Abstract
Alcohol abuse in the adult is often preceded by high alcohol consumption during adolescence. Profound changes in brain structure and function occur during this developmental period, therefore alcohol may impact essential cognitive skill development during the formal educational years. The objective of this study was to determine if chronic oral alcohol intake slows acquisition and performance of cognitive tasks in male adolescent rhesus monkeys. Treatment groups (Alcohol, N=4; Control, N=3) were evaluated on bimanual dexterity and tests of visuo-spatial memory and learning adapted from the Cambridge Neuropsychological Test Automated Battery. Animals were trained daily in 30 min sessions and had subsequent access to alcohol/Tang® solutions (Alcohol group) or Tang® only (Control group) Monday through Friday for 11 months. Recordings of brainstem auditory evoked potentials (BSAEP) were conducted periodically before and during the chronic drinking.
Results
Chronic alcohol drinking (ave of 1.78g/kg alcohol per session) impaired behavioral performance assessed ~22 hrs after the prior drinking session. The Alcohol group required more trials than the Control group to reach criterion on the visuo-spatial memory task and showed increased sensitivity to trial difficulty and retention interval. Alcohol animals also had slowed initial acquisition of the bimanual task. The latency of P4 and P5 BSAEP peaks were also delayed in the Alcohol group. Chronic alcohol consumption impaired the acquisition and performance of a spatial memory task and disrupted brainstem auditory processing, thus these results show that repeated alcohol exposure in adolescence interferes with a range of brain functions including complex visuo-spatial mnemonic processing.
Keywords: Cognition, brain functioning, CANTAB, adolescence, executive function, Rhesus Macaque
1. Introduction
Alcoholic beverages remain the recreational psychoactive substances most widely used by adolescents (Johnston et al. 2006). Chronic heavy drinking has been associated with adverse effects on the central nervous system and brain functioning in animals and humans (for review, see (Hiller-Sturmhofel and Swartzwelder 2004; Tapert et al. 2004), yet it remains unclear when in the course of a person’s exposure to alcohol these central nervous system changes may occur. Since alcohol use often begins in adolescence, consumption during this critical developmental period may have particularly profound effects on brain structure and function.
The cognitive functions that appear to be especially vulnerable to the effects of alcohol in human adolescents are subserved by brain regions which undergo significant changes in structure and function during adolescence (De Bellis et al. 2000; De Bellis et al. 2001; De Bellis et al. 2005; Huttenlocher 1979; Huttenlocher et al. 1982). Specifically, interference in the development of the prefrontal cortex (Durston et al. 2001; Giedd et al. 1999; Paus et al. 2001; Pfefferbaum et al. 1994; Thompson et al. 2000) may underlie impairments in visuospatial skills, attention and executive function which are observed in recently detoxified adolescents (Brown et al. 2000; Tapert and Brown 1999); some impairments may last up to 4 years after detoxification. These cognitive domains are similar to those reported disrupted in adult alcoholics (O'Mahony and Doherty 1996) suggesting that adolescents may suffer cognitive impairment that is similar to adults who have abused alcohol over comparatively longer time spans. Thus adolescent humans may be particularly sensitive to the cognitive impairing effects of alcohol. Rodent studies have also shown that adolescent animals are more sensitive than are adults to alcohol impairment of cognitive and electrophysiological measures of spatial memory (Markwiese et al. 1998; Swartzwelder et al. 1995a; b) and exhibit more severe neurotoxicity associated with heavy doses of ethanol in brain regions linked to mnemonic function (Crews et al. 2000).
