Abstract
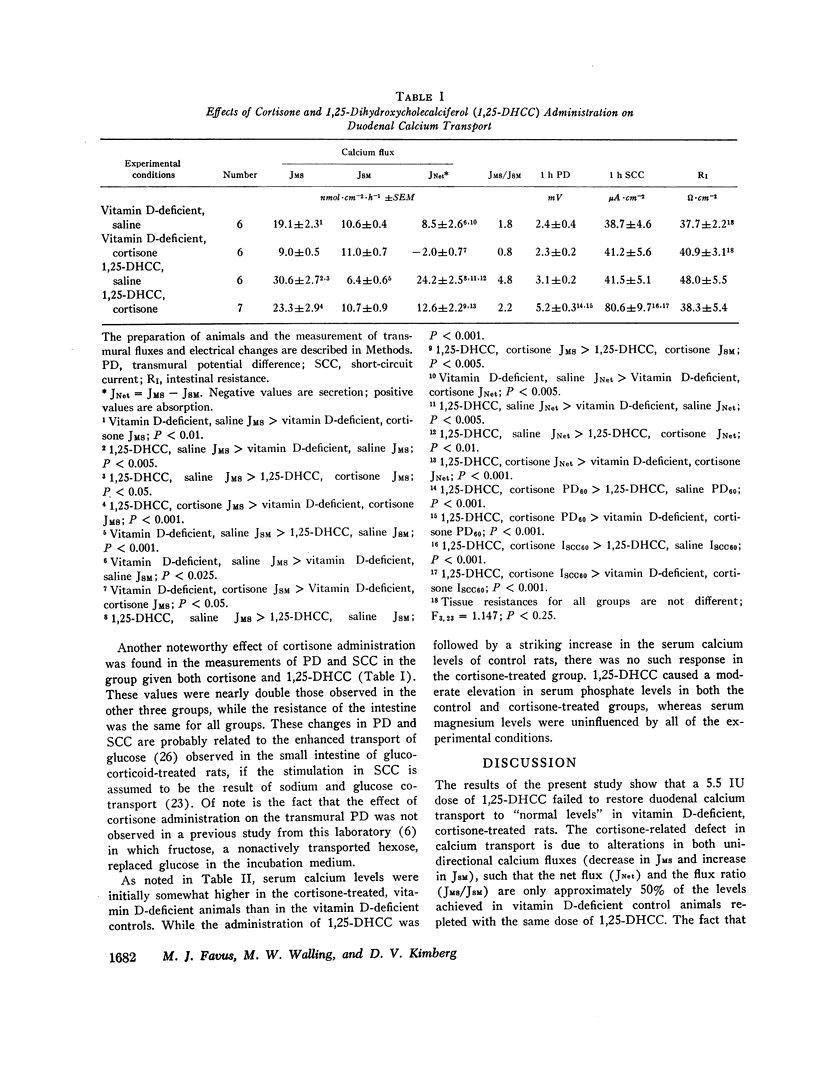
The administration of glucocorticoids may decrease intestinal calcium absorption in vivo and the active transport of calcium in rat duodenum in vitro. It has been suggested that this apparent “anti-vitamin D-like” effect of steroid hormones may be related to alterations in vitamin D metabolism. In order to test this hypothesis, vitamin D-deficient control and cortisone-treated rats were given an intraperitoneal injection of 5.5 IU of 1,25-dihydroxycholecalciferol (1,25-DHCC), the probable end-organ active vitamin D metabolite in the intestine, and 16 h later studies of duodenal calcium transport were performed in modified Ussing chambers. In the vitamin D-deficient state, cortisone administration was associated with a diminution in JMS, JNet, and the flux ratio (JMS/JSM). While the magnitude of the increases in JMS and JNet that resulted from 1,25-DHCC treatment were approximately the same in control and cortisone-treated animals, 1,25-DHCC failed to restore these parameters to “normal levels” in the steroid-treated rats. Furthermore, contrary to the results obtained in the saline-treated controls, 1,25-DHCC failed to reduce JSM in the duodenum from cortisone-treated rats. The cortisone-related defect in calcium transport was due to alterations in both unidirectional calcium fluxes (decrease in JMS and increase in JSM), such that the JNet and the flux ratio (JMS/JSM) were only approximately 50% of the levels achieved in vitamin D-deficient control animals repleted with the same dose of 1,25-DHCC.
