Abstract
Purpose
This study was designed to determine the effect of dose and fractionation schedule of prophylactic cranial irradiation (PCI) on the incidence of chronic neurotoxicity (CNt) and changes in quality of life (QoL) for selected patients (pts) with limited disease small cell lung cancer (LD SCLC).
Methods and Materials
Pts with LD SCLC who achieved a complete response (CR) following chemotherapy and thoracic irradiation were eligible for randomization to undergo PCI to a total dose of 25 Gy in 10 daily fractions (Fxs) (Arm 1) vs. the experimental cohort of 36 Gy. Those receiving 36 Gy underwent a secondary randomization between daily 18 Fxs (Arm 2) and twice daily 24 Fxs (Arm 3). Enrolled pts participated in baseline and follow-up neuropsychological test batteries (NPTB) along with QoL assessments.
Results
265 pts were accrued with 131 in Arm 1, 67 in Arm 2, and 66 in Arm 3 being eligible. There are 112 (42.2%) pts alive with 25.3 months (mos) of median follow-up. There were no significant baseline differences among groups regarding QoL measures and one of the NPTB, namely the Hopkins Verbal Learning Test. However, there was a significant increase in the occurrence at 12 mos post-PCI of CNt in the 36 Gy cohort (p=0.02). Logistic regression analysis revealed increasing age was the most significant predictor of CNt (p=0.005).
Conclusions
Due to the increased risk of developing CNt in study patients with 36 Gy, a total PCI dose of 25 Gy remains the standard of care for patients with LD SCLC attaining a CR to initial chemoradiation.
Keywords: Limited disease small cell lung cancer, prophylactic cranial irradiation, neuropsychological testing, quality of life, chronic neurotoxicity
INTRODUCTION
Small cell lung cancer (SCLC) comprises approximately 13% of all cases of lung cancer being diagnosed more recently in the United States of which roughly 30% are found to have limited disease [1]. Despite the impact of chemotherapy and chest irradiation for treating the primary disease, brain relapses are still a major concern and may occur in about 50% of the time in long-term survivors [2]. Although a meta-analysis [3] demonstrated the impact of prophylactic cranial irradiation (PCI) on improving survival in patients with limited disease small cell lung cancer (LDSCLC) who sustained a complete response (CR) to chemoradiation, the optimal total dose and fractionation schedule of delivering PCI still remain uncertain.
Recently, the results of an international phase III trial (PCI 01-EULINT1; ClinicalTrials.gov identifier: NCT 00005062) of over 700 patients comparing 25 Gy in 10 daily fractions (Fxs) versus 36 Gy total dose of PCI delivered at institutional choice in either 18 daily Fxs or in 24 twice daily delivered Fxs showed no survival benefit in the higher dose group [4]. In fact, this latter study found a lower 2-year overall survival of 37% in the cohort receiving 36 Gy versus 42% for those receiving 25 Gy (p=0.05). This multi-national PCI trial was already accruing patients at the time of concept development for RTOG 0212.
Therefore, with the support of the National Cancer Institute (NCI), RTOG 0212 was designed with two specific goals. First, the study was to contribute patients to the international PCI phase III trial that evaluated the impact of total dose of PCI on the incidence of brain metastasis at two years, overall survival, and disease-free survival. The accrual to the international study was achieved in December 2005 of which 146 patients were enrolled from RTOG 0212 [4]. Second, since there was a two-part randomization scheme not only between 25 Gy versus 36 Gy total PCI dose but also between daily and twice daily fractionation with the 36 Gy total PCI dose arms, RTOG 0212 was planned as a phase II trial to determine the impact of PCI total dose and treatment schedule on incidence of chronic neurotoxicity (CNt) and on quality of life (QoL). This study required a total of 264 evaluable patients, including the first 146 patients co-enrolled into the international study, in order to perform its objectives. Thus, this report is an analysis of the initial results of RTOG 0212.
METHODS and MATERIALS
Patient Selection
Histological or cytological evidence of SCLC was required. After appropriate staging workup and Zubrod performance status of ≤ 1 along with RTOG neurological function class of 1 or 2, patients had to have limited disease and achieved a CR to chemotherapy and consolidative chest radiotherapy that was documented at least on standard chest X-rays within one month of study entry. Patients may still have been receiving chest irradiation but must have completed chest irradiation by at least one week before being enrolled. All subjects had negative MRI or CT scans of the brain at most one month before protocol entry. Pretreatment laboratory studies included absolute granulocyte count ≥1.5 ul; Hgb ≥ 10.0 gm/100 mL; and platelet count of ≥ 75,000 ul. All enrolled subjects had to provide informed consent and sign an Institutional Review Board –approved consent form before beginning protocol specified PCI.
Patients were excluded from study for the following reasons: cytological evidence of a malignant pleural effusion; prior external beam irradiation to the head & neck region; concurrent chemotherapy or immunotherapy during PCI; epilepsy requiring chronic medication; a malignancy (excluding nonmelanomatous skin cancer or carcinoma in situ of the cervix) within the prior 5 years of undergoing study PCI; a serious medical or psychiatric condition that would impede appropriate study participation; or persistent inability to give informed consent.
At time of study enrollment, patients were stratified by age (≤ or > 60 years) and time interval from the start of induction chemotherapy (≤ 90 days, 91–180 days, or 181–240 days). Patients underwent an initial randomization to either 25 Gy total dose delivered in 10 daily fractions of 2.5 Gy (control Arm 1, 131 patients) or a higher dose of 36 Gy. Those selected for the higher total dose then had a secondary randomization to receive PCI in 18 daily fractions of 2.0 Gy (experimental Arm 2, 67 patients) or in 24 twice daily fractions of 1.5 Gy with 6–8 hour interval between fractions (experimental Arm 3, 66 patients). PCI was to be initiated within 15 days after randomization. Patients were not treated on weekends.