Interpretation of human studies can be complicated by several factors. Variables such as the amount of ethanol consumed by the individual, the duration of exposure to ethanol and concurrent use of other illicit drugs are difficult to experimentally control or reliably estimate. In addition, studies in humans cannot distinguish whether individuals predisposed to become alcoholics/alcohol abusers exhibit innate cognitive differences or whether exposure to ethanol during adolescence, or other developmental period, induces the observed alterations in cognitive functioning. For example children who are at high risk of developing alcoholism or alcohol abuse due to a positive family history of alcoholism perform worse than adolescents without a family history of alcoholism on tests of verbal IQ, visuospatial perception, attention, memory, executive function in the absence of significant alcohol exposure (Begleiter et al. 1984; Harden and Pihl 1995; Hill et al. 1990; Ozkaragoz et al. 1997; Polich et al. 1994; Whipple et al. 1991). Therefore innate cognitive differences likely exist in some individuals who will later develop alcoholism and/or alcohol abuse, thus the independent or additive role of alcohol exposure is unclear. Thus it is difficult to clearly establish a causal role of alcohol exposure in producing neuropsychological deficits in adolescent alcohol users. Nonhuman models are advantageous, since they can control for these variables in a more systematic manner.
The aim of the present study was to examine the impact of ongoing alcohol exposure on cognitive performance using an adolescent nonhuman primate model. Nonhuman primates are genetically more similar to humans than rodents, exhibit wide cognitive capabilities, consume alcohol to the point of intoxication readily and are similar in many of the physiological, neuroanatomical and behavioral systems potentially affected by alcohol (Grant and Bennett 2003). Such similarities may allow more direct generalization of results from preclinical models to the human condition. The study used a monkey model of adolescent alcohol drinking (Katner et al. 2004; Katner et al. 2007) in combination with longitudinal evaluation of cognitive function (Taffe et al. 2004; Weed et al. 1999). Adolescent male rhesus monkeys were chronically exposed to alcohol and were compared with a control group on tests of visuo-spatial memory and motor function. It was hypothesized that alcohol-exposed monkeys would exhibit a slower rate of acquisition on behavioral tasks including reduced accuracy and decreased psychomotor speed. A brain stem auditory evoked response paradigm was incorporated because of evidence that electrophysiological investigations may be sensitive to fetal alcohol exposure (Lieu and Champion 2006), chronic alcoholism (Smith and Riechelmann 2004) and even alcohol-induced disturbance when no behavioral changes are evident (Maurage et al. 2009).
2. Methods
2.1. Subjects
Seven adolescent male rhesus monkeys (Macaca mulatta, Chinese origin) participated in the present study. The monkeys were approximately 4–5 years of age and weighed an average of 7.7 kg at the beginning of the study. The definition of adolescence in monkeys can vary depending on definition by hormonal status, growth rate and even brain development just as it would for humans. In our experience male rhesus increase their rate of bodyweight gain/month around 32 months of age and do not reach stable mature weight of 12–16 kg until about 8–9 years of age. This is consistent with an increase in plasma testosterone observed in intact male monkeys across the 34–50 month interval (Morris et al. 2010) and observation of brain growth tapering off at about 40–50 months of age (Knickmeyer et al. 2010). Thus, our age range is consistent with a start in the immediate peri-pubertal timepoint and then stretching into late adolescence, similar to the high school population of humans. Animals were pair-housed and fed in the home cage after completion of the daily testing and ethanol access sessions. The animals’ chow (Lab Diet 5038, PMI Nutrition International) was supplemented with fruit or vegetables 7 days per week, and water was available ad libitum in the home cage at all times, except as noted below. The National Institutes of Health guidelines for laboratory animal care (Clark et al. 1997) were followed, and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. All animals were immobilized with ketamine in doses of 5 to 10 mg/kg (intramuscularly) no less than semiannually for the purposes of routine care and health monitoring and as described below for evoked potential recording and blood collection.
2.2. Behavioral Testing
All animals were evaluated on three behavioral tasks as specified below. In general, two of these tasks required monkeys to respond by touching the touch-sensitive computer screen and were reinforced with the delivery of food pellet reinforcers. The third task required the animal to extract raisins from holes in a transparent plastic board. Animals performed ~daily (M-F) in behavioral sessions lasting approximately 30–45 minutes. Once alcohol exposure was initiated (see above) the behavioral test sessions were conducted before any alcohol drinking, i.e., approximately 22 hrs after the prior drinking session T-F and about 70 hrs after the prior session on Mondays.
