Abstract
Background
Statin use and serum cholesterol reduction have been proposed as preventions for dementia and mild cognitive impairment (MCI).
Methods
1,604 and 1,345 eligible participants from the Baltimore Longitudinal Study of Aging (BLSA) were followed after age 50 for a median time of around 25 years, to examine incidence of dementia (n=259) and MCI (n=138), respectively. Statin use (ever-use and time-dependent use), total cholesterol levels (TC; first-visit and time-dependent), TC change trajectory from first-visit, and high-density lipoprotein (HDL-C):TC ratio (first-visit and time-dependent) were main exposures of interest. Cox proportional hazards models were used.
Results
Participants with incident dementia had higher first-visit TC compared to participants who remained free of dementia and MCI, while first-visit TC was higher among statin ever-users compared to never-users (age-unadjusted associations). Statin users had two to three-fold lower risk of developing dementia (HR=0.41; 95% CI: 0.18–0.92), but not MCI, when considering time-dependent “statin use” with propensity score model adjustment. This association remained significant independently of serum cholesterol exposures. An elevated first-visit TC was associated with reduced MCI risk (Upper quartile (Q4) vs. Q1: HR=0.51; 95% CI=0.29–0.90). Compared to the lowest quartile (Q1: 0.00–0.19), HDL-C:TC (time-dependent) in (Q2: 0.19–0.24) was associated with reduced MCI risk (HR=0.53; 95%CI: 0.30–0.94). Among men only, TC decline from first-visit was significantly associated with increased dementia risk (HR=4.21; 95% CI: 1.28–13.85).
Conclusions
Statins may have multifactorial effects on dementia but not MCI risk. Future interventions may be warranted and research should focus on optimal serum TC, HDL-C:TC ratio and TC change trajectories.
Keywords: Statins, serum cholesterol, dementia, mild cognitive impairment, aging
Statins have been proposed as agents for preventing dementia and other neurological disorders [1–6, 7], though a recent meta-analysis of prospective cohort and case-control studies suggested that statins are less beneficial in reducing dementia risk than expected [8]. More recent cohort studies conducted since this meta-analysis suggested that statins may have a protective effect against incidence of dementia, mild cognitive impairment (MCI), and Alzheimer’s disease or their combination [9–11], although at least one other study did not find an association[12].
The direct effects of plasma total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) on the incidence of dementia and cognitive decline are controversial based on recent epidemiological evidence (e.g. [13–18]). Due to the potential multifactorial actions of statins [19], it is biologically plausible that statin therapy may reduce risk of dementia and even delay onset of MCI, independently of the effects of statin on serum cholesterol.
We analyzed data from a large prospective study with median follow-up time of over 20 years. Our main study aims were to examine: 1) The association of statin use with incidence of dementia and MCI, and whether it is altered by serum cholesterol levels; 2) The putative independent effect of first-visit or time-dependent serum TC and TC changes on incidence of dementia and MCI; 3) The influence of first-visit and time-dependent HDL-C:TC ratio on dementia and MCI risks.
METHODS
Study Design and participants
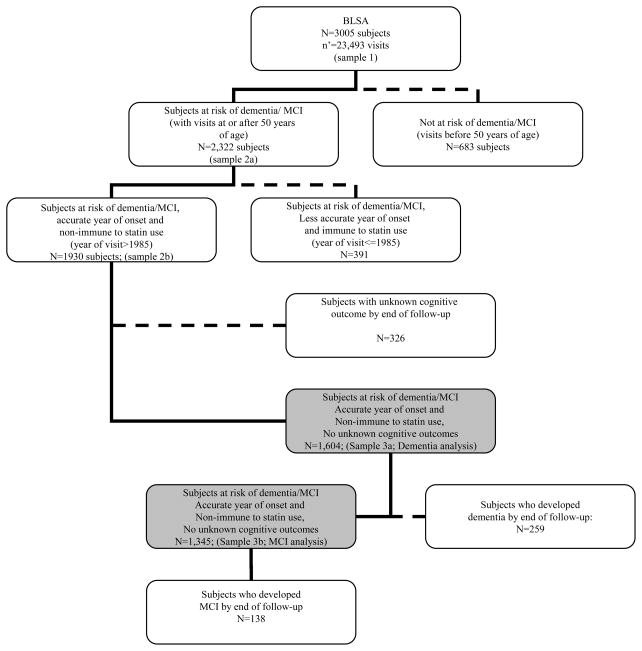
We used data from the Baltimore Longitudinal Study of Aging (BLSA), an ongoing prospective study of community-dwelling adults [20]. BLSA participants were initially recruited in 1958, new participants were continuously enrolled since then, and most participants had at least one follow-up interview after 1–2 years interval, though frequency of follow-up and inter-wave period varied for each BLSA participant. In our present analysis, with dates of visits ranging between Feb. 6th, 1958 and August 3rd, 2006, about 75% of the total sample (n=3,005) had two visits or more. Participants became at risk at age 50 years and exited follow-up at first failure, defined as incident MCI or dementia at or beyond age ≥50 years or when censored at last examination visit (end of follow-up), or due to death or loss to follow-up. Of the original sample (n=3,005 BLSA participants, age range: 17–97 at first-visit; 60.1% men), 2,322 were at risk of dementia or MCI, given that they had at least one visit 50 years of age. As both statin use and case conferencing were initiated in the mid-1980s, participants having all visits prior to 1985 were excluded from this study (n=391), leaving 1,930 eligible subjects. Participants with unknown outcomes by the end of follow-up (n=326 out of 1,930) were also excluded, resulting in 1,604 eligible participants in our final analysis with dementia as the main outcome. In analyses in which MCI was the main outcome, participants with incident dementia during follow-up were excluded leaving 1,345 at risk for MCI (Figure 1). The protocol of the BLSA was reviewed by the Institutional Review Board on Human Subjects at the National Institute on Aging, Intramural Research Program.
Figure 1.
Diagram for inclusion and exclusion of BLSA participants into main analyses
Notes: BLSA=Baltimore Longitudinal Study on Aging; Sample 1 was used for prediction of TC trajectories with linear mixed models; Samples 3 was used for fitting Cox proportional hazard models and running Kaplan-Meier survival curves. “Subjects immune to statin use” are those with visits that preceded the introduction of statins into the market (i.e. prior to 1985). “Subjects with less accurate year of onset” are those whose diagnosis of dementia or MCI was done retrospectively since case conferencing was initiated in the mid-80s.
