Abstract
Objective
This study was designed to evaluate the effects of the HIV protease inhibitor lopinavir/ritonavir on gingival epithelium growth, integrity and differentiation.
Methods
Organotypic (raft) cultures of gingival keratinocytes were established and treated with a range of lopinavir/ritonavir concentrations. To examine the effect of lopinavir/ritonavir on gingival epithelium growth and stratification hematoxylin and eosin staining was performed. To investigate the effect of this drug on tissue integrity, transmission electron microscopy (TEM) was performed on untreated and drug treated tissues. Further, immunohistochemical analysis of raft cultures was performed to assess the effect of lopinavir/ritonavir on the expression of key differentiation and proliferation markers including cytokeratins, PCNA and cyclin A.
Results
Lopinavir/ritonavir treatments drastically inhibited the growth of gingival epithelium when the drug was present throughout the growth period of the tissue. When drug was added at day 8 of tissue growth, lopinavir/ritonavir treatments compromised tissue integrity over time and altered the proliferation and differentiation of gingival keratinocytes. Expression of cytokeratins 5, 14, 10, 6, PCNA and cyclin A were induced, and their expression patterns were also altered over time in treated rafts.
Conclusions
The findings of our studies suggest that lopinavir/ritonavir treatments compromised tissue integrity over time and deregulated the cell cycle/proliferation and differentiation pathways resulting in abnormal epithelial repair and proliferation. Our study provides a potential model which gives insight in the area studying antiretroviral drugs affects in vitro.
Keywords: HIV infections, antiretroviral therapy, oral complications, lopinavir/ritonavir, cytokeratins
Introduction
Infection with human immunodeficiency virus (HIV) is a major health problem, with an estimated 33.4 million people living with HIV worldwide [1]. The introduction of antiretroviral drugs, especially protease inhibitors, have markedly decreased mortality and greatly improved the life expectancy of HIV positive patients [2,3]. In addition, the prevalence of some oral complications in these patients, especially oral candidiasis and oral hairy leukoplakia have dropped significantly [4–6]. In contrast, other complications such as Kaposi’s sarcoma and oral apthous ulceration have shown no significant changes [5–7]. Despite having many beneficial effects on HIV positive patients, highly active antiretroviral therapy (HAART) can give rise to several adverse oral effects. Long term use of HAART has been associated with oral warts [5,7], erythema multiforme [8,9], xerostomia [8,9], toxic epidermal necrolysis, lichenoid reactions [8,10], exfoliative cheilitis [8], oral ulceration and paresthesia [9,11]. Therefore, in HIV patients undergoing HAART treatment, adverse oral health may compromise adherence to drug regimens resulting in suboptimal exposure to the drugs. As a consequence, drug resistance could compromise future therapy [12].
Lopinavir/ritonavir (trade name Kaletra), manufactured by Abbott Laboratories, was approved in 2000 by the U.S. Food and Drug Administration (FDA) for the treatment of HIV infection in adults and children. This drug is a co-formulation of lopinavir and sub-therapeutic dose of ritonavir. Administered alone, lopinavir exhibits poor bioavailability, however, the sub-therapeutic dose of ritonavir included in this drug (a potent cytochrome P450 [CYP] 3A4 inhibitor) inhibits the metabolism of lopinavir, resulting higher blood levels of lopinavir [13]. Further, lopinavir is the active ingredient in this drug that provides the anti-HIV activity. Abbott therefore pursued a strategy of co-administering lopinavir with sub-therapeutic doses of ritonavir. Therefore, lopinavir is only marketed as a co-formulation with ritonavir. It is the first combination pill to contain a drug (lopinavir) not available individually [13]. Similar to other protease inhibitors, prolonged use of lopinavir/ritonavir was reportedly associated with several adverse orofacial effects [14–16].
The oral epithelium functions as a protective barrier against environmental stress. A compromised epithelial layer allows microorganisms and toxic materials to access the underlying tissues. To maintain a functional epithelial lining, epithelial cells undergo a well defined differentiation program resulting in the expression of several structural proteins whose design is to maintain the integrity of the epithelial tissues [17]. Normal structural integrity and function of the oral epithelium is still susceptible to damage resulting from its masticatory function. Normally, the high rate of growth allows for a rapid wound healing response when there is a breach in the epithelial lining. Therefore, differential changes in the rate of epithelial turnover during treatment with HAART may significantly affect acquisition of oral disease.
Cytokeratins are a subfamily of intermediate filament proteins and are the fundamental markers of epithelial differentiation. These proteins show considerable heterogeneity and specificity among epithelial tissues, and their expression varies with proliferation and differentiation and state of development [18]. Cytokeratin filaments specifically interact with the specialized plasma membrane domains termed desmosomes. Desmosomes are a major component of cellular adhesion by acting both as cell to cell connection points, and as attachment sites for the intermediate filaments. As a result desmosomes are important for the maintenance of tissue integrity [19].
The protease inhibitors including lopinavir/ritonavir have been shown to inflict several adverse oral complications. However, the effects of these drugs on oral epithelium have not been studied widely. We have initiated studies to analyze the effect of antiretroviral drugs on the growth of oral epithelium. Previously, we have reported the effects of Amprenavir, a protease inhibitor, on the growth and differentiation of gingival epithelium, using the organotypic (raft) tissue culture model system [20]. In the present study, we explore the effects of lopinavir/ritonavir on gingival epithelium growth and differentiation as determined from the expression patterns of key proliferation and differentiation markers.
Methods
Isolation of gingival keratinocytes and growth in organotypic cultures
Gingival keratinocytes were isolated from human gingival tissue as previously described [20]. Briefly, the mixed pool of gingival tissues was obtained from patients undergoing dental surgery. To maintain confidentiality, gingival samples were devoid of any identification such as name, race, age and religion. Approval to collect patient samples was obtained from the Penn State University College of Medicine Institutional Review Board (IRB# 25284). The connective tissue and dermis was removed from the epithelium and discarded. The epithelial tissue was washed three times with PBS containing 50 µg/ml gentamycin sulfate (Gibco BRL, Bethesda, MD, USA) and 1X nystatin (Sigma Chemical Co., St. Louis, MO, USA). The epithelial tissue was then minced with scissors and trypsinized into a single cell suspension in a spinner flask. The suspension was removed, 20 ml of E media containing 5% FCS was added and cells were pelleted by centrifugation. The supernatant was aspirated and the cell pellet was resuspended in 1 ml of 154 media (Cascade Biologics, Inc.) supplemented with Human Keratinocyte Growth Supplement Kit (Cascade Biologics, Inc.) then added to a 10 cm tissue culture plate containing an additional 7 ml of 154 media. This process was repeated three times. When cultures reached ≈70% confluent they were split 1:3, when the plates of the first passage were more than 70% confluent, the cells were used for growing raft cultures.