2.3. Apparatus
Animals were tested in the home colony room, unrestrained, in transport cages similar to the home cage. The transport cage was placed in front of a computer monitor fitted with a touch-sensitive screen on which visual stimuli were presented. Stimulus presentation and response detection were controlled by a computer equipped with a version of the CAmbridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK) designed for use with non-human primates. A dispenser delivered 190 mg flavored Noyes precision pellets (Research Diets, Inc.; New Brunswick, NJ) to a bin mounted on the front of the cage after correct responses.
2.4. Behavioral Procedures
2.4.1. Initial shaping to use the touch-screen manipulandum
A colored square nearly filling the monitor’s screen was presented and each touch was reinforced with food pellets. The monitor screen was initially “baited” with banana smeared across the screen until animals were touching reliably, usually 1–3 sessions. Sessions ranged from 10 to 30 min and were conducted 3–5 days per week. Responding outside the colored square resulted in a brief blanking of the screen without reinforcer delivery. The size of the colored square was reduced after 25 correct responses to a given size square until monkeys were responding reliably to the smallest (~3 cm) square. Next the square was randomly repositioned around the screen from trial to trial for the final sessions of training until animals had completed at least 5 sessions with 70% correct responses.
2.4.2. Intradimensional/Extradimensional Attentional Set Shift
The animals in this study completed the 8-stage ID/ED sequence twice prior to alcohol exposure to include both Shape-to-Line and Line-to-Shape versions of the task. The task can be evaluated with minimal training and therefore can serve as a rapid assay for individual differences in cognitive/behavioral capability in adolescent monkeys. The ID/ED procedure consists of a series of two-alternative visual discriminations in which two patterns are presented; complete details may be found in (Weed et al. 1999). The task is to determine by trial-and-error which pattern is associated with reinforcement; successful selection of the correct alternative on 18 of 20 consecutive trials constitutes a “pass” of a discrimination stage. Data are represented as a square root transformation (to normalize the distribution) of the number of errors made prior to reaching criterion in each stage. The available stimulus dimensions are irregular polygons (“Shape” stimuli) and multi-segment line patterns (“Line” stimuli). For a given completion of the task, one of the stimulus dimensions (e.g., Shape) is the relevant dimension for the first six stages thereupon the relevant dimension shifts to the other (e.g. Line) dimension to evaluate the Extradimensional Shift capability.
2.4.3. Visuo-spatial paired-associates learning task (vsPAL)
Training on this task was initiated after the initial alcohol induction period and assignment to experimental groups, see below.
2.4.3.1. Acquisition
The vsPAL is a learning and memory task involving an association of a given stimulus with particular spatial location on a trial-by-trial basis. The stimuli for the task consisted of 68 distinct colored patterns. To begin a trial, one large, colored abstract sample stimulus was presented in one of 4 positions on the computer screen (top center, bottom center, left middle, right middle). The subject was required to touch the sample stimulus (sample phase), which then disappeared. The same pattern re-appeared after a 1 sec delay in 1–3 of the four possible locations on the screen (choice phase). The subject was required to touch the stimulus that was presented in the same location as the sample item within 30 seconds to obtain a reinforcer. Touches directed to the stimulus in an incorrect location (error) or a failure to touch within 30 s (omission) initiated a 5 s screen blank and scored as a failed attempt (see (Taffe et al. 2004) for a full description of the task).