Incident Dementia and MCI
All participants were followed every one to two years depending on age and were reviewed at a consensus conference if their Blessed Information Memory Concentration score [21] was ≥3, if their informant or subject Clinical Dementia Rating (CDR) [22] score was ≥0.5, or if their Dementia Questionnaire (DQ) [23] was abnormal. All participants, regardless of screening tests, were evaluated by case conference at the time of death or withdrawal.
Dementia diagnosis was determined at a consensus conference based on criteria from the DSM-III-R [24] and from the National Institute of Neurological and Communication Disorders—Alzheimer’s Disease and Related Disorders Association[25]. Diagnosis required evidence of a progressive cognitive syndrome, including memory decline and functional loss based on self-reported and informant-reported Clinical Dementia Ratings [22, 26–27]. The method of diagnosis has been detailed and validated elsewhere[28].
A diagnosis of MCI not meeting criteria for dementia was made following the Petersen criteria [29] when participants had either single domain cognitive impairment (usually memory) or cognitive impairment in multiple domains without significant functional loss in activities of daily living (ADL; assessed with the CDR and Pfeffer Functional Activities Questionnaire). Cases of MCI were retained in the “at risk for dementia” group. The year of onset for MCI was estimated using the same methodology as for dementia. In our present analysis, incident MCI was considered as one of the main outcomes of interest. Among the population at risk of developing either MCI or dementia, those who developed dementia by end of follow-up were removed from this analysis at the beginning of follow-up, leaving the contrast between MCI and the non-demented group.
Statin use
A detailed inventory of all over-the-counter and prescription medications in current use were obtained at each visit. Participants using lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin, or fluvastatin were considered as having used statins at a particular visit. Two exposure variables were constructed to examine effect of statin use on MCI and dementia risk, alternatively. Definition 1 (Ever-use of statins, time-independent): A person is exposed if they ever-used statins beyond age 50 years but prior to incidence of the dementia or MCI outcomes or censoring at end of follow-up. Eligible participants are considered unexposed if they never-used statins (n=1,486) or if they used them only at or prior to age 50 (n=3) or only at or after onset of dementia or MCI (n=5). Definition 2 (Time-dependent use of statins): A person is considered a user at first use and afterwards as long as they were free of outcome at that point in time and as long as statins are used beyond 50 years of age.
Serum cholesterol exposures
Antecubital venous blood samples were drawn following an overnight fast and used to determine plasma lipid levels. TC (mg/dL) was determined by enzymatic methods (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, TX 75015). HDL-C concentration (mg/dL) was assessed by dextran sulfate-magnesium precipitation procedures [30]. Measurements on TC and HDL-C were carried out at different times for each participant. TC and the HDL-C:TC ratio were examined at first-visit (i.e. visit 1) as well as time-dependent variables. In the latter, missing values were imputed with values of TC or HDL-C in the preceding non-missing visit, using Stata’s stfill command with forward option [31]. Analysis was conducted using quartiles of cholesterol exposures, which were classified as such based on available data for eligible participants at first-visit (“time-independent” approach) or over the follow-up period (“time-dependent” approach). Moreover, TC change trajectory between first-visit and age 50 (when follow-up started for dementia and MCI risk started) was also considered. To this end, a multivariable linear mixed model was carried out and empirical Bayes estimator of slope (annual rate of change) at first-visit age was predicted (See Appendix 1 for more detail). The slope was then dichotomized in our final analyses as “same or upward sloping” trajectory (when the annual rate of change was 0 or positive) and “downward sloping” trajectory (when the annual rate of change was negative).
Covariates
Potentially confounding covariates were measured at first-visit for eligible participants. Covariates included: (1) Demographic and lifestyle factors such as age at first-visit, sex, race and ethnicity, education (years of schooling), and smoking status (never, former or current smoker); (2) Self-reported history of type 2 diabetes, hypertension, cardiovascular disease or CVD (stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation) and dyslipidemia; (3) Directly measured metabolic outcome variables: Body mass index (BMI=weight in kg over squared height in m2), blood pressure (systolic and diastolic in mm Hg) and fasting blood glucose (in mg/dL). Due to appreciable missing data on many metabolic variables, only BMI and SBP were considered in the multivariable analyses as potential confounders.
Statistical Analysis
All analyses were performed using Stata version 10.0 [31]. First, statin users were compared to non-users and dementia and MCI cases were compared to non-cases, using ANOVA, t-test and chi-square tests. Second, to examine the associations between statin use and cholesterol exposures on one hand and incidence of dementia or MCI on the other, survival analyses were conducted (See aims 1, 2 and 3 in introduction). To this end, Kaplan-Meier survival curves and log-rank tests were used to compare the number of incident dementia or MCI cases by exposure category. We further conducted Cox proportional hazards (PH) models to examine if dementia or MCI risks were associated with statin use (time-dependent), after adjusting for various socio-demographic, lifestyle and metabolic factors. Another set of analyses examined the effects of serum cholesterol on dementia and MCI risks, adjusting for statin use. In both analyses, the dependent variables were age at dementia or MCI onset or the last observed (censored) age of non-cases, adjusting for covariates (See covariates section). A sensitivity analysis was conducted to account for confounding by indication[32]. In particular, we estimated probability of statin use from a multivariable logistic model in which socio-demographic, first-visit smoking status, metabolic factors (mainly BMI, SBP and DBP at first-visit) and history of co-morbid conditions (including dyslipidemia and CVD) at first-visit were included as predictors. This predicted probability, also known as the propensity score (PS) [33] was then grouped into quintiles and introduced into a Cox PH model where observed statin use was the only exposure variable predicting risk of dementia (“PS adjustment method”). Effect modification by sex was examined in part of the analysis. Type I error used for statistical significance was 0.05 for all analyses.