Patients received whole brain PCI that involved 4–6 MV photons delivered with opposed lateral portals with at least normal tissue sparing of the lens. Fields had to include at least 1 cm margin on the calvarium. At the discretion of the treating radiation oncologist, the inferior border could be no more inferior than the interspace level of the second and third cervical vertebrae. All fields had to have fluoroscopic simulation prior to treatment; however, central review of simulation films for study patients was not done for this trial. CT simulation was not allowed for RTOG 0212.
Neurocognitive and Quality of Life Instruments
The neuropsychological test batteries (NPTB) employed for this trial included the Hopkins Verbal Learning Test (HVLT) [5] for memory (both immediate and delayed recall and recognition), Controlled Oral Word Association Test (COWAT) [6] for language/verbal fluency, Trail Making Test Part A (TMT-Part A) [7] for visual and spatial scanning, attention, sequencing, and speed, and Trail Making Test Part B (TMT – Part B) [7] for executive/frontal lobe skills. Each study institution was required to have a RTOG certified reviewer to conduct all NPTB in this study. Quality of Life (QoL) measures included the EORTC Quality of Life Questionnaire (QLQ-C30) and Brain Cancer Module 20 (BN 20). Both the EORTC QLQ-C30 and the BN20 have previously been shown to be reliable and valid instruments for evaluating recurrent high-grade gliomas [8, 9]. Previous investigations have demonstrated the EORTC QLQ-C30 questionnaire had adequate reliability in patients with lung, breast, ovarian and head & neck cancer [10, 11, 12, 13], as well as other cancer diagnoses [14, 15]. Compliance rates in multicenter, randomized clinical trials have been high for this questionnaire [16, 17]. The BN20 is a supplemental questionnaire specifically developed for use with the general questionnaire (QLQ-C30) in patients with brain cancer [9].These instruments were collected at baseline, at 6 and 12 months for the first year post-treatment, then annually for 3 years and also at disease progression or relapse and at death.
Statistical Methods
This analysis was undertaken since all patients had been potentially followed for a minimum of 12 months per protocol. NPTB and QOL assessments within ± 4 weeks of the scheduled 6- and 12-month assessments were included in this analysis. Neurological deterioration (ND) was defined as a decrease of one standard error measurement (SEM) in any of the NPTB with each patient serving as his/her own control in evaluating a cognitive decline. Any differences between the pre-treatment baseline and follow-up assessments among the treatment groups were confirmed by the reliable change index (RCI) [18]. This index is derived from the SEM [19] for each test in the battery.
The change score of each test from baseline and the score at baseline were tested using a Kruskal-Wallis rank test statistics [20]. Hommel’s stagewise rejective multiple test procedure [21] was employed for multiple testing problem. The percentages of patients having ND at 12 months post-treatment were estimated for all evaluable patients. Deterioration in at least one neurocognitive test (HVLT, COWAT, or TMT- Part A or B), without documentation of brain metastases was considered a chronic neurotoxicity (CNt). The proportion of study patients with a CNt was computed using a 90% confidence interval (CI). The Chi-square test [22] was used to test the difference of CNt and logistic regression [23] was used to associate CNt as a categorical response (yes or no) with important prognostic factors. The following prognostic factors were considered initially: Treatment arm (Arm 1(2.5 Gy × 10, reference level [RL]) versus (vs) Arm 2 (2.0 Gy × 18) vs Arm 3 (1.5 Gy × 24)), age (continuous), gender (male [RL] vs. female), education level (≤ high school vs > high school [RL]), and marital status (married/living as married [RL] vs single/divorced/widowed). A backward variable selection method was used to build the model at the criteria of p-value <0.1. QLQ-C30 was scored according to methods described in the EORTC QLQ-C30 scoring manual. The BN 20 was scored in a manner analogous to the QLQ-C30 symptom scales.
As specified in the statistical design of this protocol, an absolute difference of 10% from baseline on any question indicated a clinically significant difference. The following domains were evaluated: role functioning, social functioning, global QoL, visual disorder, motor dysfunction, communication deficit, drowsiness, memory/concentration. Time to development of brain metastasis (without CNt) was estimated using the cumulative incidence method [24]. All statistical comparisons were considered statistically significant with a (unadjusted and adjusted) p-value of <0.05. A Statistical Analysis System (SAS Institute, Cary, NC) software package was used for all statistical analyses.
RESULTS
RTOG 0212 opened on February 19, 2003 and closed on February 12, 2008. A total of 265 patients were accrued of which 264 were eligible for analysis. This evaluation was performed using those eligible patients in RTOG 0212 as of February 23, 2009.
At the time of this analyssis, there are 112 patients alive with 25.3 months of median follow-up (0–53.0 months) with 16 patients having less than 12 months of follow-up. There was one patient in Arm 2 for which no follow-up data has been submitted. Table 1 depicts the pretreatment characteristics of the patients by treatment arm and they were well balanced with the exception of there being more males than females in Arm 1. Note that most patients received induction chemotherapy consisting of cisplatin and etoposide chemotherapy along with thoracic consolidative irradiation.
Table 1.