Training was initiated with sessions which presented 15 trials consisting of one sample stimulus and one choice stimulus (1-stim (1-location)) and 15 trials consisting of one sample stimulus and two choice stimuli (1-stimuli (2 location)), until performance averaged >80% correct trials on the 2-location trials for three consecutive sessions. Next, monkeys received sessions of 5 1-stim (1 loc) trials, 20 1-stim (2 loc) trials and 20 1-stim (3 loc) trials until performance averaged >80% correct trials on the 1-stim (2 and 3 loc) trials over three consecutive sessions. Finally, monkeys received sessions of 5 1-stim (2 loc) trials, 5 1-stim (3 loc) trials and 30 2-stim (2 loc) trails. In this latter condition (2-stim (2 loc)) the two sample stimuli are presented sequentially with the 2nd stimulus appearing 1 second after the first sample response in a different location. After a 1 second delay, one of the sample patterns (randomly selected) re-appeared in two locations on the screen and the monkey was required to touch the stimulus that was presented in the same location as the first sample. If the monkey made an incorrect choice (error) or did not respond (omission), the trial was terminated, the screen was blanked for 5 seconds and a new trial began. If the monkey responded correctly to the first choice, the 2nd choice then immediately appeared. The trial was scored as accurately completed only if both stimulus-location associations were correctly selected during the choice phase.
2.4.3.2. Incremental Learning Probe
For the learning probe up to 5 additional attempts at a given trial were allowed if an error occurred in attempting to complete a given trial. In a trial repetition, the same stimulus-location associations were presented in a randomly selected sample order, followed by another choice phase. Performance was measured by accuracy on the initial attempt to complete a trial (percent correct initial) and the accuracy for trials successfully completed within the allowed attempts (percent correct overall). The initial attempt score is considered a memory measure and the difference between overall completion rates and initial attempt rates are considered a measure of learning. This probe was conducted for five sequential sessions starting about 4 months after the initiation of this task.
2.4.4. Spatial Delayed Response (SDR) Probe
The standard vsPAL task was modified to parametrically test spatial working memory by increasing the memory delay between the response to the sample stimuli and the presentation of the choice stimuli. Delays were increased from the 1 sec vsPAL standard interval to 5, 10, 20 and 30 seconds. Monkeys received 15 trials of 1-stim (4-loc) with each of the delays being evaluated over sequential sessions in a randomized order for each animal. This probe was assessed 3 months after the initiation of vsPAL training.
2.4.5. Bimanual Motor Skill (BMS) Task Acquisition
A transparent plastic board drilled with 15 holes and filled with raisins was mounted perpendicular to the door of the transport cage. The diameter of the hole is such that for efficient bimanual retrieval of raisins, the animal must push the raisin partially out of the hole with one finger before retrieving it with the other hand. With training, animals universally adopt a strategy of pushing the raisin with one hand while retrieving it with the other hand, thus entailing bimanual dexterity. To acquaint the animals with the task, raisins were only partially inserted into the hole, leaving half of the raisin sticking out of one end of the hole for the first 5 sessions. Raisins were fully inserted for the remaining sessions. The time required to retrieve all 15 raisins was recorded by stopwatch and recorded as retrieval latency. This task was routinely provided at the end of each touch-screen task session.
2.5. Brain Stem Auditory Evoked Potential (BSAEP) Recordings
Brainstem auditory evoked potentials (BSAEPs) were recorded under ketamine anesthesia (20 mg/kg, i.m.), prone, as previously described (Taffe et al. 2003; Taffe et al. 2001), on four occasions throughout the study. Comparison baseline recordings were obtained prior to any introduction of ethanol and then at 2, 6 and 9 mo after the initiation of the chronic ethanol/vehicle phase (see below). For these latter sessions recordings were conducted approximately 23 hours after the prior ethanol/vehicle session. Electrodes were placed subcutaneously at the cranial vertex (active), over the greater alar cartilage (reference) and in the musculature of the neck (ground) for recordings. Raw electroencephalographic signals (EEG) were amplified and filtered within a bandwidth of 30–3000 Hz. Binaural condensation stimulation, produced by clicks generated by 0.10 ms square waves and delivered at a 70 dB sound pressure level at a rate of 10 Hz, was used to generate BSAEPs. BSAEPs were calculated by averaging the first 10 ms of EEGs recorded for 1024 samples. Several component peaks are identified in the average waveform for individual BSAEPs including P1, P2a, P2b, P3, P4 and P5.