RESULTS
First-visit Characteristics of the BLSA study sample
Of 1,604 eligible participants at risk and after a median follow-up of 24.9 years, two-hundred and fifty-nine participants developed dementia (70%; n=182 were AD). Among the population at risk excluding dementia cases that developed by end of follow-up (n=259), 138 developed MCI after a median 25.1 years of follow-up. Statin ever-users (n=110) differed from statin never-users (n=1,494) on most first-visit covariates, except for type 2 diabetes, hypertensive status, systolic and diastolic blood pressures (Table 1). Compared to never-users, statin ever-users were older, had higher proportions women and minority ethnic groups, and were less likely to be current smokers. Ever-users also had a greater prevalence of CVD, dyslipidemia (p<0.05 based on χ2 test); and they had higher TC, lower HDL-C and higher glucose levels on average compared to non-users (p<0.05 based on two-sided t-test). The lack of correspondence between statin ever-use and dyslipidemia at first-visit is due to the differences in the pre-defined time frames for the two variables. Comparing participants by dementia or MCI status, both MCI and dementia cases were older at first-visit, less likely to be current smokers and had a higher prevalence of self-reported hypertension at first-visit (p<0.05 for χ2 test) compared to non-cases (Table 1). They also had higher mean levels of SBP and DBP (p<0.05 based on two-sided t-test with Bonferroni correction). Dementia cases, but not MCI cases, had significantly higher TC compared to non-cases (229.4 vs. 218.9, p<0.05 based on two-sided t-test with Bonferroni correction). However, dementia cases were the least likely to report having dyslipidemia at first-visit (0.8% vs. 5.1% among MCI and 6.0% among non-cases, p<0.05 for χ2 test). In fact, statin use was lowest among dementia cases, followed by MCI and was the highest among non-cases (p<0.05 for χ2 test). Moreover, incident MCI cases had significantly higher glucose levels and lower HDL-C compared to non-cases (p<0.05 based on two-sided t-test with Bonferroni correction). Statins used were mostly lipophilic and mean age at self-reported statin use (n=110) was 72.7 years with a SD=9.0, with range of 51.2–92.4 years.
Table 1.
Characteristics of participants included in main analysis according to “statin ever-use” (definition 1) ‡ and Dementia or MCI status; Baltimore Longitudinal Study of Aging
| Statin ever-use, total‡ |
Dementia/MCI status |
|||||
|---|---|---|---|---|---|---|
| All | Statin never-user | Statin ever-user | No Dementia or MCI | Incident Dementia | Incident MCI | |
| N | 1,604 | 1,494 | 110 | 1,205 | 259 | 138 |
| Sex (% women) | 38.5 | 37.7 | 49.1* | 39.6 | 36.3 | 33.3 |
| Race/ethnicity (%) | ||||||
| NH white | 91.4 | 91.8 | 86.4 | 89.8 | 97.1 | 95.7* |
| NH black | 7.3 | 7.0 | 10.9 | 8.6 | 2.5 | 4.3 |
| Others | 1.3 | 1.2 | 2.7 | 1.6 | 0.4 | 0.0 |
| Education (yr.); mean (SD) | 16.6 (2.8) | 16.7 (2.8) | 15.7 (2.8)* | 16.7 (2.7) | 16.7 (2.8) | 16.0 (3.3)a* |
| Smoking status | ||||||
| Never | 40.3 | 40.1 | 42.1* | 38.7 | 42.4 | 50.0* |
| Former | 38.7 | 38.0 | 49.5 | 38.0 | 44.0 | 36.0 |
| Current | 21.0 | 21.9 | 8.4 | 23.3 | 13.6 | 14.0 |
| Type 2 diabetes (%) | 2.1 | 2.0 | 3.6 | 2.3 | 0.4 | 3.6~ |
| Hypertension (%) | 35.5 | 35.2 | 38.2 | 30.1 | 56.4 | 45.6* |
| Cardiovascular disease† (%) | 5.0 | 4.5 | 10.9* | 4.6 | 5.8 | 7.2 |
| Dyslipidemia (%) | 5.2 | 4.1 | 20.0* | 6.0 | 0.8 | 5.1* |
| Age at first-visit (yr.); mean (SD) | 57.6 (18.4) | 57.6 (18.4) | 63.5 (8.4)* | 51.5 (15.7) | 81.9 (7.7) | 65.7 (13.7)* |
| ≤20 | 0.1 | 0.1 | 0.0* | 0.1 | 0.0 | 0.0* |
| 21–29 | 6.0 | 6.4 | 0.0 | 8.0 | 0.0 | 0.0 |
| 30–39 | 15.1 | 16.3 | 0.0 | 19.6 | 0.0 | 5.1 |
| 40–49 | 18.1 | 19.4 | 0.0 | 22.9 | 0.0 | 10.1 |
| 50–59 | 15.3 | 13.6 | 38.2 | 17.9 | 1.2 | 17.4 |
| 60–69 | 14.9 | 13.0 | 40.0 | 16.3 | 6.2 | 19.6 |
| 70–79 | 14.9 | 14.9 | 15.4 | 10.5 | 25.6 | 33.3 |
| 80+ | 15.6 | 16.3 | 6.4 | 4.7 | 67.2 | 14.5 |
| BMI (kg/m2); mean (SD) | 24.9 (3.6) | 24.9 (3.5) | 25.6 (3.7)* | 24.9 (3.6) | 24.7 (3.2) | 25.6 (3.3)* |
| Underweight (BMI≤18.5); % | 1.7 | 1.8 | 0.0~ | 1.7 | 2.3 | 0.0~ |
| Normal weight (18.5≤BMI≤24.9) | 54.2 | 54.5 | 50.9 | 55.2 | 55.0 | 44.9 |
| Overweight (25.0≤BMI≤29.9) | 36.5 | 36.5 | 39.4 | 35.1 | 36.8 | 46.4 |
| Obese (BMI≥30) | 7.6 | 7.2 | 12.7 | 7.9 | 5.8 | 8.7 |
| Systolic blood pressure (mm Hg.); mean (SD) | 127.0 (19.0) | 126.8 (19.9) | 129.3 (19.4) | 126.1 (18.7) | 134.8 (20.5) a | 135.4 (20.6)a* |
| Diastolic blood pressure (mm Hg.); mean (SD) | 79.1 (10.7) | 79.1 (10.8) | 79.3 (9.8) | 78.9 (10.6) | 80.8 (10.2) a | 81.3 (11.3)a* |
| Total cholesterol level (mg/dl.); mean (SD) | 219.9 (40.6) | 217.3 (40.0) | 245.5 (38.6)* | 218.9 (40.3) | 229.4 (36.1) a | 226.1 (41.8)* |
| HDL-C (mg/dl.); mean (SD) | 49.0 (13.0) | 49.8 (12.9) | 44.5 (13.0)* | 45.2 (10.5) | 45.1 (8.0) | 42.6(7.9) a* |
| Fasting plasma glucose (mg/dl.); mean (SD) | 98.5 (14.2) | 98.0 (13.5) | 102.5 (19.3)* | 97.7 (13.3) | 97.9 (10.4) | 103.7 (15.7)a* |
| Ever-used statins, total‡ | — | — | — | |||
| % | 8.0 | 2.3 | 5.1* | |||
| n | 97 | 6 | 7 | |||
| Ever-used statins, lipophilic | — | — | — | |||
| % | 7.3 | 2.3 | 3.6 | |||
| n | 88 | 6 | 5 | |||
| Ever-used statins, hydropophilic | — | — | — | |||
| % | 1.7 | 0.0 | 1.5 | |||
| n | 21 | 0 | 2 | |||
| Mean frequency of statin ever-use, (SD) | 0.26 (1.1) | 0.03 (0.24)a | 0.09 (0.56) | |||
| Range | 0–11 | 0–2 | 0–6 | |||
Abbreviations: BMI=Body Mass Index; HDL-C=High Density Lipoprotein-Cholesterol; Hg=Mercury; MCI=Mild Cognitive Impairment; NH=Non-Hispanic; SD=Standard Deviation.
p<0.10;
p<0.05 based on χ2 test or ANOVA.