Pretreatment Characteristics
| Arm 1 2.5 Gy × 10 (n=131) |
Arm 2 2.0 Gy × 18 (n=67) |
Arm 3 1.5 Gy × 24 (n=66) |
p-value* | ||||
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Median (years) | 62 | 62 | 61 | 0.59 | |||
| Range (years) | 39–86 | 39–78 | 44–77 | ||||
| n | % | n | % | n | % | ||
| Gender | |||||||
| Male | 78 | 60 | 34 | 51 | 34 | 52 | 0.39 |
| Female | 53 | 40 | 33 | 49 | 32 | 48 | |
| Education Level | |||||||
| 8th Grade or Less | 10 | 8 | 3 | 4 | 2 | 3 | NA† |
| 9–11th Grade | 20 | 15 | 12 | 18 | 7 | 11 | |
| High School Graduate/GED | 44 | 34 | 29 | 43 | 28 | 42 | |
| Vocational/Technical School | 7 | 5 | 3 | 4 | 4 | 6 | |
| Associate Degree/Some College | 14 | 11 | 10 | 15 | 9 | 14 | |
| Bachelor's Degree | 13 | 10 | 4 | 6 | 3 | 5 | |
| Advanced Degree | 9 | 7 | 1 | 1 | 4 | 6 | |
| Other | 3 | 2 | 1 | 1 | 0 | 0 | |
| Prefers not to Answer | 1 | 1 | 1 | 1 | 2 | 3 | |
| Not reported | 10 | 8 | 3 | 4 | 7 | 11 | |
| ≤ High School | 74 | 63 | 44 | 71 | 37 | 65 | 0.58 |
| > High School | 43 | 37 | 18 | 29 | 20 | 35 | |
| Marital Status | |||||||
| Married | 82 | 63 | 40 | 60 | 34 | 52 | NA† |
| Widowed | 11 | 8 | 10 | 15 | 7 | 11 | |
| Single | 13 | 10 | 3 | 4 | 6 | 9 | |
| Divorced/Separated | 15 | 11 | 10 | 15 | 11 | 17 | |
| Living as Married | 3 | 2 | 2 | 3 | 3 | 5 | |
| Prefers not to Answer | 0 | 0 | 0 | 0 | 2 | 3 | |
| Not reported | 7 | 5 | 2 | 3 | 3 | 5 | |
| Married/Living as Married | 85 | 69 | 42 | 65 | 37 | 61 | 0.56 |
| Single/Divorced/Widowed | 39 | 31 | 23 | 35 | 24 | 39 | |
| Induction Chemotherapy | |||||||
| No | 3 | 2 | 0 | 0 | 1 | 2 | NA† |
| Yes | 127 | 97 | 66 | 99 | 65 | 98 | |
| Unknown | 1 | 1 | 1 | 1 | 0 | 0 | |
| Thoracic RT | |||||||
| No | 9 | 7 | 6 | 9 | 8 | 12 | 0.42‡ |
| Yes | 118 | 90 | 59 | 88 | 54 | 82 | |
| Unknown | 4 | 3 | 2 | 3 | 4 | 6 | |
| IPCI+(Days) | |||||||
| ≤90 | 1 | 1 | 2 | 3 | 3 | 5 | |
| 91–180 | 97 | 74 | 52 | 78 | 52 | 79 | 0.58∘ |
| 181–240 | 28 | 21 | 12 | 18 | 10 | 15 | |
| > 240 | 5 | 4 | 1 | 1 | 1 | 2 | |
F-test for continuous variables (age) and Chi-square test for categorical variables (others);
NA = Not Applicable
Insufficient cell counts;
Comparing ‘No’ versus ‘Yes’ only;
IPCI = Interval to Prophylactic Cranial Irradiation
Comparing 91–180 vs. 181–240 only.
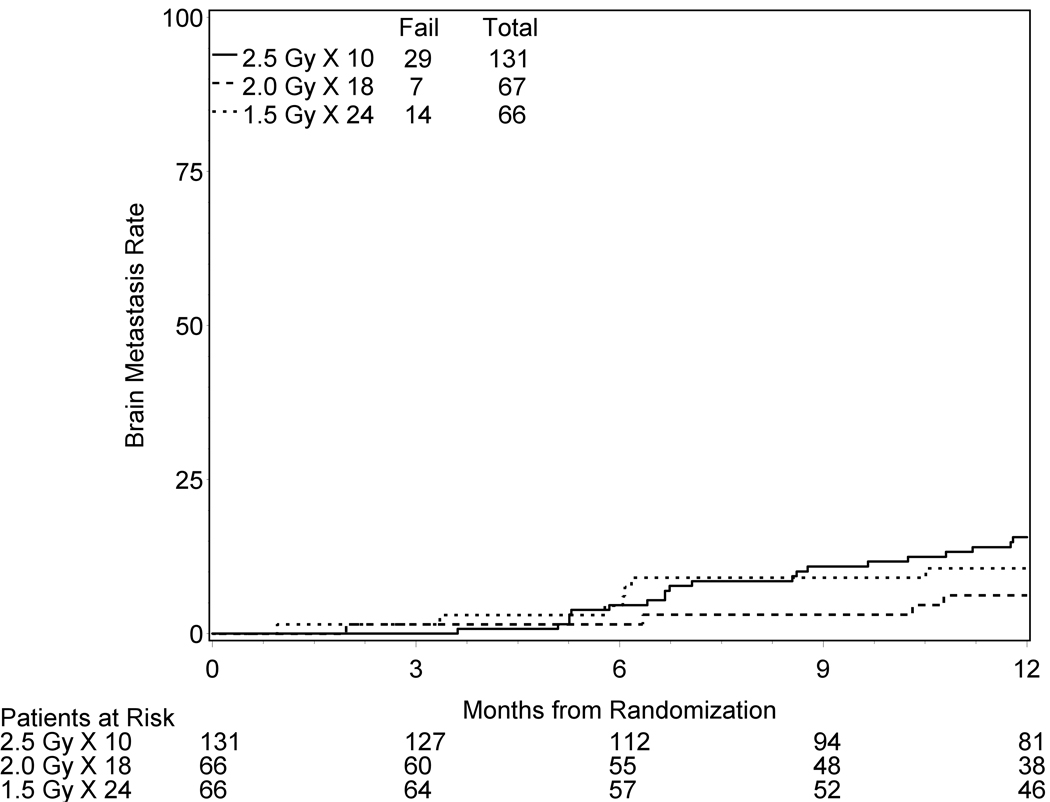
The total patients with brain metastases at any time within the first year of followup was 29 Arm 1 (22%), 7 in Arm 2 (10%), and 14 in Arm 3 (21%). The brain metastases rates determined by cumulative incidence at 12 months were 15.6%, 6.2%, and 10.6% for Arms 1, 2, and 3 respectively (Figure 1). Death without brain relapse occurred in 53 patients in Arm 1 (40%), 32 in Arm 2 (48%), and 26 in Arm 3 (39%). However, note that this study was not powered to evaluate the impact of treatment for either the incidence of brain metastases or patient survival.
