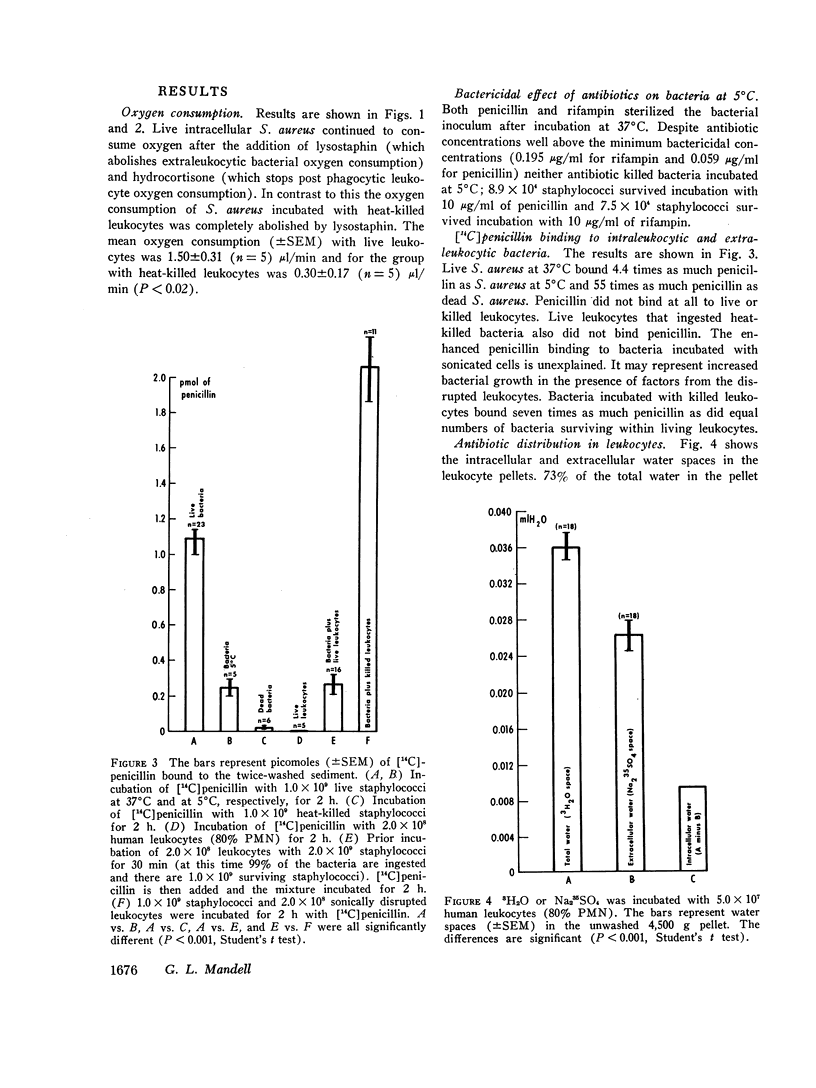
Bacteria that survive inside polymorphonuclear neutrophils (PMN) following phagocytosis are protected from the bactericidal action of most antibiotics. Two possible explanations are altered metabolism by intraleukocytic bacteria or failure of antibiotics to enter the phagosome. The oxygen consumption of intraleukocytic and extraleukocytic bacteria was measured as an index of bacterial metabolism. PMN respiration and bactericidal activity were suppressed with large doses of hydrocortisone and extraleukocytic bacterial oxygen consumption was abolished by the addition of lysostaphin. Intraleukocytic bacterial continued to consume oxygen suggesting that surviving ingested micro-organisms are metabolically active. Neither penicillin (which cannot kill intraleukocytic bacteria) nor rifampin (which can kill intraleukocytic bacteria) was bactericidal for staphylococci at 5°C. Thus, rifampin is not uniquely able to kill “resting” bacteria.