Statistically significant (p<0.05) two-side t-test after bonferroni correction, comparing MCI or dementia group to “No Dementia or MCI” group.
Reported any of the following conditions at first-visit: stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation.
Statin use prior to onset of dementia or MCI among dementia or MCI cases and after age 50 for all subjects. The mean age of statin use among eligible participants at risk of dementia was 72.7 (Standard Deviation, SD=9.0; Range: 51.2–92.4 years).
Statin use, dementia and MCI risk
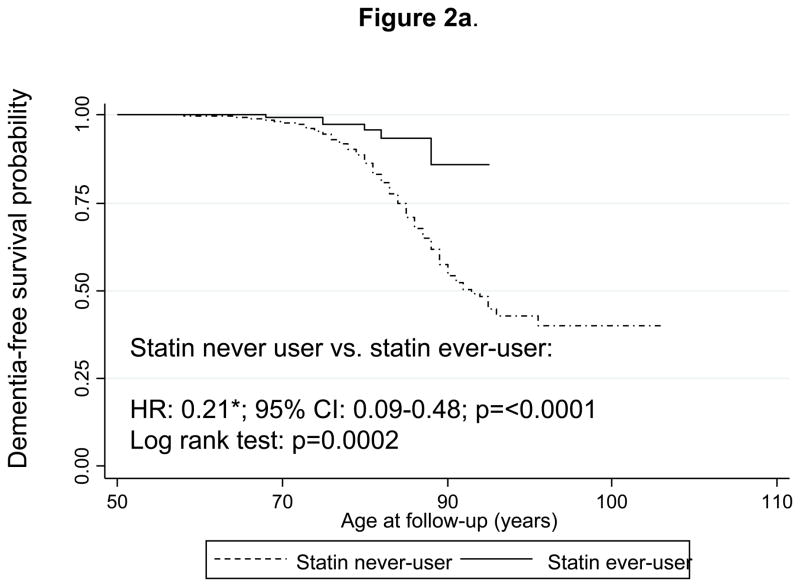
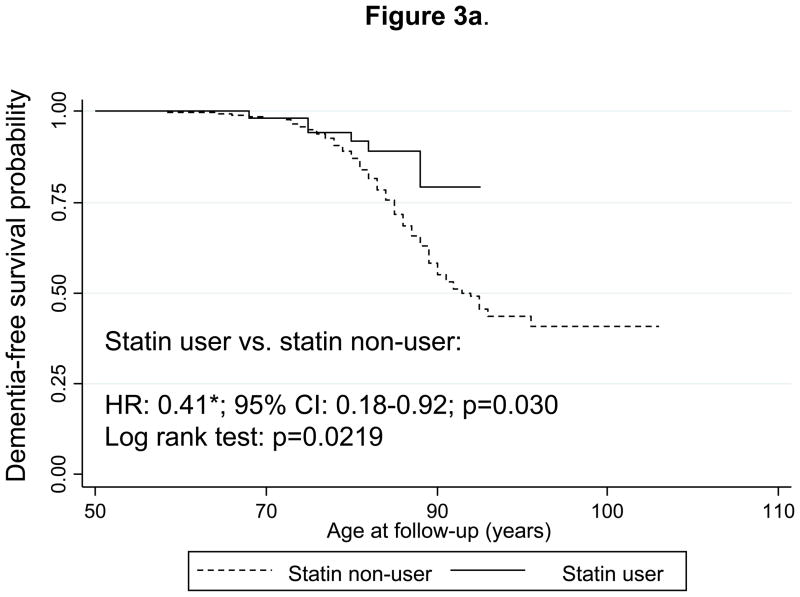
Figure 2a shows Kaplan-Meier survival curves for incident dementia comparing “statin ever-users” with “statin never-users” (time-independent variable). The curves indicate that statin ever-users are at a reduced risk of developing dementia over time with a log-rank test p-value of 0.0002. Multivariable-adjusted Cox PH models yielded a hazard ratio indicating that statin users were at around five-fold lower risk of developing dementia compared to statin non-users (HR: 0.21; 95% CI: 0.09–0.48). Substituting the statin ever-use exposure (definition 1) with the time-dependent definition of statin use (definition 2, See Methods section) and combining it with the PS modeling approach, yielded a HR of 0.41 with a 95% CI:0.18–0.92 (Figure 3a). This same analysis conducted on only AD cases (n=178 failures; N=1,561 participants at risk in this model) yielded a HR of 0.30 with a 95% CI: 0.10–0.95. In addition, when all participants with unknown outcomes were re-included into the risk set in a sensitivity analysis, both HRs with their 95% CI were not appreciably altered (data not shown).
Figure 2.
Figure 2a. Kaplan-Meier survival curve of time to incidence of dementia by use of statins (time-independent): Cox Proportional Hazards model and Log-rank test.
Notes: A participant is defined as a statin user prior to onset of dementia or MCI or by end of follow-up if a non-case but beyond age 50 years. Cox PH model analysis controlled for sex, education, race/ethnicity, age at first-visit (continuous), smoking status at first-visit, chronic conditions at first-visit (type 2 diabetes, hypertension, dyslipidemia and CVD), body mass index and systolic blood pressure (continuous) at first-visit and was based on 1,561 participants at risk for dementia and for use of statins (visits at or after 1985) and 252 failures (person-years= 37,860). Log-rank test was based on 259 incident cases. Six statin-users were observed incident dementia cases, while 22.7 were expected to become incident cases by chance, which yielded a Log-rank χ2 test of 13.93 (1 d.f.); p=0.0002.
*p<0.05 for null hypothesis that Loge(HR)=0.