Figure 1.
Cumulative incidence of brain metastasis at one year. There is 1 patient for which there has been no followup-information submitted to RTOG Head Quarters.
The baseline data for the NPTB and QoL measures were collected for more than 90% of eligible patients in all three treatment cohorts. A review of the compliance to the NPTB and QoL measurements for the first 12 month follow-up study is displayed in Table 2 and revealed a continued downward trend in eligible patients actually undergoing study-mandated evaluations. There were no statistically significant differences among treatment groups regarding HVLT, TMT B, QLQ-C30, and BN20 at 12 months versus baseline evaluations. However, there were statistically significant differences for the COWAT (p-value = 0.03) and TMT-A (adjusted p-value=0.03) at baseline among the three treatment groups. There were no statistically significant differences in score changes at 12 months from baseline among the treatment arms with respect to NPTB and QoL (unadjusted or adjusted p-values > 0.05). Confirmation of these observations was made when each neuropsychological test was subjected to the RCI (results not shown). However, it should be noted that the number of patients evaluable at 12 months for all instruments was too small to yield any statistically significant results. Although there was no significant difference in the change scores among all three treatment arms, there was a noted decline in cognitive functioning in QLQ-C30 across all groups (the mean change scores −14.0 in Arm 1, −13.5 in Arm 2, and −19.6 in Arm 3).
Table 2.
Neurocognitive and Quality of Life (QoL) Assessment Compliance
| Assessment | Treatment Arm | Evaluation Status | Baseline | At 6 months | At 12 months |
|---|---|---|---|---|---|
| HVLT | 2.5 Gy × 10 | Expected | 131 | 123 | 104 |
| Dead / alive and not evaluated | 0 / 12 | 8 / 57 | 19 / 59 | ||
| Received (%) | 119 (91%) | 66 (54%) | 45 (43%) | ||
| 2.0 Gy × 18 | Expected | 67 | 58 | 49 | |
| Dead / alive and not evaluated | 0 / 5 | 9 / 24 | 9 / 29 | ||
| Received (%) | 62 (93%) | 34 (59%) | 20 (41%) | ||
| 1.5 Gy × 24 | Expected | 66 | 63 | 57 | |
| Dead / alive and not evaluated | 0 / 5 | 3 / 27 | 6 / 38 | ||
| Received (%) | 61 (92%) | 36 (57%) | 19 (19%) | ||
| TMT A | 2.5 Gy × 10 | Expected | 131 | 123 | 104 |
| Dead / alive and not evaluated | 0 / 12 | 8 / 58 | 19 / 60 | ||
| Received (%) | 119 (91%) | 65 (53%) | 44 (42%) | ||
| 2.0 Gy × 18 | Expected | 67 | 58 | 49 | |
| Dead / alive and not evaluated | 0 / 5 | 9 / 24 | 9 / 29 | ||
| Received (%) | 62 (93%) | 34 (59%) | 20 (41%) | ||
| 1.5 Gy × 24 | Expected | 66 | 63 | 57 | |
| Dead / alive and not evaluated | 0 / 6 | 3 / 27 | 6 / 39 | ||
| Received (%) | 60 (91%) | 36 (57%) | 18 (32%) | ||
| TMT B | 2.5 Gy × 10 | Expected | 131 | 123 | 104 |
| Dead / alive and not evaluated | 0 / 17 | 8 / 60 | 19 / 61 | ||
| Received (%) | 114 (87%) | 63 (51%) | 43 (41%) | ||
| 2.0 Gy × 18 | Expected | 67 | 58 | 49 | |
| Dead / alive and not evaluated | 0 / 5 | 9 / 24 | 9 / 29 | ||
| Received (%) | 62 (93%) | 34 (59%) | 20 (41%) | ||
| 1.5 Gy × 24 | Expected | 66 | 63 | 57 | |
| Dead / alive and not evaluated | 0 / 7 | 3 / 27 | 6 / 39 | ||
| Received (%) | 59 (89%) | 36 (57%) | 18 (32%) | ||
| COWAT | 2.5 Gy × 10 | Expected | 131 | 123 | 104 |
| Dead / alive and not evaluated | 0 / 13 | 8 / 57 | 19 / 60 | ||
| Received (%) Expected | 118 (90%) | 66 (54%) | 44 (42%) | ||
| 2.0 Gy × 18 | Expected | 67 | 58 | 49 | |
| Dead / alive and not evaluated | 0 / 5 | 9 / 24 | 9 / 29 | ||
| Received (%) | 62 (93%) | 34 (59%) | 20 (41%) | ||
| 1.5 Gy × 24 | Expected | 66 | 63 | 57 | |
| Dead / alive and not evaluated | 0 / 5 | 3 / 27 | 6 / 38 | ||
| Received (%) | 61 (92%) | 36 (57%) | 19 (33%) | ||
|
QoL Measures + |
2.5 Gy × 10 | Expected | 131 | 122 | 94 |
| Dead / alive and not evaluated | 0 / 11 | 9 / 51 | 28 / 47 | ||
| Received (%) | 120 (92%) | 71 (58%) | 47 (50%) | ||
| 2.0 Gy × 18 | Expected | 67 | 58 | 47 | |
| Dead / alive and not evaluated | 0 / 5 | 9 / 18 | 11 / 25 | ||
| Received (%) | 62 (93%) | 40 (69%) | 22 (47%) | ||
