Abstract
The cylindromatosis tumor suppressor (CYLD) is a deubiquitinating enzyme that has been implicated in various aspects of adaptive and innate immune responses. Nevertheless, the role of CYLD in the function of specific types of immune cells remains elusive. In this report we have used conditional gene targeting in mice to address the role of the deubiquitinating activity of CYLD in the myelomonocytic lineage. Truncation of the deubiquitinating domain of CYLD specifically in myelomonocytic cells impaired the development of lethal LPS-induced endotoxic shock and the accumulation of thioglycollate-elicited peritoneal macrophages. Our data establish CYLD as a regulator of monocyte-macrophage activation in response to inflammatory stimuli and identify it as a potential target for therapeutic intervention in relevant inflammatory disorders in humans.
Introduction
Cyld was identified as the predisposition gene for the tumor syndrome of familial cylindromatosis. It mediates the suppression of the NF-κB, JNK, p38 and Wnt pathways in a manner that depends on its deubiquitinating activity (reviewed in [1]). The use of mouse models of CYLD deficiency has implicated the protein in the regulation of multiple physiological processes including T cell development and activation, B cell homeostasis and function, osteoclastogenesis and spermatogenesis (Reviewed in [2]). Moreover, CYLD downregulation sensitizes mice to various pathological conditions that include chemically induced skin tumor formation as well as colitis-associated cancer [3]–[4]. Interestingly, in all these cases a broad range of CYLD-targeted proteins could be recognized, establishing CYLD as a multi-tasking deubiquitinating enzyme implicated in the fine tuning of many developmental and physiological processes.
A number of studies have provided compelling evidence for the implication of CYLD in various aspects of innate immunity. More specifically, it has been shown that CYLD constitutes a key negative regulator of NF-κB and JNK in macrophages from Cyld null mice that have been treated with CD40 or ligands against TLR4 and TLR2 [4]. Another study showed that Cyld-deficient mice were more resistant to lethal pulmonary infection caused by Streptococcal pneumoniae. This was attributed to enhanced p38 activation and subsequent elevated expression of PAI-1 [5], [6]. However, a third study did not recognize any difference in the activity of NF-κB or MAPKs in macrophages following treatment with TNF or TLR stimulation, using an independently derived mouse model of Cyld deficiency [7]. In all the aforementioned studies mice bearing obligatory null alleles were used making it impossible to discern the cell-specific contribution of CYLD to the observed phenotype [8].
In the present study we employed a conditional gene targeting approach to introduce a cell-specific deubiquitinating-domain-truncating mutation in the Cyld locus (CyldΔ9) and investigate a plausible implication of CYLD in the development and function of the myelomonocytic lineage. In accordance with previous studies no obvious hematopoietic defects were observed. However, CyldΔ9 mice exhibited increased resistance to LPS induced endotoxic shock. Moreover, in an in vivo model of induced peritonitis CYLD-deficient macrophages failed to accumulate to the inflammatory site. Overall, our data demonstrate that Cyld is dispensable for hematopoiesis but unravel a cell intrinsic function of Cyld in monocyte-macrophage response to inflammatory stimuli.