2.6. Ethanol Exposure Prodecures
2.6.1. Ethanol Induction/Group Assignment
Oral ethanol self-administration was induced using a procedure in which the concentration (%) and/or amount (g/kg) of ethanol in a palatable solution was gradually increased over a series of daily limited-access sessions (see (Katner et al. 2004) for detailed description). All monkeys were initially given the opportunity to consume 1% (w/v) ethanol plus 6% (w/v) Tang® (Kraft Foods, Inc.) in tap water with an absolute ethanol limit of 0.5 g/kg per session via a drinking bottle placed on each animal’s home cage. The ethanol concentration was gradually increased to 6% (w/v) ethanol plus 6% (w/v) Tang® with an absolute ethanol limit of 2.0 g/kg per session (Table 1). The inclusion of all seven monkeys in the induction procedure was to determine individual ethanol preference levels (i.e. high and low) in order to balance the groups on this factor.
Table 1.
Treatment phases for the induction of oral ethanol self-administration.
| Ethanol Concentration plus 6% (w/v) Tang | Number of Sessions | EtOH Limit |
|---|---|---|
| 1% (w/v) | 6 | 0.5 g/kg |
| 2% (w/v) | 5 | 0.5 g/kg |
| 4% (w/v) | 4 | 0.5 g/kg |
| 4% (w/v) | 5 | 1.0 g/kg |
| 6% (w/v) | 7 | 1.0 g/kg |
| 6% (w/v) | 6 | 1.5 g/kg |
| 6% (w/v) | 6 | 2.0 g/kg |
Our laboratory has shown that the flavorant-fade induction process provides a strong indication of stable individual preferences for ethanol (Katner et al. 2004); therefore the alcohol fade procedure, described below, was conducted in all monkeys up the point where 2.0 g/kg of alcohol (6% w/v solution in Tang®) was available. Total intake in the initial stages was used to match the groups for high vs. low preferring drinkers. Thereafter, the alcohol sessions were discontinued in the Control animals and maintenance of drinking 6% EtoH with 6% Tang for a maximum of 3.0g/kg EtoH in the Alcohol group continued throughout the study.
2.6.2. Chronic Oral Ethanol Self-Administration
Solutions of 6% ethanol with 6% Tang (Ethanol solution; 3.0 g/kg session limit) or 6% Tang in water (Tang solution) were made available in the home cage of the Alcohol and Control groups, respectively, during daily (Monday through Friday) sessions of 1 hr duration (i.e., limited access) via normal drinking bottles. Drinking sessions were provided in the afternoon, after behavioral sessions had been completed. Cage water was not available for the 1-hr period preceding the alcohol session to reduce variability in initial fluid satiation; we have shown that consumption of large fluid volumes may reduce immediately subsequent ethanol solution intake (Katner et al. 2007). The ethanol or Tang solution was the only liquid available in the home cage for the duration of the session, after which the ethanol or Tang was removed and the drinking water was restored.
The amount of the ethanol and Tang solution consumed during this and all subsequent sessions was recorded during the initial 5 min of the session, then at 10, 15, 20, 30, and 60 min after ethanol availability. Drinking bottles were tested before use to ensure minimal leakage, and the investigators were careful to note any leakage (or bottles knocked off an animal’s cage entirely) that occurred during sessions and to adjust the amount of ethanol consumption recorded.