| 1.5 Gy × 24 | Expected | 66 | 63 | 52 | |
| Dead / alive and not evaluated | 0 / 5 | 3 / 26 | 11 / 29 | ||
| Received (%) | 61 (92%) | 37 (59%) | 23 (44%) | ||
HVLT = Hopkins Verbal Learning Test
TMT = Trail Making Test
COWAT = Controlled Oral Word Association Test
QoL Measures = Quality of Life Questionnaire (QLQ-C30) and Brain Cancer Module 20 (BN 20)
Table 3 depicts the incidence of neurological deterioration and shows that ND defined as a significant decrease at 12 months in at least one neurocognitive test (HVLT, COWAT, or TMT A & B) from baseline regardless of brain metastasis is statistically significantly different among the treatment arms (62% [Arm 1] vs. 85% [Arm 2] vs. 89% [arm 3], p-value = 0.03). Specifically, the higher dose arms (Arms 2 & 3) had a significantly higher incidence of ND than the lower dose group (Arm 1). Table 3 further demonstrates that the incidence of CNt (a decline in at least one of the aforementioned tests and without evidence of brain metastasis at 12 month follow-up) also was significantly limited only to the treatment arms delivering 36 Gy total dose (Arms 2&3) versus the lower dose arm (Arm 1) (p = 0.02). Table 4 shows the results of a logistic regression model of CNt. It found that both higher total dose arms (p = 0.03) and age (p = 0.005) were statistically significant factors for the development of CNt. This finding is compelling. However, the number of patients available at 12 months is too small due to the high drop-out rate. Therefore, these findings should be taken with caution. Lastly, Table 5 shows that at the 12 month follow-up interval, the overwhelming majority (at least 88%) of patients with ND did not have concomitant brain metastasis.
Table 3.
The Incidence of Neurologic Deterioration and Chronic Neurotoxicity** at 12 months
| No Neurologic Deterioration |
Neurologic Deterioration |
95% CI of Deterioration Percentage |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Comparison | n | % | n | % | p-value† | |
| Treatment Arm | 2.5 Gy × 10 | 17 | 38 | 28 | 62 | (50, 74) | 0.03 |
| 2.0 Gy × 18 | 3 | 15 | 17 | 85 | (72, 98) | ||
| 1.5 Gy × 24 | 2 | 11 | 17 | 89 | (78, 100) | ||
| Gender | Male | 13 | 28 | 33 | 72 | (61, 83) | 0.64 |
| Female | 9 | 24 | 29 | 76 | (65, 88) | ||
| Education Level | ≤ High School | 11 | 34 | 21 | 66 | (52, 79) | 0.12 |
| > High School | 8 | 19 | 35 | 81 | (72, 91) | ||
| Marital Status | Married/Living as married | 14 | 28 | 36 | 72 | (62, 82) | 0.59 |
| Single/Divorced/Widowed | 7 | 23 | 24 | 77 | (65, 90) | ||
| Age | ≤ 60 years | 13 | 41 | 19 | 59 | (45, 74) | 0.02 |
| >60 years | 9 | 17 | 43 | 83 | (74, 91) | ||
|
No Chronic Neurotoxicity |
Chronic Neurotoxicity |
95% CI of Chronic Neurotoxicity |
|||||
| Variable | Category | n | % | n | % | p-value† | |
| Treatment Arm | 2.5 Gy × 10 | 18 | 40 | 27 | 60 | (48, 72) | 0.02 |
| 2.0 Gy × 18 | 3 | 15 | 17 | 85 | (72, 98) | ||
| 1.5 Gy × 24 | 2 | 11 | 17 | 89 | (78, 100) | ||
| Gender | Male | 13 | 28 | 33 | 72 | (61, 83) | 0.84 |
| Female | 10 | 26 | 28 | 74 | (62, 85) | ||
| Education Level | ≤ High School | 11 | 34 | 21 | 66 | (52, 79) | 0.20 |
| > High School | 9 | 21 | 34 | 79 | (69, 89) | ||
| Marital Status | Married/Living as married | 14 | 28 | 36 | 72 | (62, 82) | 0.83 |
| Single/Divorced/Widowed | 8 | 26 | 23 | 74 | (61, 87) | ||
| Age | ≤ 60 years | 14 | 44 | 18 | 56 | (42, 71) | 0.009 |
| >60 years | 9 | 17 | 43 | 83 | (74, 91) | ||
Neurologic deterioration is defined as the deterioration in at least one of the following regardless of the development of brain metastasis at 12 months: Hopkins Verbal Learning Test (HVLT)-recall, HVLT-recognition, HVLT-delayed recall, Controlled Oral Word Association Test (COWAT), Trail Making Test (TMT) - A, or TMT - B.
Chronic neurotoxicity is defined as the deterioration in at least one of the following without development of brain metastasis at 12 months: HVLT-recall, HVLT-recognition, HVLT-delayed recall, COWAT, TMT - A, or TMT - B.
CI = Confidence Interval
From Cochran-Mantel Haenzel chi-square test.
Table 4.