Raft cultures were grown as previously described [20–22]. Briefly, human gingival epithelial keratinocytes were seeded onto collagen matrices containing J2 3T3 mouse fibroblast feeders. When the epithelial keratinocytes were attached to the dermal equivalent, the collagen matrices were lifted onto stainless steel grids at the air-liquid interface. The raft cultures were fed by diffusion from below with E-media supplemented with lopinavir/ritonavir. We chose Cmax (peak concentration of this drug in blood serum) as our baseline concentration plus two lower and two higher concentrations from the Cmax for our treatments. Earlier studies have shown that the drugs level is almost same in blood serum and in saliva [23,24,25]. We also assumed that the blood level of lopinavir/ritonavir would be the same as in the saliva. The Cmax of lopinavir/ritonavir is 9.8 ± 3.7 µg/ml [13]. In the first set of experiments, the rafts were treated from the first day with a range of lopinavir/ritonavir concentrations: 3, 6, 9.8, 13.5 and 16 µg/ml. Vehicle control rafts were fed with E-media containing an equivalent volume of 70% ethanol. Based on our standard lab protocol [22], rafts cultures were fed every other day with fresh medium at which time the drugs treatments were also replenished. The rafts were harvested at day 4, 8, 12 and 16.
In the second set of experiments, the rafts were fed with E-media only for 7 days and on the day 8th the rafts were treated with lopinavir/ritonavir at the concentrations stated above. The rafts were fed every other day and harvested at 2, 4, 6 and 8 days post treatment.
Histochemical analyses
Raft cultures were harvested, fixed in 10% buffered formalin, and embedded in paraffin. Four micrometer sections were cut and stained with hematoxylin and eosin as described previously [21].
Immunostaining was done using a Vectastain Elite ABC kit (Vector laboratories Burlingame, CA, USA) [21]. Briefly, slides were baked at 55°C in a vacuum oven for 1 h. Tissue sections were dehydrated in xylene and rehydrated in alcohol gradients. Endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide. Then sections were blocked for 1 h with 3% normal horse serum and 20% normal goat serum for primary mouse antibodies and rabbit antibodies, respectively. Primary antibodies used were mouse monoclonal keratin 5 (clone XM26, dilution 200 µg/ml), keratin 14 (clone LL002, dilution 200 µg/ml), keratin 10 (clone DE-K10, dilution 200 µg/ml), keratin 6 (clone LHK6B, dilution 10 ng/ml) (all from Lab Vision, Fremont, CA, USA), rabbit polyclonal PCNA (clone FL-261, dilution 2 µg/ml) and cyclin A (clone H-432, dilution 4 µg/ml) (both from Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) and incubated for 1 h. After two washings in PBS, a biotin labeled secondary antibody was applied for 30 min and then rinsed two times in PBS. A streptavidin/peroxidase complex was used to bind the biotin tag and color visualization of the complex was achieved with 3, 3’-diaminobenzidine (DAB).
Transmission electron microscopy (TEM)
Epithelial tissues were cut into small pieces and fixed in fixative solution (2.5% glutaraldehyde and 2% paraformaldehyde buffered with 0.1 M sodium cacodylate, pH 7.3). Following fixation, tissues were washed in 0.1 M sodium cacodylate buffer. Tissues were then dehydrated in a graded series of ethanol and embedded in EmBed-812. Thin sections (70–90 nm) were cut on a Sorvall MT-2B ultramicrotome using a diamond knife and mounted on 200 mesh copper grids and stained with uranyl acetate followed by lead citrate. Thin sections were viewed in a JEOL JEM1400 Transmission Electron Microscope (JEOL USA, Inc, Peabody, MA, USA).
Results
Effect of lopinavir/ritonavir on morphological differentiation and stratification of gingival keratinocytes in raft cultures
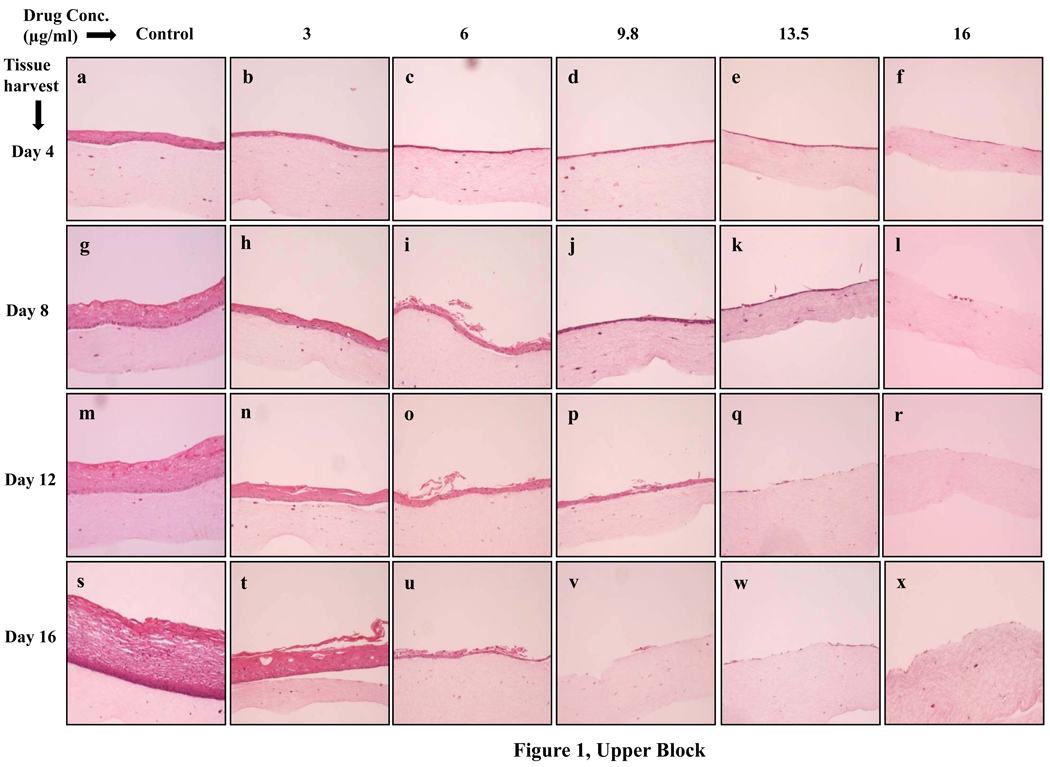
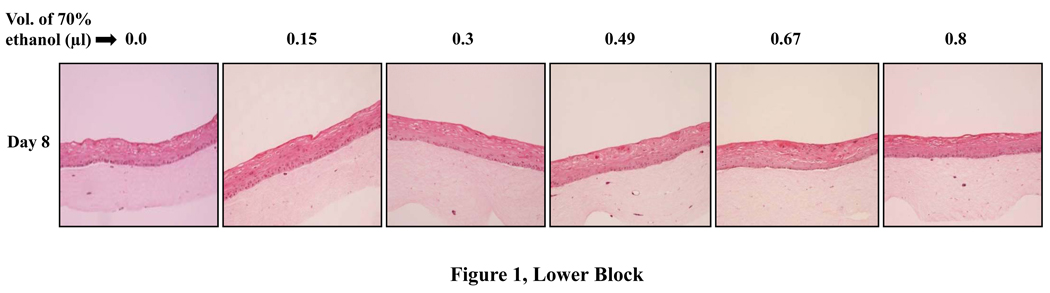
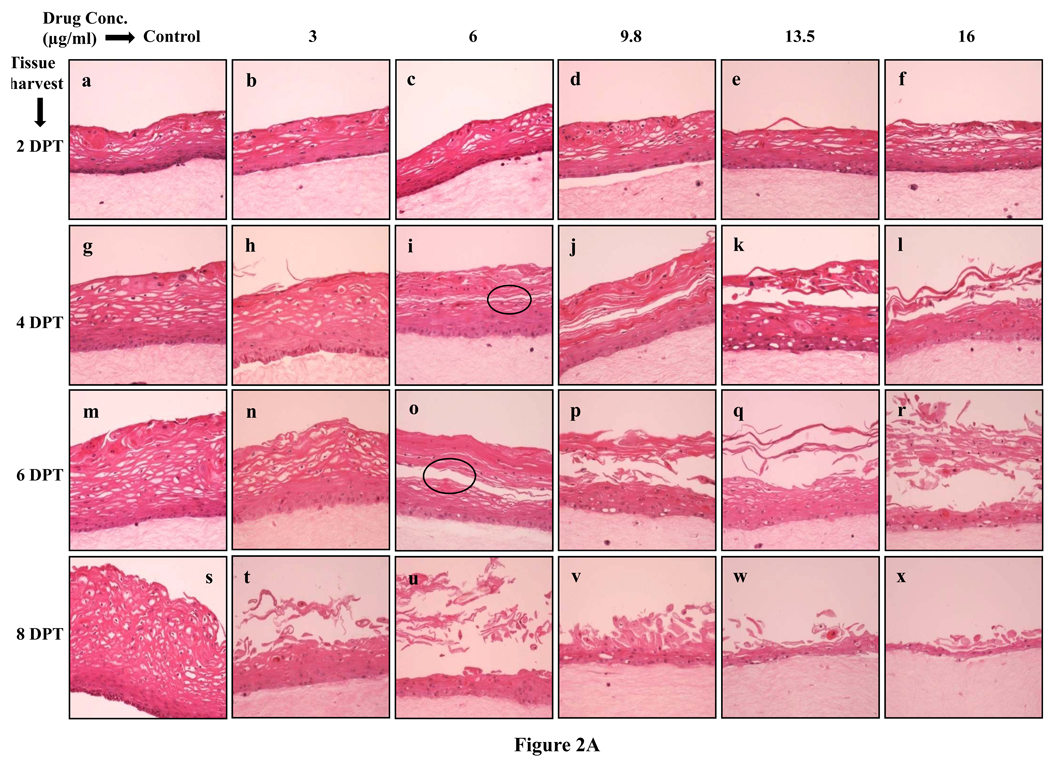