2.6.3. Blood Ethanol Levels
After one month and ten months of chronic alcohol consumption for the Alcohol group, a single 30 minute alcohol session was scheduled with 3.0 g/kg of 6% ethanol available to all animals (Alcohol and Control) for the determination of BELs. Blood samples were drawn from the femoral vein under ketamine (10 mg/kg, intramuscularly) anesthesia 30 minutes after the drinking session to determine BELs reached by each animal. Serum was separated from blood cells by centrifugation and analyzed for ethanol content with an Analox AM1 ethanol analyzer (Analox Instruments USA, Lunenburg, MA).
2.7. Data analysis
Analysis of the number of sessions to reach criteria in the vsPAL task employed two-way analysis of variance (ANOVA) with between-subjects factor of treatment group (Alcohol, Control) and within-subjects factor of acquisition stage (see Table 2). The analysis of the vsPAL accuracy data was conducted using three-way ANOVA with between-subjects factor of treatment group (Alcohol, Control) and within-subjects factors of trial difficulty (1-stim (2-loc), 1-stim (3-loc)) and blocks of 5 sequential sessions. Analysis of the accuracy data for the Incremental Learning probe included between-subjects factor of treatment group and within-subjects factors of trial difficulty (1-stim (2-loc), 1-stim (3-loc), 2-stim (2-loc)) and Initial/Overall attempts. Analysis of the accuracy data in the Spatial Delayed Response probe employed two-way analysis of variance (ANOVA) with between-subjects factor of treatment group (Alcohol, Control) and within-subjects factor of retention interval. Data for the bimanual motor skill task were analyzed by two way repeated-measures ANOVA with factors of treatment group and block of training sessions. Post hoc analyses of significant main effects were conducted using the Fisher’s LSD test including all pairwise comparisons; the criterion for significance was p< 0.05. Analyses were conducted with commercially available software packages (GB-STATv7.0; Dynamic Microsystems, Silver Spring MD; SPSS v13.0 for Windows, SPSS Inc, Chicago, IL).
Table 2. Training sequence and advancement criteria for the vsPAL task.
The indicated trial types were included in each training session at each stage of the initial training and for introduction of the incremental learning component. Trial abbreviations follow the same convention, e.g. 1-stim (3-loc) indicates trials in which one sample stimulus was presented and the stimulus was presented in three target locations on the choice display; 2-stim (2-loc) trials present two sequential samples followed by choice displays containing stimuli in two target locations, etc. For the initial training, the listed performance criteria were met prior to advancement to the subsequent training stage.