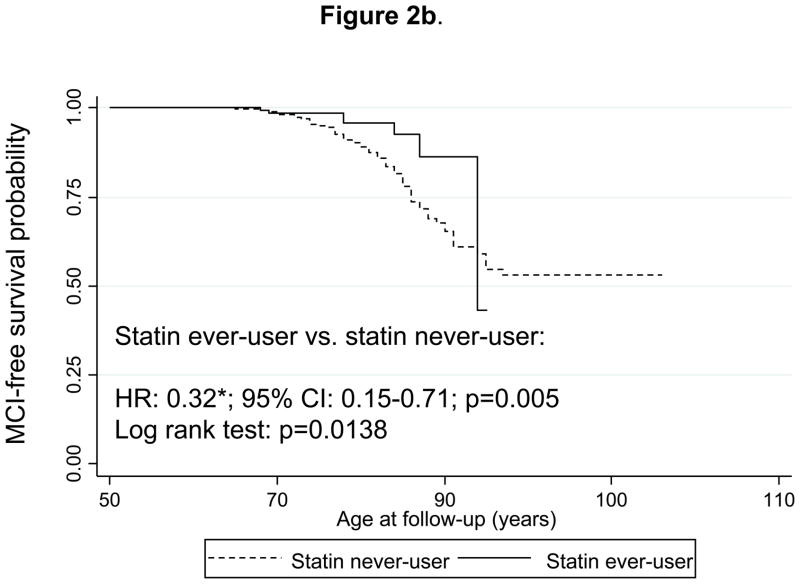
Figure 2b. Kaplan-Meier survival curve of time to incidence of mild cognitive impairment (MCI) by use of statins (time-independent): Cox Proportional Hazards model and Log-rank test.
Notes: Statin use was defined the same way as in Figure 1a. Cox PH model analysis controlled for the same covariates as in Figure 1a, but was based on 1,308 subjects at risk for MCI and at risk of using statins (visits at or after 1985), with 133 MCI failures (person-years= 29,600). Log-rank test was based on 138 incident MCI cases. Seven statin-users were observed incident MCI cases, while 16.2 were expected to become incident cases by chance, which yielded a Log-rank χ2 test of 6.07 (1 d.f.); p=0.0138.
*p<0.05 for null hypothesis that Loge(HR)=0.
Figure 3.
Figure 3a. Kaplan-Meier survival curve of time to incidence of dementia by use of statins (time-dependent): Cox Proportional Hazards model and Log-rank test.
Notes: A participant is defined as a statin user at first prescription onwards, prior to onset of dementia or MCI or by end of follow-up if a non-case but beyond age 50 years. Cox PH model analysis controlled for the same covariates as in Figure 1a, but was based on 1,560 participants at risk for dementia and for use of statins (visits at or after 1985) and 252 dementia failures (person-years=37842). Log-rank test was based on 259 incident cases. Six statin-users were observed incident dementia cases, while 14.3 were expected to become incident cases by chance, which yielded a Log-rank χ2 test of 5.26 (1 d.f.); p=0.0219.
*p<0.05 for null hypothesis that Loge(HR)=0.
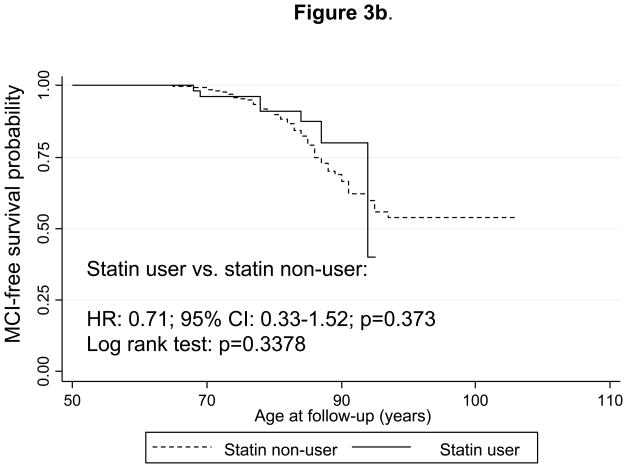
Figure 3b. Kaplan-Meier survival curve of time to incidence of mild cognitive impairment (MCI) by use of statins (time-dependent): Cox Proportional Hazards model and Log-rank test.
Notes: Statin use was defined the same way as in Figure 2a. Cox PH model analysis controlled for the same covariates as in Figure 1a, but was based on 1,308 subjects at risk for MCI and for use of statins (visits at or after 1985) and 133 failures (person-years= 29,600). Log-rank test was based on 138 incident cases. Seven statin-users were observed incident MCI cases, while 9.9 were expected to become incident cases by chance, which yielded a Log-rank χ2 test (1 d.f.) of 0.92; p=0.3378.
Similarly, Figure 2b shows survival curves for incident MCI and comparing “statin ever-users” with “statin never-users” (time-independent variable, definition 1). Both the log-rank test and the multivariable-adjusted hazard ratio indicated that “statin ever-users” were at around three-fold lower risk of developing MCI compared to statin never-users (HR = 0.32; 95% CI 0.15–0.71). Using the time-dependent statin exposure (definition 2) combined with PS approach (Figure 3b), the HR was non-significant and attenuated to 0.71 with a 95% CI: 0.33–1.52. In both analyses, dementia cases were excluded from the risk set. In a sensitivity analysis where dementia cases were re-included into the risk set, and using time-dependent statin use as main exposure combined with PS approach, the HR remained statistically non-significant (HR=1.07 with a 95% CI: 0.49–2.30).
Serum cholesterol and risks of dementia and MCI
Table 2 shows results from Cox PH models predicting dementia and MCI risk in relation to statin use (“time-dependent variable”) and serum cholesterol exposures. Model 1 (initial visit total cholesterol, dementia risk) indicated that statin use was inversely and significantly related to dementia risk, independently of first-visit TC. In the total population, none of the other serum cholesterol exposures (Models 2 through 5, dementia risk) considered was associated with dementia risk, independently of statin use. However, when stratifying the analysis by sex (Models 1 through 5, dementia risk, data not shown), declining TC trajectory over time as predicted at initial visit was found to be significantly associated with dementia risk among men (HR=4.21; 95% CI: 1.28–13.85), but not among women, independently of statin use. In fact, the interaction term between sex and TC trajectory variable was statistically significant (p=0.012) in a separate model with main effect of sex added.
Table 2.