To examine the effects of lopinavir/ritonavir on gingival epithelial morphology and stratification in raft cultures hematoxylin and eosin staining was performed. Among the numerous techniques used to culture gingival epithelial cells, the raft culture system has proven to accurately mimic the in vivo physiology of the gingival epithelium [26,27]. Using 70% ethanol alone as the vehicle control did not adversely affect the growth of the tissues (Fig. 1, Lower Block). However, lopinavir/ritonavir treatments severely affected the growth of gingival epithelium in a dose dependent manner when compared with control rafts (Fig. 1, Upper Block, Panels a to x). Further, at 9.8, 13.5 and 16 µg/ml of lopinavir/ritonavir treatments, the growth of gingival epithelium was completely obliterated over time (Fig. 1, Upper Block, Panels v, q, w, f, l, r and x). These results suggested that lopinavir/ritonavir treatments severely inhibited the growth and differentiation of gingival epithelium when the drug was present throughout the growth period. We then decided to start treating the rafts with lopinavir/ritonavir at day 8. Under normal conditions, a developing epithelium with all four strata: basal, spinosum, granulosum, and corneum can be visualized on collagen matrices by day 8 of tissue growth. Further, starting treatment on 8 day epithelium would provide the opportunity to examine the effect of this drug on developing epithelium as present in the human oral cavity. The raft cultures treated with lopinavir/ritonavir were morphologically similar to control rafts at 2 days post treatment, (Fig. 2A, Panels a to f). However, at 4 and 6 days post treatments, the cell-cell contacts within the stratified layers appear to be less tight in rafts treated with 6, 9.8, 13.5 and 16 µg/ml of lopinavir/ritonavir, compared with untreated controls (Fig. 2A, Panels g to r). Further, lopinavir/ritonavir treatments at all concentrations interfered with the epithelial stratification and severely compromised gingival epithelial growth and structure at 8 days post treatment (Fig. 2A, Panels t to x).
Fig. 1.
Upper Block. Effect of lopinavir/ritonavir on gingival epithelium growth and stratification. Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of lopinavir/ritonavir throughout the growth period. (Panels a, g, m, and s) untreated rafts; (Panels b, h, n and t) rafts treated with 3 µg/ml lopinavir/ritonavir; (Panels c, i, o and u) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels d, j, p and v) rafts treated with 9.8 µg/ml lopinavir/ritonavir; (Panels e, k, q and w) rafts treated with 13.5 µg/ml lopinavir/ritonavir; (Panels f, l, r and x) rafts treated with 16 µg/ml lopinavir/ritonavir. Rafts were harvested at different points and stained with hematoxylin and eosin. (Panels a to f) rafts were harvested at day 4; (Panels g to l) rafts were harvested at day 8; (Panels m to r) rafts were harvested at day 12; (Panels s to x) rafts were harvested at day 16. Images are at 10 × original magnification.
Lower Block. Effect of 70% ethanol corresponding to the different drug treatments on gingival epithelium growth and stratification. Treatment with 3, 6, 9.8, 13.5 and 16 µg/ml lopinavir/ritonavir corresponds to exposure of raft cultures with 0.15, 0.3, 0.49, 0.69 and 0.8 µl of 70% ethanol per ml of E-media respectively.
Fig. 2.
A. Effect of lopinavir/ritonavir on gingival epithelium morphology and stratification. Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of lopinavir/ritonavir at day 8. (Panels a, g, m, and s) untreated rafts; (Panels b, h, n and t) rafts treated with 3 µg/ml lopinavir/ritonavir; (Panels c, i, o and u) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels d, j, p and v) rafts treated with 9.8 µg/ml lopinavir/ritonavir; (Panels e, k, q and w) rafts treated with 13.5 µg/ml lopinavir/ritonavir; (Panels f, l, r and x) rafts treated with 16 µg/ml lopinavir/ritonavir. Rafts were harvested at different points and stained with hematoxylin and eosin. (Panels a to f) rafts were harvested at 2 days post treatment; (Panels g to l) rafts were harvested at 4 days post treatment; (Panels m to r) rafts were harvested at 6 days post treatment; (Panels s to x) rafts were harvested at 8 days post treatment. Images are at 20 × original magnification. Circles in images i and o indicate less tight cell-cell contacts within the stratified layers or tissues with compromised integrity.