Results and Discussion
Generation and characterization of M-CyldΔ9 mice
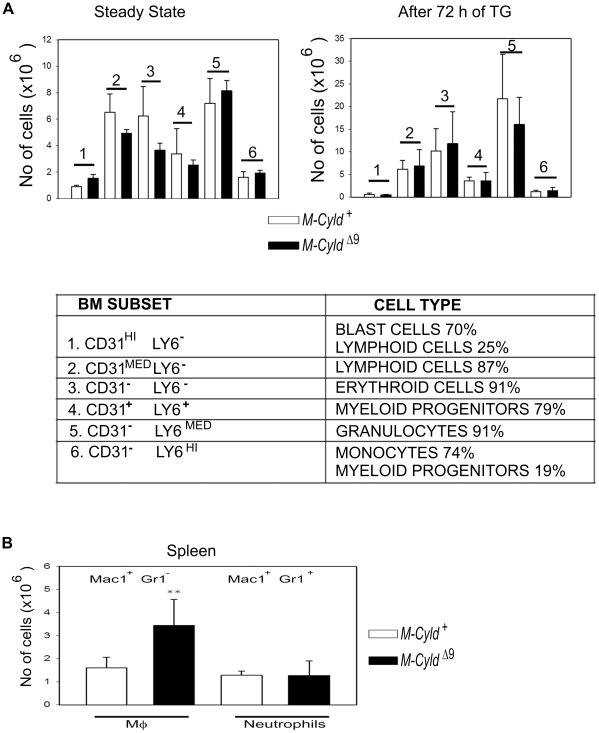
The generation of mice with loxP sites flanking the exon 9 of the Cyld locus (Cyld flx9/flx9), has been described elsewhere [9]. By crossing these mice to LysMcre transgenic mice we aimed to render murine CYLD catalytically inactive in myelomonocytic cells, by truncating its catalytic domain through Cre-mediated excision of exon 9[9], [10]. In accordance with the expression of the Cre recombinase in myelomonocytic cells in LysMCre-Cyldflx9/flx9 mice (termed M-CyldΔ9 mice from this point onwards), the effective excision of Cyld exon 9 was readily evident both in bone marrow purified progenitor cells (Fig. 1A) and in splenic sorted macrophages (Fig. 1B). Finally, immunoblotting with an anti-CYLD antibody indicated a nearly complete absence of full length CYLD protein in M-CyldΔ9 bone marrow derived macrophages (BMDMs, Fig. 1C). M-CyldΔ9 mice were viable and normal in appearance. Since CYLD has been implicated in T cell development [7], [11] the hematopoietic development in M-CyldΔ9 mice was evaluated. No gross hematopoietic defect was detected by flow cytometric analysis of the combined expression of Ly6C and CD31 surface markers both in steady state conditions and after thioglycollate-induced peritonitis that was adopted in order to evaluate the hematopoietic capacity of M-CyldΔ9 mice under stress (Fig. 2A). Interestingly, the higher numbers of splenic macrophages in M-CyldΔ9 mice (Figure 2B) further support the fact that there is not a hematopoietic defect.
Figure 1. Generation and characterization of mice with inactivation of CYLD specifically in myelomonocytic cells.
Cyld recombination is detected by genomic PCR in Lin− precursor cells isolated from bone marrow (A) as well as in mature macrophages sorted from spleen (B). C) Immunoblotting of whole cell extracts from BMDMs isolated from control and M-Cyld Δ9 mice with anti-CYLD and anti-ACTIN antibodies.
Figure 2. Normal hematopoietic development in M-CyldΔ9 mice.
A) Enumeration of the indicated hematopoietic lineage cells (lower panel) by flow cytometric analysis of bone marrow cells for the expression of Ly6c and CD31 surface markers at steady state conditions (upper left panel) and after 72 h of thioglycollate treatment (upper right panel). In the first case 5 control and 3 M-CyldΔ9 mice were evaluated whereas in the second case 7 control and 5 M-CyldΔ9 mice were evaluated. B) Monocyte lineage populations in spleen. Enumeration of cells in the spleen that were collected from control (M-Cyld+) and mutant (M-CyldΔ9) mice 72 hours after thioglycollate injection. Data are depicted as mean absolute numbers (±SEM) from n = 7 control and 5 M-CyldΔ9 mice at 8 weeks of age. The statistically significant difference in the macrophage populations (Mac1+ Gr1−) of control and mutant mice is depicted by two stars (p<0,001, Student's unpaired t test).