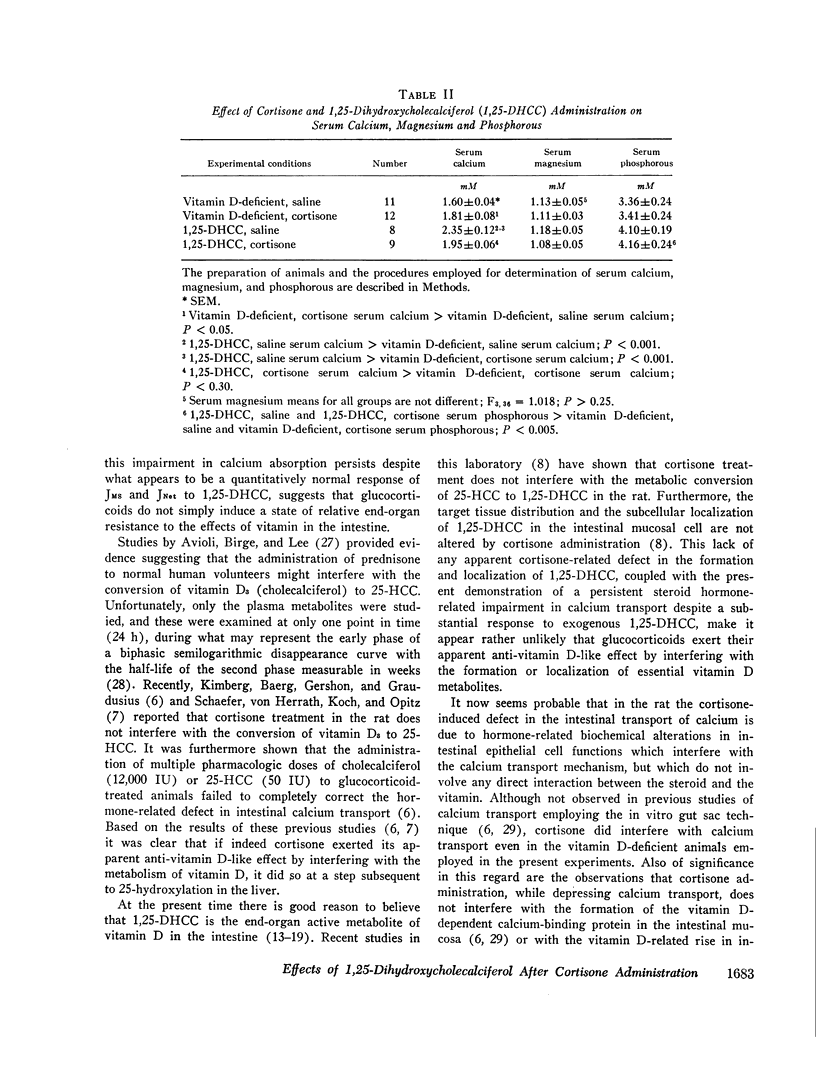
The administration of 1,25-DHCC was accompanied by a marked increase in the serum calcium levels of control rats, but there was no such response in the cortisone-treated group.