Risk of dementia/MCI by statin use prior to dementia (time-dependent) or MCI onset, TC (first-visit and time-dependent), HDL-C:TC ratio (first-visit and time-dependent), and TC trajectory from first-visit†: Results from Cox Proportional hazard models; Baltimore Longitudinal Study of Aging
| Dementia |
MCI |
|||||||
|---|---|---|---|---|---|---|---|---|
| N‡ | n§ | N‡ | n§ | |||||
| HR | 95% CI | HR | 95% CI | |||||
| Model 1: First-visit TC | 1,290 | 199 | 1,091 | 106 | ||||
| Statin user | 0.34* | (0.14; 0.84) | 0.68 | (0.29; 1.60) | ||||
| TC (mg/dL) | ||||||||
| Q1: 118.2 to 191.1 | 1 | 1 | ||||||
| Q2: 191.3 to 215.4 | 1.45 | (0.89; 2.35) | 0.64 | (0.35; 1.15) | ||||
| Q3: 215.6 to 244.0 | 1.13 | (0.69; 1.87) | 0.72 | (0.42; 1.25) | ||||
| Q4: 244.4 to 476.0 | 1.31 | (0.82; 2.09) | 0.51* | (0.29; 0.90) | ||||
| Model 2: First-visit HDL- | 1,113 | 138 | 975 | 96 | ||||
| C:TC ratio | ||||||||
| Statin user | 0.40~ | (0.16; 1.01) | 0.55 | (0.23; 1.30) | ||||
| HDL-C:TC ratio | ||||||||
| Q1: 0.08 to 0.17 | 1 | 1 | ||||||
| Q2: 0.17 to 0.21 | 1.30 | (0.81; 2.07) | 0.94 | (0.54; 1.64) | ||||
| Q3: 0.21 to 0.25 | 1.14 | (0.68; 1.93) | 1.10 | (0.61; 2.00) | ||||
| Q4: 0.24 to 0.48 | 1.09 | (0.58; 2.06) | 1.65 | (0.82; 3.33) | ||||
| Model 3: Time-dependent TC | 1,458 | 234 | 1,216 | 124 | ||||
| Statin user | 0.35* | (0.15; 0.80) | 0.49~ | (0.22; 1.09) | ||||
| TC (mg/dL) | ||||||||
| Q1: 56.3–186.2 | 1 | 1 | ||||||
| Q2: 186.5–211.0 | 1.11 | (0.77; 1.59) | 1.10 | (0.69; 1.76) | ||||
| Q3: 211.3–238.7 | 1.03 | (0.71; 1.51) | 0.53* | (0.30; 0.94) | ||||
| Q4: 239.0–476 | 0.96 | (0.65; 1.40) | 0.70 | (0.42; 1.16) | ||||
| Model 4: Time-dependent | 1,244 | 164 | 1,073 | 110 | ||||
| HDL-C:TC ratio | ||||||||
| Statin user | 0.39* | (0.17; 0.89) | 0.48~ | (0.22; 1.05) | ||||
| HDL-C:TC ratio | ||||||||
| Q1: 0.00–0.19 | 1 | 1 | ||||||
| Q2: 0.19–0.24 | 1.09 | (0.69; 1.70) | 0.58* | (0.34; 0.98) | ||||
| Q3: 0.24–0.29 | 0.95 | (0.60; 1.52) | 0.65 | (0.38; 1.12) | ||||
| Q4:0.29–0.59 | 1.00 | (0.62; 1.62) | 0.72 | (0.41; 1.26) | ||||
| Model 5: TC change trajectory | 1,290 | 199 | 1,091 | 106 | ||||
| Statin user | 0.33* | (0.13; 0.82) | 0.67 | (0.28; 1.60) | ||||
| TC trajectory from first-visit | ||||||||
| Same or upward sloping | 1 | 1 | ||||||
| Downward sloping | 1.16 | (0.78; 1.73) | 1.04 | (0.60; 1.80) | ||||
| TC (mg/dL) at first-visit | ||||||||
| Q1: 118.2 to 191.1 | 1 | 1 | ||||||
| Q2: 191.3 to 215.4 | 1.44 | (0.89; 2.34) | 0.64 | (0.35; 1.15) | ||||
| Q3: 215.6 to 244.0 | 1.14 | (0.69; 1.88) | 0.72 | (0.42; 1.26) | ||||
| Q4: 244.4 to 476.0 | 1.33 | (0.83; 2.13) | 0.51* | (0.29; 0.90) | ||||
Abbreviations: BMI=Body Mass Index; HDL-C=High Density Lipoprotein-Cholesterol; Hg=Mercury; HR=Hazard Ratio; MCI=Mild Cognitive Impairment; NH=Non-Hispanic; Q= Quartile; SD=Standard Deviation; TC=Total Cholesterol.
p<0.10;
p<0.05 based on Wald test for Loge(HR)=0;
Cox PH hazards models with dementia as the outcome controlled for sex, education, race/ethnicity, smoking status, age at first-visit, chronic conditions at first-visit (type 2 diabetes, hypertension, cardiovascular disease, dyslipidemia), body mass index and systolic blood pressure at first-visit. These potential confounders were selected based on results from Table 1, specifically their association with the outcome of interest. Adding diastolic blood pressure to the model did not alter the findings. Plasma glucose level was not included due to large % of missing data at first-visit.
N=number of subjects in the analysis;
n=number of failures observed in the analysis.
An elevated initial visit TC (Model 1, MCI risk) was predictive of a lower risk of incident MCI (TC>244.4 vs. TC<191.1; HR=0.51 with 95% CI: 0.29–0.90). Looking at time-dependent TC (Model 3, MCI risk), the third quartile (TC ranging between 211-238 mg/dL) was significantly protective against MCI compared to the lowest quartile (TC between 56.3 and 186.2) (HR=0.53; 95% CI: 0.30–0.94). Moreover, examining the balance between HDL-C and TC (Model 2, MCI risk), initial visit ratios were not significantly associated with the risk of MCI. However, examining this variable in a time-dependent fashion (Model 4, MCI risk) indicated that a ratio of 0.19-0.24 may be optimal in preventing MCI incidence among men and women, combined, compared to a lower ratio of 0.00–0.19, independently of statin use (HR=0.58; 95%CI: 0.34–0.98), whereas a higher ratio had no significant association with MCI. TC trajectory (Model 5, MCI risk) was not associated with MCI in the total population or within each sex group.