Logistic Regression Model* of Chronic Neurotoxicity at 12 Months (n=75)
| Variable | Comparison | Odds Ratio | (95% CI) | p-value |
|---|---|---|---|---|
| Treatment Arm | 2.5 Gy × 10 vs. | RL | ||
| 2.0 Gy × 18 vs. | 8.00 | (1.29, 49.50) | 0.03 | |
| 1.5 Gy × 24 | 4.37 | (0.81, 23.60) | 0.09 | |
| Age | Continuous | 1.12 | (1.04, 1.21) | 0.005 |
| Education Level | ≤ High School | 2.96 | (0.85, 10.29) | 0.09 |
| > High School | RL |
A backward method was used. The following variables were initially entered into the model: Treatment arm (2.5 Gy × 10 (referent) vs. 2.0 Gy × 18 vs. 1.5 Gy × 18), age (continuous), gender (male (referent) vs. female), education level (≤ high school vs. > high school (referent)), and marital status (married/living as married (referent) vs. single/divorced/widowed). Parameters with a p < 0.1 remained in the model.
CI = Confidence Interval; vs. = versus; RL = Reference Level
Table 5.
Chronic Neurotoxicity/Neurologic Deterioration and Brain Mets at 12 Month
| Treatment Arm | Brain Mets Status | No Neurologic Deterioration |
Neurologic Deterioration |
No Chronic Neurotoxicity |
Chronic Neurotoxicity |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| 2.5 Gy × 10 | No brain mets | 76 | 74 | 26 | 93 | 76 | 73 | 26 | 96 |
| Brain mets | 27 | 26 | 2 | 7 | 28 | 27 | 1* | 4 | |
| 2.0 Gy × 18 | No brain mets | 44 | 88 | 16 | 94 | 44 | 88 | 16 | 94 |
| Brain mets | 6 | 12 | 1 | 6 | 6 | 12 | 1* | 6 | |
| 1.5 Gy × 24 | No brain mets | 37 | 76 | 15 | 88 | 37 | 76 | 15 | 88 |
| Brain mets | 12 | 24 | 2 | 12 | 12 | 24 | 2 | 12 | |
Brain metastasis developed after Month 12 evaluation
Mets = metastasis
DISCUSSION
Since the sentinel meta-analysis published in 1999 regarding 987 patients from 7 randomized trials [3], the standard of care for patients with LD SCLC who achieve a CR following chemoradiation for the primary disease has been to incorporate PCI into their therapeutic regimen. In addition to reducing the risk of brain metastases from 58.6% without PCI to 33.3% at 3 years, PCI was found to result in a 16% reduction in mortality rate that translated into a 5.4% increase in 3-year patient survival (from 15.3% in the group not having treatment to 20.7% in the PCI cohort) [3].
The international PCI trial [4] was a prospectively randomized Phase III that enrolled 720 patients from September 1, 1999 through December 31, 2005 from 157 medical centers in 22 countries that was established to address the primary issue of the effect of increased total dose on the incidence of brain relapses as well as on patient survival. When RTOG 0212 was first being designed, it was deemed of utmost importance by the NCI for the RTOG study to contribute patients to the then still open multinational PCI study. By the close of this collaborative international effort two years later, the RTOG trial was able to contribute 146 patients (20.2%). One noteable difference between the European- initiated study and RTOG 0212 is that the former did not have a secondary randomization between conventional and altered fractionated PCI that did occur in the latter study. In fact, only 22% of patients that were randomized to receive 36 Gy were given accelerated hyperfractionated PCI (1.5 Gy twice daily for 24 Fxs) versus 78% who underwent conventional (2 Gy daily for 18 Fxs) elective brain irradiation in the international trial [4]. Nevertheless, the Phase III multi-national study [4] did demonstrate that there was no significant difference (p = 0.18) in the 2-year brain failure rates between the standard dose arm of 25 Gy total dose (29%) versus the 36 Gy higher dose group (23%). Furthermore, there actually was a significantly higher (p = 0.05) 2-year overall survival in the standard cohort (42%) versus 37% in the higher dose one that was attributed to an increase in the cancer-related deaths in the latter group [4].
Since RTOG 0212 was constructed with a two tiered randomization scheme, it was powered to evaluate the impact of total PCI dose on the development of CNt and deterioration in QoL as defined by this study. The initial results at approximately 12 months post-PCI found a significant deterioration in neurocognitive functions among those that received the higher total dose of 36 Gy to the brain (p = 0.03) as well as a significant increase in the occurrence of chronic neurotoxicity (p = 0.02) as defined by the parameters of this study. In order to compare the impact of treatment schedules on study endpoints, a logistic regression analysis determined that those patients who were randomized to Arm 2 (p = 0.03) were the most likely to have abnormalities in neuropsychological testing compared to Arm 1.
Several factors in the literature have been reported as being associated with the risk of long-term neurotoxicity, including age greater than 60 years [25], a daily fraction size of greater than 3 Gy [25, 26, 27, 28, 29], and concurrent use of chemotherapy during PCI [25, 26, 28, 30, 31, 32, 33]. In RTOG 0212, age as a continuous variable was the most significant predictor of CNt (p = 0.005). Of note is the fact that this present study did not use any daily fraction size of greater than 3 Gy or permit chemotherapy to be administered during the PCI.