M-CyldΔ9 mice are less susceptible to LPS-induced endotoxic shock
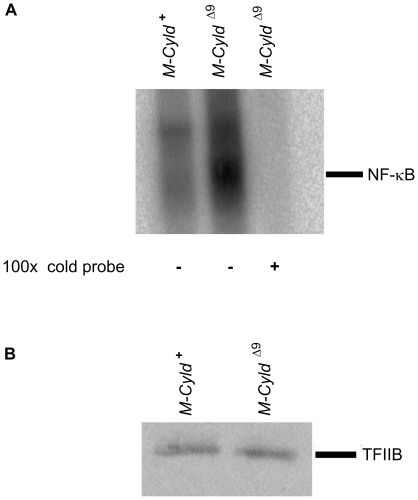
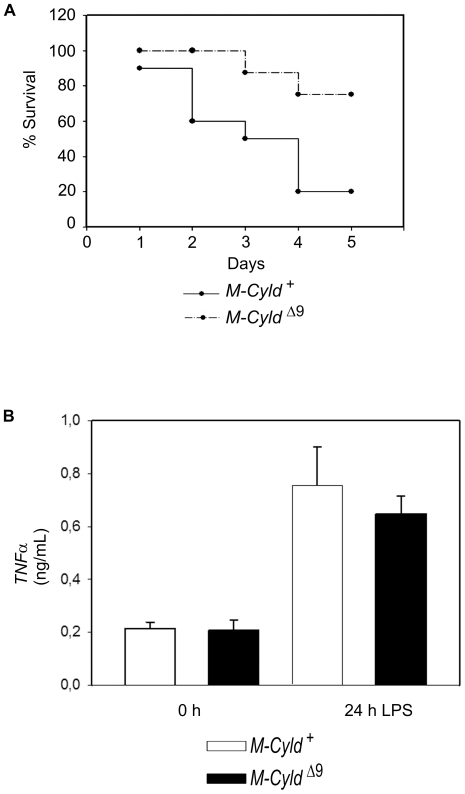
Macrophages constitute a central player of the innate immune response. The NF-κB pathway has been implicated in many aspects of macrophage activation and function. p50/NF-κB1 −/− mice show increased susceptibility to LPS-induced endotoxic shock due to decreased IL-10 production [12]. Interestingly, increased ubiquitination and degradation of p50 was observed in Bcl3 −/− macrophages leading to the abolishment of LPS tolerance and the increased susceptibility of mice that lack BCL3 to LPS-induced shock [13]. Since CYLD has been shown to be a negative regulator of the NF-κB pathway in various cell types, its ability to modulate the activity of NF-κB in CyldΔ9 BMDMs was evaluated by EMSA. As expected, the basal NF-κB activity was significantly elevated in CyldΔ9 BMDMs in comparison to control BMDMs (Fig. 3). This finding prompted an investigation into the response of M-CyldΔ9 mice to LPS. M-CyldΔ9 and control mice were injected with 32 mg/kg of bacterial LPS. While only 20% of the control mice survived, the survival rate of M-CyldΔ9 mice reached 80%. Interestingly, M-CyldΔ9 mice not only showed increased resistance but also increased endurance since they died later than the control animals (Fig. 4A). Moreover, the levels of secreted TNFα following LPS treatment were not higher in M-CyldΔ9 BMDMs compared to control BMDMs. Instead, there was a tendency for lower secretion of TNFα by M-CyldΔ9 BMDMs following LPS treatment, even though this was not statistically significant (Figure 4B). The response of M-CyldΔ9 mice to LPS-induced endotoxemia was surprising, given the hyperactivation of NF-κB in M-CyldΔ9 BMDMs. A possible explanation for the observed phenotype could be the adoption of alternative activation by M-CyldΔ9 macrophages, which leads to the secretion of anti-inflammatory cytokines such as IL-10 [14]. Interestingly, NF-κB can exert an anti-inflammatory role besides its well known proinflammatory activity, which mediates the resolution of inflammation [15]. Moreover, it has been shown that conditional inactivation of IKK2 in myelomonocytic cells and subsequent endotoxin challenge of the resultant mice leads to increased endotoxin induced mortality and increased levels of IL-1β. Prolonged pharmacological inhibition of IKKβ, which interferes with NF-κB activation in the whole animal, also increased LPS-induced mortality and plasma IL-1β[16]. Therefore a plausible scenario would be that in M-CyldΔ9 mice the aberrant activation of NF-κB leads to suppression of IL1β generation as well as of the secretion of other inflammatory cytokines. Indeed, our findings are consistent with the resistance of Cyld null mice to lethality following acute lung injury by Streptococcus pneumoniae [6] and suggest that the lack of functional CYLD in macrophages may contribute to the increased survival of Cyld null mice. The availability of M-CyldΔ9 mice will permit the experimental evaluation of this hypothesis in order to pinpoint the cellular basis of Cyld-deficient mice resistance to lethal lung injury by Streptococcus pneumonia.