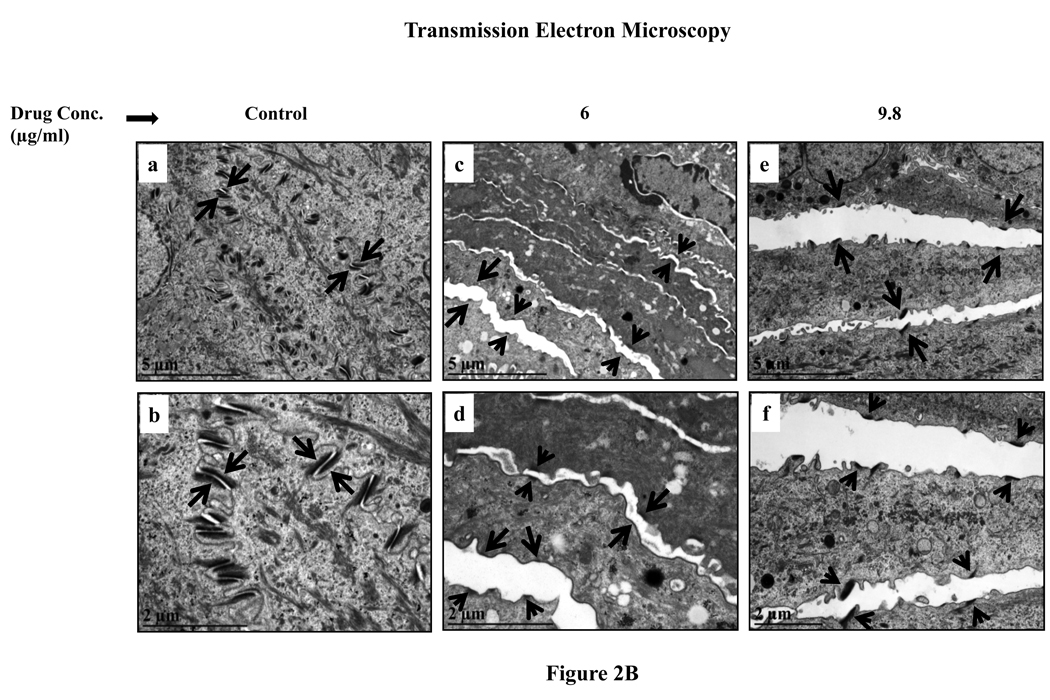
B. Transmission electron microscopy micrographs of untreated and lopinavir/ritonavir treated gingival tissue. (Panels a and b) untreated rafts; (Panels c and d) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels e and f) rafts treated with 9.8 µg/ml lopinavir/ritonavir. (Panels a, c and e) micrograph at low magnification; (Panels b, d and f) micrograph at higher magnification. Arrows indicate the desmosomes configuration.
To study in more detail the effect of this drug on tissue integrity TEM studies were performed to analyze desmosomes configuration in untreated and drug treated tissue. TEM analysis showed that in untreated tissue the desmosomes were well organized and tightly configured between cells (Fig. 2B, Panels a and b). However, in lopinavir/ritonavir treated tissues desmosomal halves were separated in the intercellular region which could potentially explain the loss of cell-cell adhesion in lopinavir/ritonavir treated tissues (Fig. 2B, Panels c to f).
Effect of lopinavir/ritonavir treatments on the expression pattern of differentiation markers in gingival epithelium
Upon commitment of gingival keratinocytes to terminal differentiation, a number of biochemical changes occur, namely, the expression of cytokeratins: 5, 14 and 10 [28]. Cytokeratins 5 and 14 are normally expressed in the basal cells of gingival stratified epithelium and have been used as proliferative cell markers [28–30]. In our study, lopinavir/ritonavir treatments increased and altered the expression patterns of cytokeratins 5 and 14 in a time and dose- dependent manner (data not shown). Lopinavir/ritonavir treatments dramatically compromised epithelium structure at 8 days post treatment thereby making it difficult to observe the staining patterns (Fig. 2A, Panels t to x).
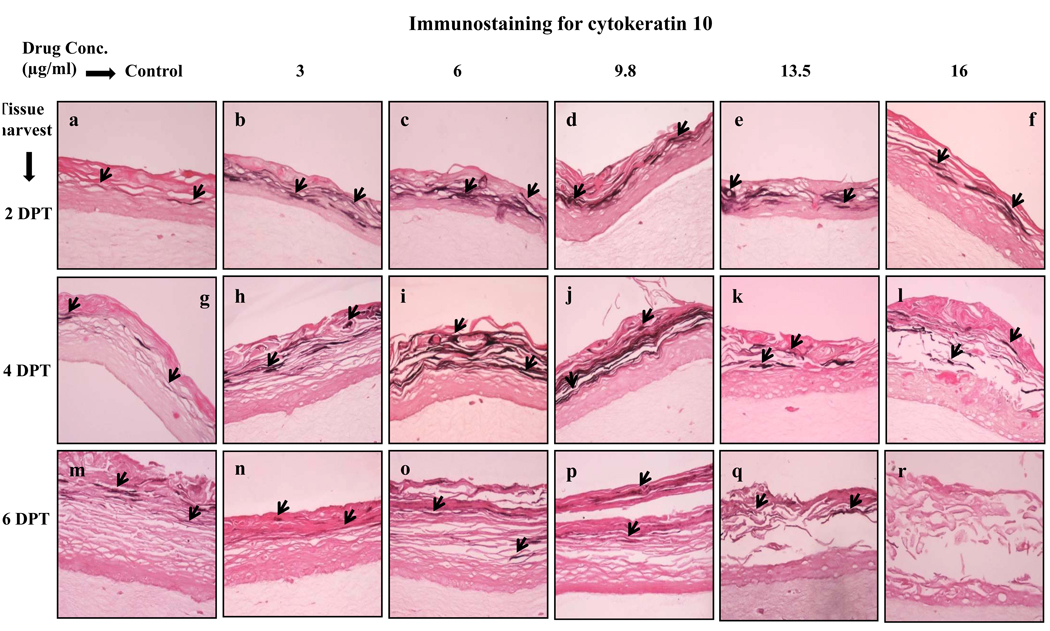
The next differentiation marker studied was cytokeratin 10, which is normally expressed in low levels in the suprabasal layers of gingival epithelium [29,31]. Cytokeratin 10 expression was induced by lopinavir/ritonavir treatments in a dose dependent manner (Fig. 3, Panels a to l). As compared with control, lopinavir/ritonavir treatments induced the expression of cytokeratin 10 at 2 and 4 days post treatment (Fig. 3, Panels a to l). However, cytokeratin 10 expression was decreased in lopinavir/ritonavir treated rafts at 6 days post treatment (Fig. 3, Panels n to r). The present results suggest the possibility that increased expression of cytokeratin 10 at early time points may be a protective response of the epithelium towards lopinavir/ritonavir induced damage.
Fig. 3.
Expression pattern of cytokeratin 10 in untreated and lopinavir/ritonavir treated gingival raft cultures. Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of lopinavir/ritonavir at day 8. (Panels a, g and m) untreated rafts; (Panels b, h and n) rafts treated with 3 µg/ml lopinavir/ritonavir; (Panels c, i and o) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels d, j and p) rafts treated with 9.8 µg/ml lopinavir/ritonavir; (Panels e, k and q) rafts treated with 13.5 µg/ml lopinavir/ritonavir; (Panels f, l and r) rafts treated with 16 µg/ml lopinavir/ritonavir. Rafts were harvested at different points and stained with anti-cytokeratin 10 antibody. (Panels a to f) rafts were harvested at 2 days post treatment; (Panels g to l) rafts were harvested at 4 days post treatment; (Panels m to r) rafts were harvested at 6 days post treatment. Arrows indicate the expression of cytokeratin 10. Images are at 20 × original magnification.