However, the use of hyperfractionated radiotherapy in this randomized trial did not yield a significant reduction in late neurologic effects that has been previously associated with altered dose fractionation schedules in other tumor sites [34] as well as for a small cohort of patients with LD SCLC having a CR to chemotherapy followed by consolidative chest irradiation [35]. If one assumes no tumor proliferation and an alpha/beta ratio of 2 for normal brain tissue and a ratio of 10 for brain tumor cells, then calculations for both tumor and normal brain tissue biologically equivalent doses (BEDs) can be determined for all three arms as follows: 1) Arm 1 – 41.4 Gy/63.0 Gy; 2) Arm 2 – 43.2Gy /72.0 Gy; and 3) 31.3 Gy/56.3 Gy. Thus, the ratios of tumor BED to normal brain BED for the three arms would be 0.56 for Arm 1, 0.6 for Arm 2, and 0.66 for Arm 3. Therefore, Arm 2 should be 7% (0.6/0.56) “better” and Arm 3 18% (0.66/0.56) “better” than Arm 1 in preventing brain relapse without significant increase in overall treatment time. Yet, despite a greater than 50% drop-out rate of patients being followed at 12 months post-PCI, this study’s results demonstrated that the higher total dose of 36 Gy and at least daily fractionation to this higher dose were significantly associated with the development of CNt.
In this study, patients did not undergo NPTB or QoL measurements before induction therapies. Yet, prior to PCI, the baseline assessments did manifest abnormalities in language, visual and spatial scanning, attention, sequencing, and speed among all three arms. Other series have clearly demonstrated that many patients with SCLC have demonstrable neurological and cognitive impairments prior to the onset of PCI [36, 37, 38, 39, 40, 41, 42, 43, 44]. Proposed causes have been attributed to the effects of chemotherapy on the brain, a paraneoplastic syndrome, aging, an immunologic dysfunction, or even microscopic cranial metastases at diagnosis resulting in frontal or subcortical cognitive defects. Future investigations should consider incorporating neurocognitive evaluations as part of the initial staging of these patients that will allow better understanding of this phenomenon. Finally, this study found that the development of ND and/or CNt at 12 months was generally not associated with the presence of brain metastasis, which contradicts other reports [45. 46] suggesting that objective declines in neurocognitive function might be an early predictor of brain relapse.
Even though the number of patients available for analysis at 12 months post-PCI is limited, it should be noted that despite the increase in CNt of patients in Arm 2, this group also had the lowest proportion of brain relapses (10%) versus those in Arm 1 (22%) and in Arm 3 (21%). In addition, more than 60% of patients receiving 25 Gy and 80–90% of those having 36 Gy PCI had documented neurotoxicity at one year. Even though there was not any observed impact on QoL measurements at 12 months, additional studies are warranted regarding methods of reducing the risk of CNt for all of these patients who still need to receive life-saving PCI.
Perhaps, with further long-term follow-up, RTOG 0212 could demonstrate a sustained reduction in brain metastases that would ultimately translate into an improvement in patient survival for those patients treated to the higher conventionally delivered total dose of 36 Gy. Of course any such finding would need to be weighed against rate of CNt in this group of patients versus the lower PCI dose group. Yet, at the present time, the standard of care for the delivery of PCI for those patients who ultimately achieve a CR to chemoradiation remains 25 Gy given in daily 2.5 Gy fractions.
Acknowledgments
Supported by RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 grants from the NCI. ClinicalTrials.gov identifier: NCT00057746. This manuscript’s contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented in part at the ASTRO 2009 Annual Meeting, November 3, 2009
“Conflicts of Interest Notification”
No conflicts of interest exist for any of the authors.
REFERENCES
- 1.Ahmedin Jemal, DVM, PhD, Rebecca Siegel, MPH, Elizabeth Ward, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. [Google Scholar]
- 2.Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep. 1981;65:811–814. [PubMed] [Google Scholar]
- 3.Auperin A, Arrriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 4.Le Pechoux C, Dunant A, Suresh S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A randomized clinical trial. Lancet Oncol. 2009;10:467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 5.Benedict RHB, Schretlen D, Groinger L, et al. Hopkins Verbal Learning Test-Revised: Normative data and abalysis of Inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 6.Benton AL, Hamsher K, De S. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- 7.Lezak MD. Neuropsychological Assessment. 3ed ed. New York: Oxford University press; 1995. [Google Scholar]
- 8.Osoba D, Aaronson N, Zee B, Sprangers M, te Velde A. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res. 1997;6:103–108. doi: 10.1023/a:1026429831234. [DOI] [PubMed] [Google Scholar]
- 9.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 11.Bergman B, Sullivan M, Sorenson S. Quality of life during chemotherapy for small cell lung cancer. II. A longitudinal study of the EORTC Core Quality of Life Questionnaire and comparison with the Sickness Impact Profile. Acta Oncol. 1992;31:19–28. doi: 10.3109/02841869209088260. [DOI] [PubMed] [Google Scholar]
- 12.Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol. 1992;31:311–321. doi: 10.3109/02841869209108178. [DOI] [PubMed] [Google Scholar]
- 13.Osoba D, Zee B, Pater J, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res. 1994;3:353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 14.Niezgoda HE, Pater JL. A validation study of the domains of the core EORTC quality of life questionnaire. Qual Life Res. 1993;2:319–325. doi: 10.1007/BF00449426. [DOI] [PubMed] [Google Scholar]
- 15.Ringdal GI, Ringdal K. Testing the EORTC Quality of Life Questionnaire on cancer patients with heterogeneous diagnoses. Qual Life Res. 1993;2:129–140. doi: 10.1007/BF00435732. [DOI] [PubMed] [Google Scholar]