Intraleukocytic or extraleukocytic Staphylococcus aurens were incubated with [benzyl-14C]penicillin for 2 h at 37°C. Live intraleukocytic bacteria bound only 13% as much penicillin as live bacteria incubated with killed PMN. To measure the penetration of antibiotics into PMN, [14C]rifampin and [14C]penicillin were measured in leukocyte pellets and in the supernatant fluid. The total water space in the pellets was quantitated using tritium water and the extracellular water space was measured using Na235SO4. All penicillin associated with the cell pellet could be accounted for in extracellular water. Thus penicillin was completely excluded from the leukocytes. Rifampin was concentrated in the cell pellet 2.2 times when compared with the supernatant concentration.
These studies suggest that a likely explanation for the survival of phagocytized bacteria in the presence of high concentrations of most antibiotics is the inability of the antibiotic to enter the phagocyte. Rifampin, which is highly lipid soluble, can enter leukocytes and kill intracellular bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Good R. A. Effect of antibiotics on the bactericidal activity of human leukocytes. J Lab Clin Med. 1968 Jun;71(6):971–983. [PubMed] [Google Scholar]
- Baron D. N., Ahmed S. A. Intracellular concentrations of water and of the principal electrolytes determined by analysis of isolated human leucocytes. Clin Sci. 1969 Aug;37(1):205–219. [PubMed] [Google Scholar]
- Bonventre P. F., Hayes R., Imhoff J. Autoradiographic evidence for the impermeability of mouse peritoneal macrophages to tritiated streptomycin. J Bacteriol. 1967 Jan;93(1):445–450. doi: 10.1128/jb.93.1.445-450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. I. Correlation between the penicillin sensitivity and combining activity of intact bacteria and cell-free extracts. J Exp Med. 1954 Mar;99(3):207–226. doi: 10.1084/jem.99.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. II. The reactivity with penicillin of resistant variants of streptococci, pneumococci, and staphylococci. J Exp Med. 1954 Jul 1;100(1):103–115. doi: 10.1084/jem.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. III. The binding of penicillin by mammalian cells in tissue culture (HeLa and L strains). J Exp Med. 1954 Jul 1;100(1):117–124. doi: 10.1084/jem.100.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPS H. E., JACKSON E. B., DANAUSKAS J. X., SMADEL J. E. Study on the growth of rickettsiae. IV. Effect of chloramphenicol and several metabolic inhibitors on the multiplication of Rickettsia tsutsugamushi in tissue culture cells. J Immunol. 1959 Feb;82(2):172–181. [PubMed] [Google Scholar]
- HOPPS H. E., SMADEL J. E., BERNHEIM B. C., DANAUSKAS J. X., JACKSON E. B. Effect of antibiotics on intracellular Salmonella typhosa. II. Elimination of infection by prolonged treatment. J Immunol. 1961 Aug;87:162–174. [PubMed] [Google Scholar]
- Lobo M. C., Mandell G. L. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973 Mar;142(3):1048–1050. doi: 10.3181/00379727-142-37173. [DOI] [PubMed] [Google Scholar]
- MAGOFFIN R. L., SPINK W. W. The protection of intracellular brucella against streptomycin alone and in combination with other antibiotics. J Lab Clin Med. 1951 Jun;37(6):924–930. [PubMed] [Google Scholar]
- Maggi N., Pasqualucci C. R., Ballotta R., Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–292. doi: 10.1159/000220462. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Vest T. K. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1972 May;125(5):486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- Patrick J., Hilton P. J. The measurement of the extracellular space in an in vitro system of human leucocytes. Clin Sci. 1972 May;42(5):647–649. doi: 10.1042/cs0420647. [DOI] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O. Protection of phagocytized bacteria against antibiotics. A new method for the evaluation of neutrophil granulocyte functions. Acta Med Scand. 1972 May;191(5):383–387. [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- WERNER C. A., KNIGHT V., McDERMOTT W. Studies of microbial populations artificially localized in vivo. I. Multiplication of bacteria and distribution of drugs in agar loci. J Clin Invest. 1954 May;33(5):742–752. doi: 10.1172/JCI102943. [DOI] [PMC free article] [PubMed] [Google Scholar]