Kaletra treatments induced the expression of cytokeratin 6
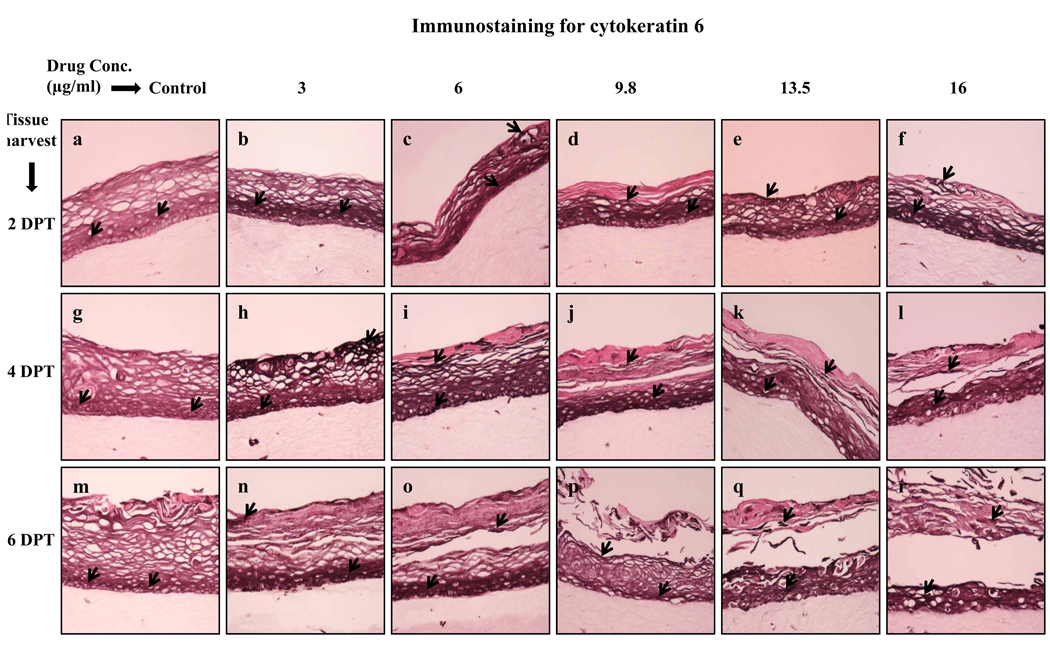
The expression of cytokeratin 6 is associated with the wound healing process and it is expressed in the suprabasal layer. In the present study, cytokeratin 6 expression was induced at 2 and 4 days post treatment in lopinavir/ritonavir treated rafts compared with untreated rafts (Fig. 4, Panels a to l). Enhanced expression of cytokeratin 6 possibly suggests a wound healing response of tissue against drug induced injury.
Fig. 4.
Expression pattern of cytokeratin 6 in untreated and lopinavir/ritonavir treated gingival raft cultures. Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of lopinavir/ritonavir at day 8. (Panels a, g and m) untreated rafts; (Panels b, h and n) rafts treated with 3 µg/ml lopinavir/ritonavir; (Panels c, i and o) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels d, j and p) rafts treated with 9.8 µg/ml lopinavir/ritonavir; (Panels e, k and q) rafts treated with 13.5 µg/ml lopinavir/ritonavir; (Panels f, l and r) rafts treated with 16 µg/ml lopinavir/ritonavir. Rafts were harvested at different points and stained with anti-cytokeratin 6 antibody. (Panels a to f) rafts were harvested at 2 days post treatment; (Panels g to l) rafts were harvested at 4 days post treatment; (Panels m to r) rafts were harvested at 6 days post treatment. Arrows indicate the expression of cytokeratin 6. Images are at 20 × original magnification..
Increased cell proliferation upon lopinavir/ritonavir treatments
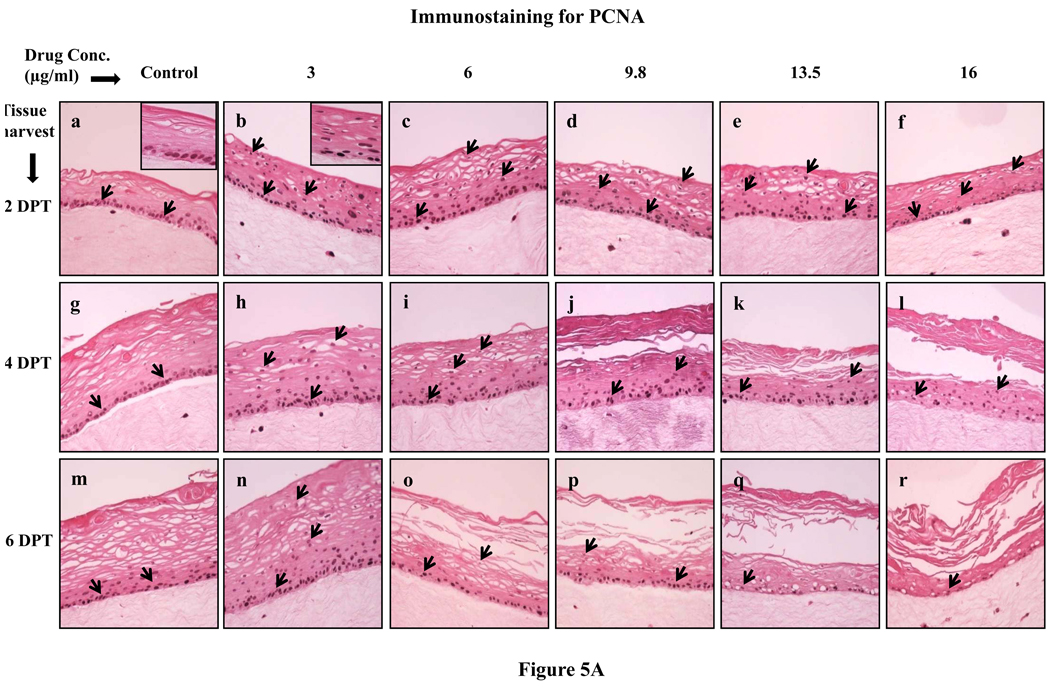
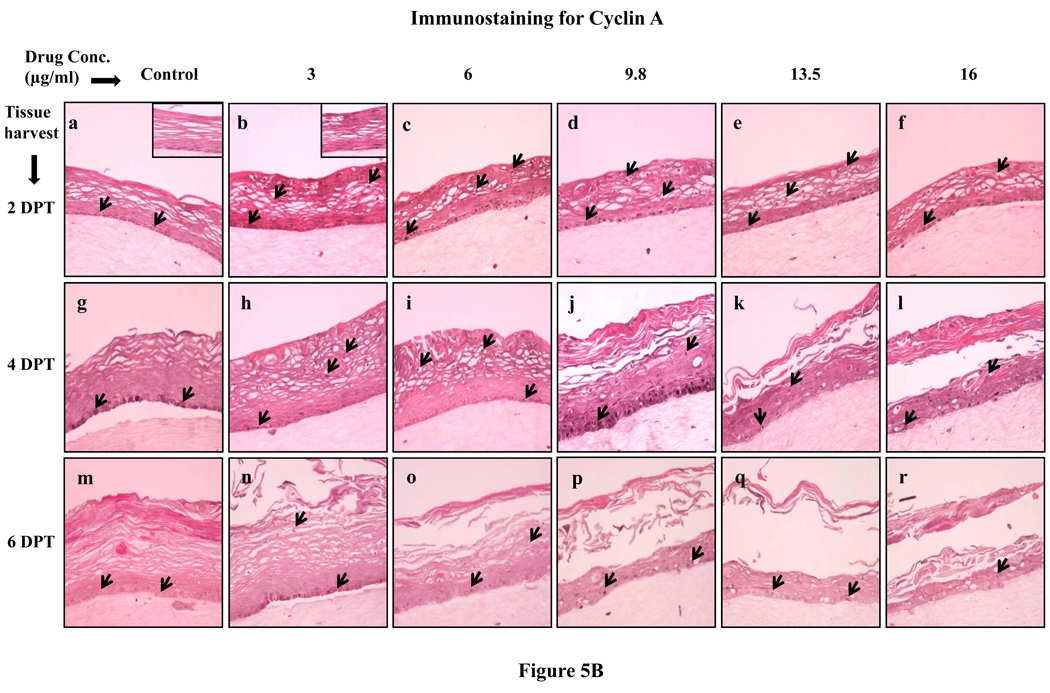
Since lopinavir/ritonavir treatments changed the expression patterns of proliferation markers cytokeratins 5, 14 and 6, we then decided to evaluate the effect of lopinavir/ritonavir on the expression of the well known cell proliferation markers PCNA and cyclin A. Cell proliferation is limited to the basal layer under normal conditions. In our study, the PCNA and cyclin A expression in untreated rafts was limited to the basal layer at 2, 4 and 6 days post treatment (Fig. 5A and B, Panels a, g and m). However, PCNA and cyclin A was strongly expressed in the basal as well as in the differentiating layers of tissue in lopinavir/ritonavir treated rafts at 2 and 4 days post treatment (Fig. 5A and B, Panels a to l). The expression of PCNA and cyclin A in lopinavir/ritonavir treated rafts lessened at 6 days post treatment (Fig. 5A and B, Panels n to r). The changed expression pattern of PCNA and cyclin A in our study indicates the activation of the wound healing pathway against drug induced damage. In addition, changed expression patterns of PCNA and cyclin A also suggest the possibility that exposure of drug induces a loss of cell cycle control which could play a role in the generation of oral complications in HIV patients under treatment with this drug.
Fig. 5.
(A) Proliferating cell nuclear antigen and (B) cyclin A expression pattern in untreated and lopinavir/ritonavir treated gingival raft cultures. Primary gingival keratinocytes were grown in organotypic (raft) cultures and treated with different concentrations of lopinavir/ritonavir at day 8. (Panels a, g and m) untreated rafts; (Panels b, h and n) rafts treated with 3 µg/ml lopinavir/ritonavir; (Panels c, i and o) rafts treated with 6 µg/ml lopinavir/ritonavir; (Panels d, j and p) rafts treated with 9.8 µg/ml lopinavir/ritonavir; (Panels e, k and q) rafts treated with 13.5 µg/ml lopinavir/ritonavir; (Panels f, l and r) rafts treated with 16 µg/ml lopinavir/ritonavir. Rafts were harvested at different points and stained with anti-PCNA and cyclin A antibody. (Panels a to f) rafts were harvested at 2 days post treatment; (Panels g to l) rafts were harvested at 4 days post treatment; (Panels m to r) rafts were harvested at 6 days post treatment. Arrows indicate the expression of PCNA and cyclin A. Images are at 20 × original magnification.