Figure 3. Elevated basal activity of NF-κB in CyldΔ9 BMDMs.
A) EMSA of NF-κB DNA binding activity in BMDMs from control (M-Cyld+) and mutant (M-CyldΔ9) mice in the absence or presence of 100-fold excess of unlabeled probe (100× cold probe) as indicated. The positions of NF-κB-containing complexes of the radiolabeled probe are shown. B) Immunoblot of nuclear extracts used in A with an anti-TFIIB antibody. Three mice per genotype were evaluated.
Figure 4. Attenuation of LPS-induced endotoxic shock in M-CyldΔ9 mice.
A) Ten control (4 Cyld flx9/+ and 6 Cyld flx9/+ LysMcre) and eight M-CyldΔ9 mice were subjected to LPS induced endotoxic shock (32 mg LPS/kg) and survival was monitored for the next 5 days. M-CyldΔ9 mice exhibited increased resistance to endotoxemia in comparison to control mice. Data are depicted as mean absolute numbers (±SEM) from n = 10 control and 8 M-CyldΔ9 mice at 8 weeks of age. (p value = 0,02 as assessed by Student's unpaired t test). B) Concentrations of TNFα were measured by enzyme-linked immunosorbent assay in supernatants from control and M-CyldΔ9 BMDMs before and 24 h after treatment with 100 ng/mL LPS. The results shown are the means (±S.D.) of triplicate measurements. Control BMDMs were isolated from 3 wild type and 4 Cyldflx9/+LysMCre mice and M-CyldΔ9 BMDMs were isolated from 4 mice.
M-CyldΔ9 mice show decreased accumulation of thioglycollate-elicited peritoneal macrophages
In order to investigate further the implication of Cyld in the inflammatory response we employed an in vivo model of macrophage recruitment by inducing chemically aseptic peritonitis. Mice were intraperitoneally injected with thioglycollate, an extensively used inflammatory stimulus that induces the recruitment of neutrophils and macrophages to the peritoneal cavity [17]. In this model, neutrophil number reaches a maximum at 8 hours, while macrophages migrate with a slow time course, reaching a maximum at 72 hours and remaining at this level for at least 96 hours [18]. At 24 hours after thioglycollate injection similar numbers of cells were recovered from the peritoneal cavity of control and mutant mice (data not shown). However, at 72 hours after thioglycollate injection, there were significantly fewer cells recovered from the peritoneal cavity of M-CyldΔ9 mice compared with control mice (approximately 6 million from M-CyldΔ9 mice versus more than 20 millions from control mice, p = 0,0000000248) (Fig. 5A). Interestingly, the number of the recruited macrophages (CD11b+Gr1−) and neutrophils (CD11b+Gr1+) were dramatically decreased in M-CyldΔ9 mice in comparison to control mice (Fig. 5B). Notably, the CyldΔ9 macrophages that were recruited to the inflammatory site exhibited similar expression pattern of the activation maker F4/80 (data not shown). At this point it is not clear whether CyldΔ9 macrophages cannot be recruited due to defects in the migration process itself as a result of microtubule reorganization problems [19], [20] or if they cannot «perceive» the inflammatory stimulus due to signaling defects. Alternatively it is also possible that the reduced accumulation of Cyld-deficient macrophages at the site of inflammation reflects an increased propensity of these cells to death following their recruitment to the site of inflammation. Nevertheless, it is undisputable that in the context of chemically induced aseptic peritonitis CyldΔ9 macrophages are unable to respond properly.