- 16.Sigurdardottir V, Bolund C, Brandberg Y, Sullivan M. The impact of generalized malignant melanoma on quality of life evaluated by the EORTC questionnaire technique. Qual Life Res. 1993;2:193–203. doi: 10.1007/BF00435223. [DOI] [PubMed] [Google Scholar]
- 17.Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995;13:1249–1254. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract. 2000;6:39–49. doi: 10.1046/j.1365-2753.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 20.Kruskal WH, Wallis A. Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association. 1952;47:583–621. [Google Scholar]
- 21.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- 22.Alan Agresti. Categorical data analysis. second edition. New York: Wiley; 2002. [Google Scholar]
- 23.Hosmer David W, Stanley Lemeshow. Applied Logistic Regression. 2nd ed. New York: Chichester, Wiley; 2000. [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual tatistics. 1988;16:1141–1143. [Google Scholar]
- 25.Glantz MJ, Choy H, Yee L. Prophylactic cranial irradiation in small cell lung cancer: Rationale, results, and recommendations. Seminars in Oncology. 1997;24(4):477–483. [PubMed] [Google Scholar]
- 26.Ball DL, Matthews JP. Prophylactic cranial irradiation: More questions than answers. Semin Radiat Oncol. 1995;5:61–68. doi: 10.1054/SRAO00500061. [DOI] [PubMed] [Google Scholar]
- 27.Shaw EG, Su JQ, Eagan RT, et al. Prophylactic cranial irradiation in complete responders with small-cell lung cancer : analysis of the Mayo Clinic and North Central Cancer Treatment Group data bases. J Clin Oncol. 1994;12:2327–2332. doi: 10.1200/JCO.1994.12.11.2327. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BE, Patronas Hayes W, et al. Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: Further follow-up of 6-to 13-year survivors. J Clin Oncol. 1990;8:48–56. doi: 10.1200/JCO.1990.8.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Tomio L, Romano M, Zanchin G, et al. Ultrarapid high-dose course of prophylactic cranial irradiation in small-cell lung cancer: Evaluation of late neurologic morbidity in 16 long-term survivors. Am J Clin Oncol. 1998;2:84–90. doi: 10.1097/00000421-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Frytak S, Shaw JN, Lee RE, et al. Treatment toxicities in long-term survivors of limited small cell lung cancer. Cancer Investigations. 1988;6:669–676. doi: 10.3109/07357908809078033. [DOI] [PubMed] [Google Scholar]
- 31.Frytak S, Shaw JN, O’Neill BP, et al. Leukoencephalopathy in small cell lung cancer patients receiving prophylactic cranial irradiation. Am J Clin Oncol. 1989;12:27–33. doi: 10.1097/00000421-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca R, O’Neill BP, Foote FL, et al. Cerebral toxicity in patients treated for small cell carcinoma of the lung. Mayo Clin Pro. 1999;74:461–465. doi: 10.4065/74.5.461. [DOI] [PubMed] [Google Scholar]
- 33.Ahles TA, Silberfarb PM, Herndon IIJ, et al. Psychologic and neuropsychologic functioning of patients with limited small-cell lung cancer treated with chemotherapy and radiation therapy with or without warfarin: A study by the Cancer and Leukemia Group B. J Clin Oncol. 1998;16:1954–1960. doi: 10.1200/JCO.1998.16.5.1954. [DOI] [PubMed] [Google Scholar]
- 34.Thames HD, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered dose fractionation: Implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 35.Wolfson AH, Bains Y, Lu J, et al. Twice-daily prophylactic cranial irradiation for patients with limited disease small-cell lung cancer with complete response to chemotherapy and consolidative radiotherapy. Report of a single institutional phase II trial. Am J Clin Oncol. 2001;24:290–295. doi: 10.1097/00000421-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: Results of a multicentre randomised trial. Eur J Cancer. 1997;33:1752–1758. doi: 10.1016/s0959-8049(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 37.Ahles TA, Silberfarb PM, Herndon IIJ, et al. Psychologic and neuropsychologic functioning of patients with limited small-cell lung cancer treated with chemotherapy and radiation therapy with or without warfarin: A study by the Cancer and Leukemia Group B. J Clin Oncol. 1998;16(5):1954–1960. doi: 10.1200/JCO.1998.16.5.1954. [DOI] [PubMed] [Google Scholar]
- 38.Sculier J-P, Feld R, Evans WK, et al. Neurological disorders in patients with small cell lung cancer. Cancer. 1987;60:2275–2283. doi: 10.1002/1097-0142(19871101)60:9<2275::aid-cncr2820600929>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Van Oosterhout AGM, Boon PJ, Houx PJ, et al. Follow-up of cognitive functioning in patients with small cell lung cancer. Int J Radiat Oncol Biol Phys. 1995;31(4):911–914. doi: 10.1016/0360-3016(94)00579-6. [DOI] [PubMed] [Google Scholar]
- 40.Van Oosterhout AGM, Ganzevles GJ, Wilmink JT, et al. Sequelae in long-term survivors of small cell lung cancer. Int J Radiat Oncol Biol Phys. 1996;34(5):1037–1044. doi: 10.1016/0360-3016(95)02257-0. [DOI] [PubMed] [Google Scholar]
- 41.Gregor A. Prophylactic cranial irradiation in small-cell lung cancer: Is it ever indicated? Oncology. 1998;12(1) Suppl 2:19–24. [PubMed] [Google Scholar]
- 42.Komaki R, Meyers CA, Shin DM, et al. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys. 1995;33(1):179–182. doi: 10.1016/0360-3016(95)00026-U. [DOI] [PubMed] [Google Scholar]
- 43.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12:231–235. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 44.Kanard A, Frytak S, Jatoi A. Cognitive dysfunction in patients with small-cell lung cancer: Incidence, causes, and suggestions of management. J Support Oncol. 2004;2(2):127–132. [PubMed] [Google Scholar]
- 45.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: Cognitive deterioration precedes MRI progression. Neuro-Oncology. 2003;5:89–95. doi: 10.1215/S1522-8517-02-00026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosshans DR, Meyers CA, Allen PK, et al. Neurocognitive function in patients with small cell lung cancer: Effect of Prophylactic Cranial Irradiation. Cancer. 2008;112:589–595. doi: 10.1002/cncr.23222. [DOI] [PubMed] [Google Scholar]