Discussion
In our previous study we observed that Amprenavir, a protease inhibitor, deregulated the growth, differentiation and cell cycle/proliferation pathway in human gingival tissue [20]. We wanted to further analyze the effects of another protease inhibitor and determine whether it also has the same effects on the growth patterns of gingival epithelium. Therefore, in this study we studied the effects of another HIV protease inhibitor, lopinavir/ritonavir, on the growth of gingival epithelium, and the expression patterns of key differentiation and proliferation markers. Additionally, in the present study TEM of untreated and treated tissues was performed to analyze in detail the impact of this drug on the integrity of gingival tissue.
Lopinavir/ritonavir treatments severely affected the growth of gingival epithelium when the drug was present throughout the growth period. To the best of our knowledge, the correlation between lopinavir/ritonavir levels in blood serum and in oral tissues has not been widely studied. However, earlier studies have shown that the drugs levels are almost equal in blood serum and in saliva [23,24,25]. Therefore, we assumed that the blood levels of lopinavir/ritonavir would be the same as in the saliva. As the oral cavity is directly exposed to saliva, we expect that the intracellular concentration of the drug in the oral cavity tissues would be equal or close to its Cmax (9.8 µg/ml). In the present study, at even lower concentrations of lopinavir/ritonavir (3 and 6 µg/ml), the growth of gingival epithelium was severely inhibited. To examine the effect of lopinavir/ritonavir on the epithelium integrity using TEM, we treated raft cultures at day 8. TEM observations clearly illustrate that lopinavir/ritonavir treatments affected the cell to cell packing by directly or indirectly reducing desmosomes adhesiveness. Since desmosomes are intercellular junctions that provide strong adhesion between cells and also give mechanical strength to tissues [19], the results of our study suggest that lopinavir/ritonavir treatments affected gingival epithelium integrity. The results in the present study are consistent with our previous study in which Amprenavir treatments also affected the epithelial growth and integrity [20]. However, the adverse impact of lopinavir/ritonavir on tissue growth and integrity was more severe compared to Amprenavir treatments. Our results support previous findings that indicated the use of antiretroviral drugs including protease inhibitors resulted in the development of oral complications [6,8–11]. These observations suggest the possibility that oral epithelium in HIV patients exposed to HAART encounter drug induced abnormalities in the tissue’s cellular and molecular biology which give rise to oral complications. However, our raft culture model is an in vitro model in which the study of growth kinetics is limited to a maximum of 20 days. In contrast, patients undergoing drug therapy have potentially been exposed to these drugs for a numbers of years. As a result, our raft culture system provides a snap-shot of the drug effects over a limited growth period. However, the effects of the drugs are representative of the adverse oral effects reported in patients undergoing anti-viral therapy.
Different cytokeratins are differentially expressed during development and differentiation and vary in different types of epithelia [18,32]. Normally, cytokeratins 5 and 14 are expressed only in the proliferative basal layer of gingival stratified epithelia [28–30]. In present study, lopinavir/ritonavir treatments increased and changed the expression patterns of cytokeratins 5 and 14 in a concentration dependent manner over time. The same changes in patterns of cytokeratins 5 and 14 expression were noticed in our previous study [20].