Figure 5. Impaired accumulation of thioglycollate-elicited peritoneal macrophages in M-CyldΔ9 mice.
A) Enumeration of cells in the peritoneal cavity fluid that was collected from control (M-Cyld+) and mutant (M-CyldΔ9) mice 72 hours after thioglycollate injection. Data are depicted as mean absolute numbers (±SEM) from n = 7 control and 7 M-CyldΔ9 mice at 8 weeks of age. The statistically significant difference in peritoneal cavity cellularity between control and mutant mice is depicted by two stars (p = 0,0000000064, Student's unpaired t test). (lower panel). B) Enumeration of macrophages (Mac1+Gr1−) and neutrophils (Mac1+Gr1+) from control (M-Cyld+) and mutant (M-CyldΔ9) mice as assessed by flow cytometric analysis of cells harvested from the inflamed peritoneum 72 hours after thioglycollate injection by means of CD11b and Gr1 expression. Data are depicted as mean absolute numbers (±SEM) from n = 7 control and 7 M-CyldΔ9 mice at 8 weeks of age. The statistically significant difference in peritoneal cavity cellularity between control and mutant mice is depicted by two stars (p<0,01 unpaired t test).
The development and characterization of M-CyldΔ9 mice permitted the functional evaluation of CYLD in the myelomonocytic lineage and revealed an anti-inflammatory element of the functional profile of this molecule. Our findings add to a growing list of evidence that highlight the discrete and in certain cases contradictory tissue-specific activities of CYLD. For example, inactivation of CYLD in thymocytes leads to excessive apoptosis and impaired development [7], [11] whereas in peripheral T and B cells ablation of Cyld expression causes their hyperresponsiveness which can be associated with spontaneous autoimmune and inflammatory symptoms [21], [22]. The tissue-specific biological function of CYLD can be associated at least partly, with the differential role of the transcription factor NF-κB, which is regulated by CYLD, in different cellular contexts. Taken together with previous reports, the results of the present study highlight the importance of evaluating the functional role of genes by tissue-specific targeting, in order to clarify possibly contradictory findings that may stem from the compound effect of a gene's ablation in multiple tissues.
In summary, our experiments identified a cell-intrinsic requirement of functional CYLD for the efficient response of macrophages to different inflammatory stimuli and raise the possibility of targeting CYLD with inhibitory molecules as a pharmacological approach to control certain cases of pathogenic inflammation.
Materials and Methods
Ethics statement
Experiments on live animals were approved by the Hellenic Ministry of Rural Development-(Directorate of Veterinary Services, approval ID: 3926) and by Biomedical Sciences Research Center “Al. Fleming's Animal Research and Ethics Committee for compliance to FELASA regulations (approval ID: 2140).
Mice
The generation of Cyld flx9]/flx9 mice has been described elsewhere [9]. Cyld flx9]/flx9 mice were crossed with LysMcre mice [10] in order to mutate Cyld in myelomonocytic cells. The mice were bred and maintained in the animal facilities of the Biomedical Sciences Research Centre “Alexander Fleming” under specific-pathogen free conditions.
BMDM preparation
BMDM were generated as previously described [23].
LPS induced endotoxic shock
Mice of 8 weeks age were intraperitoneally injected with a sublethal dose of 800 µg LPS (Sigma)/25 g animal weight and survival was monitored for 5 days.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared by BMDMs and EMSA was performed as previously described [9].
The sequence of the oligonucleotides used for NF-κB with two tandemly repeated NF-κB binding sites (underlined) were as follows:
NF-κBf: 5′ -ATC AGG GAC TTT CCG CTG GGG ACT TT- 3′
NF-κBr: 5′-CGG AAA GTC CCC AGC GGA AAG TCC CT-3′
In Vivo migration assay
Control and M-Cyld Δ9 mice were injected i.p. with 1 ml of 4% sterile thioglycollate (Becton Dickinson). 72 h later mice were sacrificed and the leukocyte number in the peritoneal lavage was assessed by a Coulter Counter. The percentage of the different cell types was assessed by Flow Cytometry.