The results support the concept that under the conditions of these experiments in the rat the apparent antagonism between glucocorticoids and vitamin D may be due to steroid hormone-related alterations in end organ function that are independent of any direct interaction between the hormone and the vitamin and that cannot be reversed by the vitamin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRIGHT F., CARROLL E. L., DEMPSEY E. F., HENNEMAN P. H. The cause of hypercalcuria in sarcoid and its treatment with cortisone and sodium phytate. J Clin Invest. 1956 Nov;35(11):1229–1242. doi: 10.1172/JCI103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avioli L. V., Birge S. J., Lee S. W. Effects of prednisone on vitamin D metabolism in man. J Clin Endocrinol Metab. 1968 Sep;28(9):1341–1346. doi: 10.1210/jcem-28-9-1341. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Varma S. D. Effect of diabetogenic hormones on transport of glucose in small intestine in vitro. Proc Soc Exp Biol Med. 1966 Oct;123(1):212–213. doi: 10.3181/00379727-123-31445. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Transfer of Ca45 across intestinal wall in vitro in relation to action of vitamin D and cortisol. Am J Physiol. 1960 Aug;199:265–271. doi: 10.1152/ajplegacy.1960.199.2.265. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):803–804. doi: 10.1073/pnas.68.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Baerg R. D., Gershon E., Graudusius R. T. Effect of cortisone treatment on the active transport of calcium by the small intestine. J Clin Invest. 1971 Jun;50(6):1309–1321. doi: 10.1172/JCI106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitt E. L. The role of intestinal transport proteins in cortisone-mediated suppression of Ca 2+ absorption. Biochim Biophys Acta. 1972 Jul 3;274(1):179–188. doi: 10.1016/0005-2736(72)90292-1. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Fraser D. R., Kodicek E., Morris H. R., Williams D. H. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971 Mar 26;230(5291):228–230. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawer E. B., Lumb G. A., Stanbury S. W. Long biological half-life of vitamin D3 and its polar metabolites in human serum. Nature. 1969 May 3;222(5192):482–483. doi: 10.1038/222482a0. [DOI] [PubMed] [Google Scholar]
- Myrtle J. F., Haussler M. R., Norman A. W. Evidence for the biologically active form of cholecalciferol in the intestine. J Biol Chem. 1970 Mar 10;245(5):1190–1196. [PubMed] [Google Scholar]
- Norman A. W., Midgett R. J., Myrtle J. F., Nowicki H. G. Studies on calciferol metabolism. I. Production of vitamin D metabolite 4B from 25-OH-cholecalciferol by kidney homogenates. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1082–1087. doi: 10.1016/0006-291x(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Ponchon G., Kennan A. L., DeLuca H. F. "Activation" of vitamin D by the liver. J Clin Invest. 1969 Nov;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER D., KIMBERG D. V., SCHENKER H. Active transport of calcium by intestine: action and bio-assay of vitamin D. Am J Physiol. 1961 Jun;200:1263–1271. doi: 10.1152/ajplegacy.1961.200.6.1263. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer K., von Herrath D., Koch H. U., Opitz A. Effect of cortisone on vitamin D metabolism. Isr J Med Sci. 1971 Mar;7(3):533–534. [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. Bone mineral mobilization activity of 1,25-dihydroxycholecalciferol, a metabolite of vitamin D. Arch Biochem Biophys. 1971 Oct;146(2):574–578. doi: 10.1016/0003-9861(71)90163-9. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- VERNER J. V., Jr, ENGEL F. L., McPHERSON H. T. Vitamin D intoxication: report of two cases treated with cortisone. Ann Intern Med. 1958 Apr;48(4):765–773. doi: 10.7326/0003-4819-48-4-765. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G. A., BOWSER E. N., HENDERSON W. J., UZGIRIES V. Effects of vitamin D and cortisone on intestinal absorption of calcium in the rat. Proc Soc Exp Biol Med. 1961 Mar;106:664–666. doi: 10.3181/00379727-106-26436. [DOI] [PubMed] [Google Scholar]
- Wajchenberg B. L., Pereira V. G., Kieffer J., Ursic S. Effect of dexamethasone on calcium metabolism and 47Ca kinetics in normal subjects. Acta Endocrinol (Copenh) 1969 May;61(1):173–192. doi: 10.1530/acta.0.0610173. [DOI] [PubMed] [Google Scholar]
- Walling M. W., Rothman S. S. Phosphate-independent, carrier-mediated active transport of calcium by rat intestine. Am J Physiol. 1969 Oct;217(4):1144–1148. doi: 10.1152/ajplegacy.1969.217.4.1144. [DOI] [PubMed] [Google Scholar]