Cytokeratin 10 is a specific terminal differentiation marker and is expressed in the suprabasal layer of keratinized epithelia. It has been reported that cytokeratin 10 protects epithelium from trauma and damage [31]. In our study, lopinavir/ritonavir treatments induced the expression of cytokeratin 10 in a concentration dependent manner at 2 and 4 days post treatment as compared with control. It is possible that enhanced synthesis of cytokeratin 10 in drug treated gingival epithelium may be a response by the tissue to protect itself against drug induced damage [31,33,34]. The increased level of cytokeratin 10 in drug treated rafts may also be linked to strong expression of cytokeratin 10 observed in oral lesions and hyperproliferative epidermis as compared to normal epidermis [35]. Additionally, the normal balance of cytokeratin proliferation and differentiation may be disrupted upon injury and under pathologic conditions [36–38].
The induced expression of cytokeratin 10 in lopinavir/ritonavir treated rafts indicated the possibility that this drug caused damage to the gingival epithelium. To better demonstrate this possibility, we analyzed cytokeratin 6 which is expressed in response of wound injury in the suprabasal layer of stratified epithelium. In our study, cytokeratin 6 expression was induced significantly at 2 and 4 days post treatment in treated rafts compared with untreated rafts. Damage to stratified epithelia causes induction of cytokeratin 6 in the differentiating layers of epidermis [31,39–41]. In addition to wound healing, cytokeratin 6 is also expressed in stratified epithelia undergoing hyperproliferation or abnormal differentiation including cancer [40,42]. It is therefore possible that induced expression of cytokeratin 6 in lopinavir/ritonavir treated rafts at 2 and 4 days post treatment is likely due to the wound healing attempts of the tissue after drug induced tissue damage. In addition, induction of cytokeratin 6 expression in lopinavir/ritonavir treated rafts also suggests the possibility that exposure to drug induces a hyperproliferative environment in the gingival tissue. Enhanced expression of PCNA and cyclin A in drug treated rafts in our study supports these arguments. The decreased expression of cytokeratin 6 over time suggests the possibility that lopinavir/ritonavir treatments severely compromised tissue integrity.
Enhanced cell proliferation is a sign of many disorders such as wounds, ulcers and human tumors, and the identification and use of suitable markers of proliferative activity is important in clinical practice [43,44]. PCNA and cyclin A are nuclear proteins and generally detected in cell nuclei between the G1 and M phases of the cell cycle [45,46]. Both are useful histochemical markers of cell proliferation because their expression and distribution correlate with cellular proliferation rates and DNA synthesis [47]. In normal conditions of cell proliferation, PCNA and cyclin A expression is limited to a few cells in the basal layer [48,49]. In our study, PCNA and cyclin A were strongly upregulated in the basal as well as in suprabasal layers of the drug treated tissue at 2 and 4 days post treatment. These results suggest two possibilities. First, enhanced expression of PCNA and cyclin A indicates the activation of wound healing pathways to counteract drug induced tissue damage. Enhanced expression of cytokeratins 10 and 6 in drug treated rafts also supports this argument. Second, drug treatments deregulated the cell proliferation and differentiation pathways which result in abnormal proliferation and epithelial repair which could make the oral tissue more favorable for the development of oral complications observed in HIV patients taking this drug. Further, increased and altered expression patterns of cell proliferation markers including cytokeratins 5 and 14, PCNA and cyclin A indicate that the drug induces hyperproliferative environment in the tissue which could make it more favorable for establishing opportunistic human papillomavirus (HPV) infections. Previous studies have shown a significant increase in the development of HPV positive lesions in HIV patients taking HAART including protease inhibitors [5,50,51].
In summary, our studies demonstrate that lopinavir/ritonavir severely inhibited the growth of gingival tissue when drug was present throughout the growth period. TEM observations revealed that tissue integrity of desmosomes was compromised in lopinavir/ritonavir treated gingival tissues. Further, lopinavir/ritonavir treatments changed the expression pattern of cytokeratins 5, 14, 10, 6, PCNA and cyclin A over time. Taken together, these data suggest that this drug compromised tissue integrity and deregulated the differentiation and cell cycle/proliferation pathway in human gingival tissue. The present results are consistent with our previous study in which Amprenavir treatments inhibited the epithelial growth, and deregulated the differentiation and proliferation pathway in human gingival tissue [20]. Both our previous studies with Amprenavir and the current studies with lopinavir/ritonavir display similar changes in the differentiation and proliferation markers upon treatments. These results indicate the possibly both protease inhibitors deregulate gingival epithelial growth and differentiation using similar mechanisms. However, the adverse impact of lopinavir/ritonavir on tissue growth and integrity was more severe compared to Amprenavir treatments. Identification of specific pathways affected by protease inhibitors will further our understanding of how this class of drugs compromise gingival tissue integrity and deregulated the differentiation and cell cycle/proliferation pathways.
Acknowledgments
We thank Lynn Budgeon for technical assistance in preparing histological slides and Roland Myers for his valuable help with TEM. We also thank Jeanette Gullett and Tracy Krouse for technical assistance with tissue processing. We are grateful to Brian Bowser, Michael Conway and Linda Cruse for critical reading of the manuscript and helpful suggestions. This study was supported by NIDCR grant DE018305 to Craig Meyers
References
- 1.Global summary of the AIDS epidemic. The Joint United Nations Programme on HIV AIDS (UNAIDS) [accessed 22 February 2010];2009 November; doi: 10.1016/S0968-8080(09)34466-3. Available at http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. [DOI] [PubMed]
- 2.Gortmaker SL, Hughes M, Cervia J, et al. Effects of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 3.Granados JMS, Amador JTR, De Miguel SF, et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus infected children. Pediatr Infect Dis J. 2003;22:863–867. doi: 10.1097/01.inf.0000091282.70253.5f. [DOI] [PubMed] [Google Scholar]
- 4.Patton LL, McKaig R, Strauses R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immunodeficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan D, Canchola AJ, MacPhail A, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001;357:1411–1412. doi: 10.1016/S0140-6736(00)04578-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan JS, Greenspan D. The epidemiology of the oral lesions of HIV infection in the developed world. Oral Dis. 2002;8:34–39. doi: 10.1034/j.1601-0825.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson TA, Greenspan D, Greenspan JS. Oral lesions of HIV disease and HAART in industrialized countries. Adv Dent Res. 2006;19:57–62. doi: 10.1177/154407370601900112. [DOI] [PubMed] [Google Scholar]
- 8.Scully C, Diz Dios P. Orofacial effects of antiretroviral therapies. Oral Dis. 2001;7:205–210. [PubMed] [Google Scholar]
- 9.Diz Dios P, Scully C. Adverse effects of antiretroviral therapy: focus on orofacial effects. Expert Opin Drug Saf. 2002;1:307–317. doi: 10.1517/14740338.1.4.307. [DOI] [PubMed] [Google Scholar]
- 10.Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC. Nevirapine and the risk of Stevans-Johnson syndrome or toxic epidermal necrolysis. AIDS. 2001;15:1843–1848. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 11.Jordan RA. Implications of antiretroviral therapy in oral medicine--a review of literature. Schw Mont f Zahn. 2007;117:1210–1216. [PubMed] [Google Scholar]
- 12.Hawkins T. HIV drug resistance and you. The limitations of drug resistance testing. Many factors influence the results. Posit Aware. 2006;Spec No:13–16. [PubMed] [Google Scholar]
- 13.Chandwani A, Shutter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CA, Orl-Hns FRCS, Mistry D, MRCS, Sharma R, Coatesworth AP. (Orl-Hns) FRCS Rhinological, laryngological, oropharyngeal and other head and neck side effects of drugs. J Laryngol Otol. 2006;120:1–10. doi: 10.1017/s0022215105005839. [DOI] [PubMed] [Google Scholar]
- 15.Borras-Blasco J, Navarro-Ruiz A, Borras C, Castera E. Adverse Cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother. 2008;62:879–888. doi: 10.1093/jac/dkn292. [DOI] [PubMed] [Google Scholar]
- 16.HIV and oral health. Appendix 1: selected agents used to treat HIV infection or reacted conditions. [accessed 10 February, 2010];2006 June; Available at http://www.hivguidelines.org/GuidelineDocuments/d-app1.pdf.