Flow Cytometry and Cell Sorting
Cell-associated fluorescence was analyzed by a FACS Aria or a FACSCantoII flow cytometer and the DIVA V6 software (Becton Dickinson). Sorting was performed with BD FACS Vantage SE II. Flow cytometry figures were prepared using the FlowJo software (Tree Star, Inc). Differences in populations were determined using the unpaired t test as calculated with Sigmaplot 9 statistical software.
ELISA
For the TNFα (provided by W. Buurman, Nutrition and Toxicology Research Institute Maastricht, Maastricht, Netherlands) ELISA assay, BMDMs cultures (3×105 cells/well) were incubated with 100 ng/ml LPS (Salmonella enteriditis; Sigma-Aldrich) and 24 h later the culture supernatants were collected.
Antibodies
Cells were stained with Ly6c, CD31, CD11b, Gr1, CD16/32(BD Pharmingen), F4/80 (eBioscience), Lin- enriched fraction from bone marrow was prepared by staining with biotinylated Ter119, B220, Cd11b, Gr1, CD3 (eBioscience). Cylindromatosis and TFIIB were purchased from Santa Cruz. The anti-actin mouse monoclonal antibody was purchased from MP Biomedical Inc.
Acknowledgments
The authors thank the Flow Cytometry Core Facility of B.S.R.C. Al. Fleming and its operator, Dr S. Grammenoudi for the help with the FACS sorting, and Ms Anastasia Apostolidou, the operator of the Flow Cytometry Core Facility of Biomedical Research Foundation of the Academy of Athens for her precious help during the initial flow cytometry experiments for this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the General Secretariat for Research and Technology of the Hellenic Ministry of Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25–34. doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Stirling B, Temmerman ST, Ma CA, Fuss IJ, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116:3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JH, Ha UH, Woo CH, Xu H, Li JD. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol. 2008;10:2247–2256. doi: 10.1111/j.1462-5822.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim JH, Stirling B, Derry J, Koga T, Jono H, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 8.Kolls JK. Balancing mucosal immunity: caught between CYLD and Charybdis. Immunity. 2007;27:187–189. doi: 10.1016/j.immuni.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Trompouki E, Tsagaratou A, Kosmidis SK, Dolle P, Qian J, et al. Truncation of the catalytic domain of the cylindromatosis tumor suppressor impairs lung maturation. Neoplasia. 2009;11:469–476. doi: 10.1593/neo.81424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 11.Tsagaratou A, Trompouki E, Grammenoudi S, Kontoyiannis DL, Mosialos G. Thymocyte-specific truncation of the deubiquitinating domain of CYLD impairs positive selection in a NF-kappaB essential modulator-dependent manner. J Immunol. 2010;185:2032–2043. doi: 10.4049/jimmunol.0903919. [DOI] [PubMed] [Google Scholar]
- 12.Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 2006;281:26041–26050. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 14.Timmer AM, Nizet V. IKKbeta/NF-kappaB and the miscreant macrophage. J Exp Med. 2008;205:1255–1259. doi: 10.1084/jem.20081056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 16.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellinghoff I, Daibata M, Humphreys RE, Mulder C, Takada K, et al. Early events in Epstein-Barr virus genome expression after activation: regulation by second messengers of B cell activation. Virology. 1991;185:922–928. doi: 10.1016/0042-6822(91)90574-u. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Huo L, Sun X, Liu M, Li D, et al. The tumor suppressor CYLD regulates microtubule dynamics and plays a role in cell migration. J Biol Chem. 2008;283:8802–8809. doi: 10.1074/jbc.M708470200. [DOI] [PubMed] [Google Scholar]
- 20.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 21.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin W, Reiley WR, Lee AJ, Wright A, Wu X, et al. Deubiquitinating enzyme CYLD regulates the peripheral development and naive phenotype maintenance of B cells. J Biol Chem. 2007;282:15884–15893. doi: 10.1074/jbc.M609952200. [DOI] [PubMed] [Google Scholar]
- 23.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, et al. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]