DISCUSSION
Our study investigated the effects of statins and several serum cholesterol exposures on dementia and MCI risks. There are several key findings. However, the most important one was that statin users throughout follow-up had two to three-fold lower risk of developing dementia (HR=0.41; 95% CI: 0.18–0.92), but not MCI, when considering time-dependent “statin use” with propensity score model adjustment. This association remained significant independently of serum cholesterol exposures. Moreover, participants with incident dementia by end of follow-up had higher initial TC compared to dementia-free participants. However, this association, as shown later, was strongly confounded by age. Initial TC was higher among statin users than non-users and statin use was related directly to initial self-reported dyslipidemia. In addition, an elevated initial TC was associated with reduced MCI risk (Upper quartile (Q4) vs. Q1: HR=0.51; 95% CI=0.29–0.90). Compared to the lowest quartile (Q1: 0.00–0.19), HDL-C:TC ratio (time-dependent) in (Q2: 0.19–0.24) was found to reduce the risk of MCI (HR=0.53; 95%CI: 0.30–0.94). Finally, among men, predicted decline in TC trajectory from initial visit was significantly associated with increased dementia risk (HR=4.21; 95% CI: 1.28-13.85). While these findings were mixed, they show that statin use may be protective against dementia risk independently of serum cholesterol exposures; TC decline may increase risk of dementia only in men, independently of statin use; whereas other serum cholesterol exposures including time-dependent HDL:TC ratio and initial TC may be at play in modulating risk of MCI, independently of statin use.
Studies related to statin use and dementia risk have produced conflicting results. Some case-control and cross-sectional studies [1, 4–7, 34] indicated a lower prevalence of statin use among dementia cases. Yet, a number of longitudinal epidemiologic studies did not demonstrate a decreased risk of AD, dementia, or prevention of cognitive decline and impairment with statin use according to a recent review [8]. This review and meta-analysis of several prospective cohort and case-control studies suggested that the pooled crude odds ratios in statin users compared with nonusers were 0.67 (95% confidence interval CI 0.54–0.82) for dementia risk. However, after adjustment for potential confounders, the pooled OR was 0.77 (95% CI 0.45–1.30), suggesting that statins may not be beneficial [8]. At least four other cohort studies were published since this review [9–12]; three showed a protective effect against incident dementia (AD in particular) [9–10], and a combination of dementia and MCI [11]. Moreover, a review of nine randomized clinical trials (RCT) with variable samples sizes (22 – 20,000) and follow-up times (three weeks to five years) indicated that statins do not alter cognitive functioning over time significantly[35].
Previous studies have also had mixed findings regarding the effect of TC on dementia risk and cognitive functioning. In fact, both high TC (>240 mg/dL) [13, 15, 17], low TC (<200 mg/dL) [14, 18] and decrease in TC level over time [16] have been related to deficits in cognitive performance and dementia. For instance, an earlier study conducted on 249 stroke-free community volunteers found that hypercholesterolemia was a significant independent correlate of memory dysfunction (OR: 3.0; 95% CI: 1.4 to 6.6)[13]. In contrast, another study carried out among 789 men and 1105 women found that lower naturally occurring TC levels were associated with poorer performance on cognitive measures, which placed high demands on abstract reasoning, attention/concentration, word fluency, and executive functioning [14].
Our present study found that a drop in TC starting from first-visit increased the risk of dementia among men and that a first-visit TC level above 244 mg/dL may be protective against MCI, independently of statin use. However, when comparing TC at first-visit between those who had developed dementia by the end of follow-up and those who did not, TC was higher among dementia participants compared to their non-case counterparts, a highly age-confounded association. Similar to a previous study, the adverse effect of a downward sloping TC over time on incident dementia was particularly found among men [16].
Both directions of effect, however, carry biological plausibility. For instance, TC at a specific level may be needed for normal neuronal functioning[36]. Alternatively, low cholesterol levels may presage chronic diseases [37] which in turn may be associated with poorer cognitive performance. The relationship may also be a result of a complex interaction between serum TC, TC in cells and certain neurotransmitters such as serotonin [38]. In contrast, the adverse effect of elevated serum TC level particularly on cognitive decline can be explained by the existence of subclinical vascular disease [39]. Experimental evidence suggests that cholesterol is in fact capable of shifting amyloid precursor protein (APP) metabolism from alpha to beta cleavage which accelerates the production of senile Alzheimer’s plaques (Aβ) [40].
Further, our findings regarding the associations between HDL-C:TC ratio vs. TC status at first-visit and as time-dependent variables vs. TC change trajectories on one hand and dementia and MCI risks on the other hand highlights the complexity of the association between serum cholesterol (status vs. trajectory) and the balance between “good” and “bad” cholesterol on dementia and MCI risks.
The fact that statin’s protective effects against dementia and MCI risk is independent of serum cholesterol exposures suggest that statins may be acting through a different pathway in the brain. These multifactorial actions may include preventing atherothrombosis through improvement of endothelial function and inhibition of platelet aggregation or anti-inflammatory activities such as inhibiting C-reactive protein and cytokine responses. Statins have also been shown to have a neuroprotective effect through the enhancement of nitric oxide synthase [41].
Our study has several strengths. First, it is based on a large prospective cohort study with long follow-up time (over 20 years) and statin use was measured prior to onset of dementia and MCI. Consequently, and unlike in case-control studies, it is less likely to be confounded by indication [42] (i.e. possibility that dementia cases are less likely to be prescribed statins). In order to address in part that threat to validity, we used propensity score adjustment method [33] and our main finding regarding the inverse association between statins and dementia incidence remained significant. Moreover, change trajectories and time-dependent exposure variables were studied, avoiding both issues of temporality and reliability due to single measurement. Further, it is one of few longitudinal studies combining analyses of statin use and serum cholesterol in relation to dementia and MCI[8]. Finally, it utilized advanced statistical methods including a combination of multivariable linear mixed models and survival analyses[43].
Some of our study’s limitations include the lack of complete measurements for potentially confounding variables such as plasma fasting glucose. In addition, the BLSA is a sample of convenience; the cohort is not fixed, and recruitment and dropout were continuous throughout the follow-up. Due to the BLSA study’s multi-faceted nature, some of the data were not of sufficient quality to study detailed exposure effects on cognitive outcomes, including combining dosage of statins, frequency of use and recency of use to obtain standard daily doses as was done by others [44–45]. Finally, many of the analyses involving serum cholesterol exposures (Table 2) may require adjustment for multiple testing before one can make an appropriate inference and thus must be examined with caution.