- 17.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 18.Sun TT, Eichner R, Schermer A, Cooper D, Nelson WG, Weiss RA. Classification, expression and possible mechanisms of evolution of mammalian epithelial keratins: a unifying model. In: Levine AJ, Vande Woude GF, Topp WC, Watson JD, editors. The transformed phenotype: cancer cells. New York: Cold Spring Harbor Laboratory; 1984. pp. 169–176. [Google Scholar]
- 19.Garrod D, Chidgey M. Desmosomes structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Israr M, Mitchel D, Alam S, Dinello D, Kishel JJ, Meyers C. Effect of the HIV protease inhibitor Amprenavir on the growth and differentiation of primary gingival epithelium. Antivir Ther. 2010;15:253–265. doi: 10.3851/IMP1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human Papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 22.Meyers C. Organotypic (raft) epithelial tissue culture system for the differentiation dependent replication of papillomavirus. Meth Cell Sci. 1996;18:1–10. [Google Scholar]
- 23.Rolinski B, Wintergerst U, Matuschke A, et al. Evaluation of saliva as a specimen for monitoring zidovudine therapy in HIV-infected patients. AIDS. 1991;5:885–888. doi: 10.1097/00002030-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27:147–159. [PMC free article] [PubMed] [Google Scholar]
- 25.Rakhmanina NY, Capparelli EV, vanden Anker JN, et al. Nevirapine concentration in nonstimulated saliva: an alternative to plasma sampling in children with human immunodeficiency virus infection. Ther Drug Monit. 2007;29:110–117. doi: 10.1097/FTD.0b013e31803258ed. [DOI] [PubMed] [Google Scholar]
- 26.Tomakidi P, Fusenig NE, Kohl A, Komposch G. Histomorphological and biochemical differentiation capacity in organotypic co-cultures of primary gingival cells. J Periodontal Res. 1997;32:388–400. doi: 10.1111/j.1600-0765.1997.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 27.Klausner M, Ayehunie S, Breyfogle BA, Wertz PW, Bacca L, Kubilus J. Organotypic human oral tissue models for toxicological studies. Toxicol In Vitro. 2007;21:938–949. doi: 10.1016/j.tiv.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie IC, Rittman G, Gao Z, Leigh I, Lane EB. Patterns of cytokeratin expression in human gingival epithelia. J Periodontal Res. 1991;26:468–478. doi: 10.1111/j.1600-0765.1991.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 29.Eichner R, Bonitz P, Sun TT. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984;96:1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990;97:39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Bonan PRF, Kaminagakura E, Pires FR. Cytokeratin expression in initial oral mucositis of head and neck irradiated patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:205–211. doi: 10.1016/j.tripleo.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 33.Bloor BK, Seddon SV, Morgan PR. Gene expression of differentiation specific keratins (K4, K13, K1 and K10) in oral non-dysplastic keratoses and lichen planus. J Oral Pathol Med. 2000;29:376–384. doi: 10.1034/j.1600-0714.2000.290803.x. [DOI] [PubMed] [Google Scholar]
- 34.Santos JN, Sousa SO, Nunes FD, Sotto MN, De Araujo VC. Altered cytokeratin expression in actinic cheilitis. J Cutan Pathol. 2003;30:237–241. doi: 10.1046/j.0303-6987.2002.028.x. [DOI] [PubMed] [Google Scholar]
- 35.Bloor BK, Tidman N, Leigh IM, et al. Expression of keratin K2e in cutaneous and oral lesions: association with keratinocytes activation, proliferation and keratinization. Am J Pathol. 2003;162:963–975. doi: 10.1016/S0002-9440(10)63891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetzels RH, Kujipers HJ, Lane EB, et al. Basal specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991;138:751–763. [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan PR, Su L. Intermediate filaments in oral neoplasia. 1. Oral cancer and epithelial dysplasia. Eur J Cancer B oral Oncol. 1994;30B:160–166. doi: 10.1016/0964-1955(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 38.Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Rep Reg. 2005;13:468–479. doi: 10.1111/j.1067-1927.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 39.Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996;132:381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48 and 56-kdalton keratin as molecular marker for hyperproliferative keratinocytes. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansbridge JN, Knapp AM. Changes in keratinocytes maturation during wound healing. J Invest Dermatol. 1987;89:253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- 42.Stoler A, Kopan R, Duvic M, Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988;107:427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundqvist K, Schmidtchen A. Cyclin A expression in chronic leg ulcers. Acta Derm Venereol. 2006;86:61–62. doi: 10.1080/00015550510044172. [DOI] [PubMed] [Google Scholar]
- 44.Thompson PJ, Goodson ML, Booth C, Cragg N, Hamadah O. Cyclin A activity predicts clinical outcome in oral precancer and cancer. Int J Oral Maxillofac Surg. 2006;35:1041–1046. doi: 10.1016/j.ijom.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celis JE, Celis A. Cell cycle dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci USA. 1985;82:3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuber M, Tan EM, Ryoji M. Involvement of proliferating cell nuclear antigen (cyclin) in DNA replication in living cells. Mol Cell Biol. 1989;9:57–66. doi: 10.1128/mcb.9.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bujia J, Sudhoff H, Holly A, Hildmann H, Kastenbauer E. Immunohistochemical detection of proliferating cell nuclear antigen in middle ear cholesteatoma. Eur Arch Otorhinolaryngol. 1996;253:21–24. doi: 10.1007/BF00176697. [DOI] [PubMed] [Google Scholar]
- 49.Girod SC, Pfeiffert P, Ries J, Papet HD. Proliferative activity and loss of function of tumour suppressor genes as biomarkers in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1998;36:252–260. doi: 10.1016/s0266-4356(98)90708-2. [DOI] [PubMed] [Google Scholar]
- 50.King MD, Reznik DA, O’Daniela CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus associated oral warts among human immunodeficiency virus seropositive patients in the area of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34:641–648. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- 51.Cameron JE, Mercante D, O’Brien M, et al. The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus seropositive adults. Sex Transm Dis. 2005;32:703–709. doi: 10.1097/01.olq.0000175398.34610.2e. [DOI] [PubMed] [Google Scholar]