| Stage | Blocks of trials in each session | Criterion to advance (three consecutive sessions) |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 1 | 15 trials 1-stim (1-loc) |
15 trials 1-stim (2-loc) |
>80% correct trials | |
| 2 | 5 trials 1-stim (1-loc) |
20 trials 1-stim (2-loc) |
20 trials 1-stim (3-loc) |
>80% correct trials 1-stim (2 & 3-loc) trials |
| 3 | 5 trials 1-stim (2-loc) |
5 trials 1-stim (3-loc) |
30 trials 2-stim (2-loc) |
>50% correct trials 2-stim (2-loc) trials |
3. Results
3.1. Ethanol Induction, Maintenance and Blood Ethanol Levels
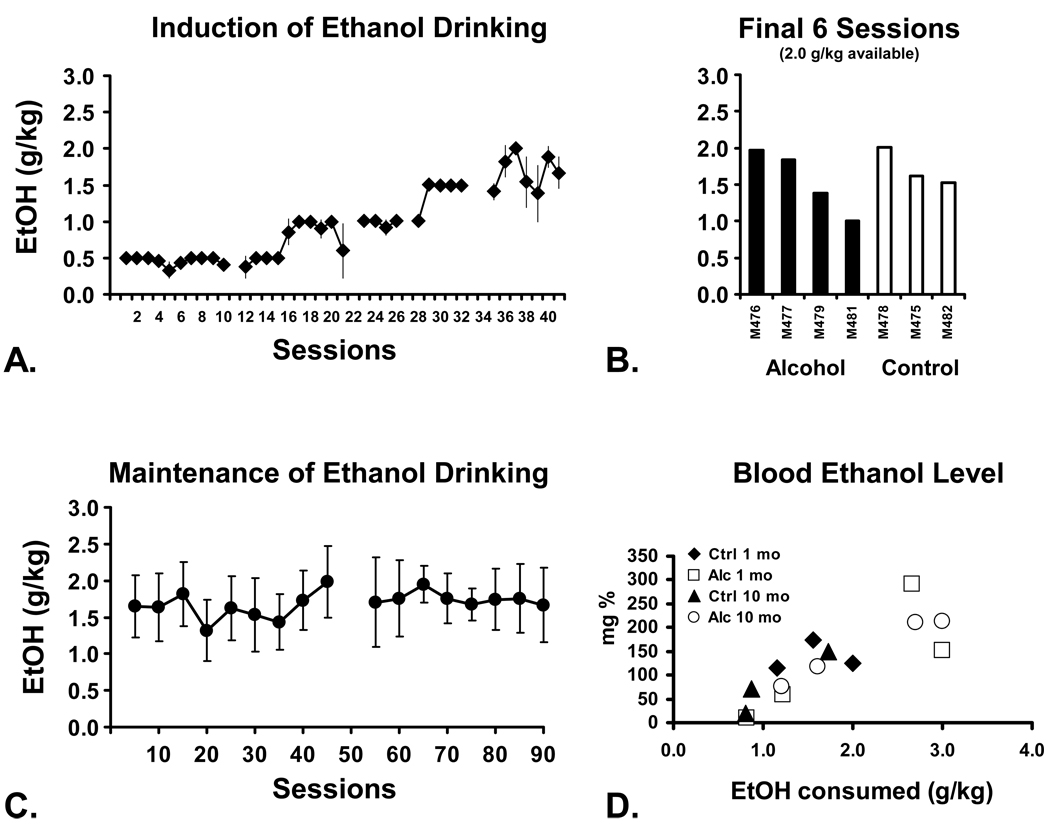
All seven animals underwent the ethanol induction process, readily consuming all of the ethanol available when the session limit was 1.5 g/kg or below (Figure 1A). Once the available ethanol was 2.0 g/kg/session or greater, animals did not consume all of the available ethanol reliably and thus appeared to titrate their dose, as in prior reports from this laboratory (Katner et al. 2004; Katner et al. 2007). The average daily intake (Figure 1B) from the last six sessions (in which 2.0 g/kg was available) was used in combination with initial behavioral data (see below) to rank the animals for group assignment. The Alcohol group was thereafter permitted to consume up to 3.0 g/kg EtOH in Tang during 1-hour daily sessions and consumed an average of 1.78 g/kg of alcohol per session during the 5 month maintenance phase (Figure 1C). Vehicle solutions of equivalent volume were made available to the Control group animals.
Figure 1.
A) Mean (N=7; ±SEM) ethanol (EtOH) intake during the induction phase. B) Individual animals’ mean EtOH intake for the final 6 sessions of induction during which 2.0 g/kg was made available. Individuals are grouped by subsequent treatment assignment. C) Mean (N=4; ±SEM) daily EtOH consumption over 90 sessions of EtOH maintenance conducted over about 5 months. Data are presented as sequential 5-session averages and a gap in the series indicates when one individual was briefly discontinued from EtOH access for health reasons unrelated to EtOH drinking. D) Blood EtOH levels for all monkeys after sessions (1 mo and 10 mo after the start of chronic exposure) in which 3.0 g/kg was made available. This is a modification of Figure 1 from a prior publication (Taffe et al. 2010).