Awaiting large randomized clinical trial (RCT), the beneficial value of statins on dementia and cognitive function can be ascertained only by observational studies. While our study found mixed results regarding serum cholesterol exposures and cognition, it calls for RCTs for statins to enhance that evidence by reducing selection bias and confounding. Moreover, future studies should examine the multifactorial effects of statins and attempt to determine the optimal HDL-C:TC and TC change trajectories for reducing risks of dementia and MCI.
KEY FINDING.
Statin users had two to three-folds lower risk of developing dementia (HR=0.41; 95% CI: 0.18–0.92), but not MCI, when considering time-dependent “statin use” with propensity score (PS) model adjustment. This association remained significant independently of serum cholesterol exposures.
WHAT THIS ADDS TO WHAT WAS KNOWN.
What is already known on this subject?
Statin use and serum cholesterol reduction have been proposed as preventions for dementia and mild cognitive impairment (MCI).
What does this study add?
This study suggests that statins may have multifactorial effects on dementia risk that is independent of cholesterol levels.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
COMPETING INTERESTS: None declared.
There was no conflict of interest disclosed. MAB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors declare no conflict of interest.
LICENSE STATEMENT
“The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JECH and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://jech.bmj.com/ifora/licence.pdf).”
References
- 1.Hajjar I, Schumpert J, Hirth V, et al. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M414–8. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 2.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65:1388–94. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 3.Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873–80. doi: 10.1212/01.wnl.0000279333.77404.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–8. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 5.Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Darvesh S. The risk of dementia in relation to statins and other lipid lowering agents. Neurol Res. 2003;25:601–4. doi: 10.1179/016164103101202039. [DOI] [PubMed] [Google Scholar]
- 7.Zamrini E, McGwin G, Roseman JM. Association between statin use and Alzheimer’s disease. Neuroepidemiology. 2004;23:94–8. doi: 10.1159/000073981. [DOI] [PubMed] [Google Scholar]
- 8.Zhou B, Teramukai S, Fukushima M. Prevention and treatment of dementia or Alzheimer’ disease by statins: a meta-analysis. Dement Geriatr Cogn Disord. 2007;23:194–201. doi: 10.1159/000099037. [DOI] [PubMed] [Google Scholar]
- 9.Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–7. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 10.Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–21. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 11.Cramer C, Haan MN, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–50. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 13.Desmond DW, Tatemichi TK, Paik M, et al. Risk factors for cerebrovascular disease as correlates of cognitive function in a stroke-free cohort. Arch Neurol. 1993;50:162–6. doi: 10.1001/archneur.1993.00540020040015. [DOI] [PubMed] [Google Scholar]
- 14.Elias PK, Elias MF, D’gostino RB, et al. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 16.Solomon A, Kareholt I, Ngandu T, et al. Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology. 2007;68:751–6. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 17.Stewart R, Richards M, Brayne C, et al. Vascular risk and cognitive impairment in an older, British, African-Caribbean population. J Am Geriatr Soc. 2001;49:263–9. doi: 10.1046/j.1532-5415.2001.4930263.x. [DOI] [PubMed] [Google Scholar]
- 18.Wada T, Matsubayashi K, Okumiya K, et al. Lower serum cholesterol level and later decline in cognitive function in older people living in the community, Japan. J Am Geriatr Soc. 1997;45:1411–2. doi: 10.1111/j.1532-5415.1997.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 19.Crisby M. The role of pleiotropic effects of statins in dementia. Acta Neurol Scand Suppl. 2006;185:115–8. doi: 10.1111/j.1600-0404.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 20.Shock N, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin J. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. [Google Scholar]
- 21.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–6. doi: 10.1017/s1041610297004870. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 23.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–6. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 27.Vitaliano PP, Breen AR, Russo J, et al. The clinical utility of the dementia rating scale for assessing Alzheimer patients. J Chronic Dis. 1984;37:743–53. doi: 10.1016/0021-9681(84)90043-2. [DOI] [PubMed] [Google Scholar]
- 28.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–7. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 30.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 31.STATA. Statistics/Data Analysis: Release 10.0. Texas: Stata Corporation; 2007. [Google Scholar]
- 32.Rockwood K. Epidemiological and clinical trials evidence about a preventive role for statins in Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:71–7. doi: 10.1111/j.1600-0404.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 33.Sturmer T, Joshi M, Glynn RJ, et al. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–47. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolozin B, Kellman W, Ruosseau P, et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–43. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 35.Xiong GL, Benson A, Doraiswamy PM. Statins and cognition: what can we learn from existing randomized trials? CNS Spectr. 2005;10:867–74. doi: 10.1017/s1092852900019817. [DOI] [PubMed] [Google Scholar]
- 36.Muldoon MFFJ, Ryan C. Serum cholesterol, the brain, and cognitive functioning. Mahwah; NJ: 2001. [Google Scholar]
- 37.Alexopoulos CG, Pournaras S, Vaslamatzis M, et al. Changes in serum lipids and lipoproteins in cancer patients during chemotherapy. Cancer Chemother Pharmacol. 1992;30:412–6. doi: 10.1007/BF00689971. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan JR, Manuck SB, Fontenot MB, Muldoon MF, Shively CA, Mann JJ. The cholesterol-serotonin hypothesis: interrelationships among dietary lipids, central serotonergic activity, and social behavior in monkeys. Washington, DC: American Psychology Association; 1997. [Google Scholar]
- 39.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59:378–84. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 40.Refolo LM, Malester B, LaFrancois J, et al. Hypercholesterolemia accelerates the Alzheimer’ amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–31. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 41.Rajanikant GK, Zemke D, Kassab M, et al. The therapeutic potential of statins in neurological disorders. Curr Med Chem. 2007;14:103–12. doi: 10.2174/092986707779313462. [DOI] [PubMed] [Google Scholar]
- 42.Birkenhager WH, Wang JG, Staessen JA. Dementia and statins. Lancet. 2001;357:880. doi: 10.1016/S0140-6736(00)04187-8. author reply −1. [DOI] [PubMed] [Google Scholar]
- 43.Morell CH, Brant LJ, Pearson JD, Verbeke GN, Fleg JL. Applying linear mixed-effects models to the problem of measurement error in epidemiologic studies. Communications in statistics. 2003;32:437–59. [Google Scholar]
- 44.Breitner JC, Haneuse SJ, Walker R, et al. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology. 2009;72:1899–905. doi: 10.1212/WNL.0b013e3181a18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.in t’Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’ disease. N Engl J Med. 2001;345:1515–21. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 46.Singer JD, Willet JB, editors. Applied Longitudinal Data Analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.