Blood samples were obtained about a month after assignment to treatment groups (20 sessions of maintenance) for determination of BEL (Figure 1D). The goal was to demonstrate that BELs reached after an observed consumption level were consistent with prior observations in macaque monkeys. Given the primary purpose was behavioral evaluation, however, close determination of pharmacokinetics within and between groups was not part of the study. Samples were collected 30 min after consumption in a 30-minute ethanol session in which all animals (including Controls) had the opportunity to consume 3.0 g/kg ethanol in the standard solution. As we (Katner et al. 2004; Katner et al. 2007) and others (Vivian et al. 2001) have shown, BEL increases as a function of ethanol consumed and ranged from the limit of detection to 292 milligrams / 100 mL (mg%).
3.2. Baseline Behavioral Testing and Group Assignment
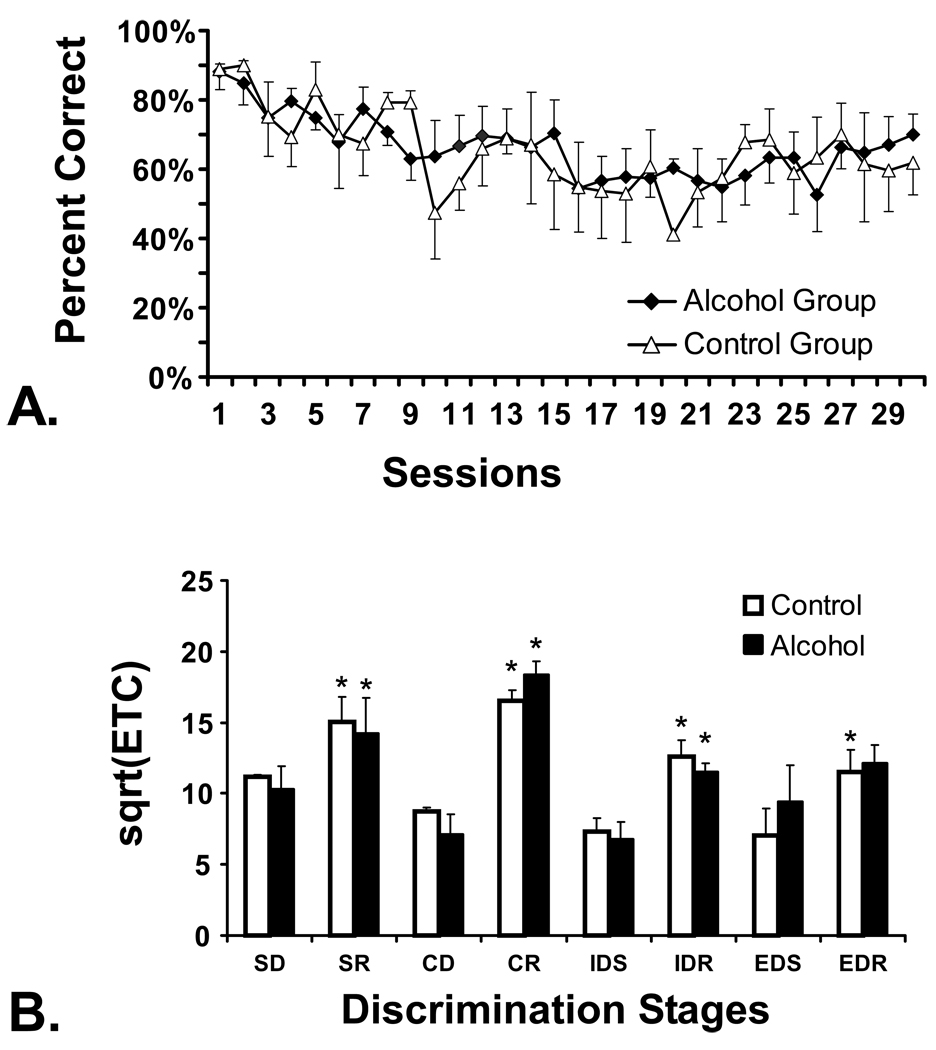
All monkeys were trained to respond to visual stimuli on the touch-screen computer apparatus (Figure 2A) prior to the administration of any ethanol and prior to assignment to treatment group. The data are presented by the eventual treatment groups to illustrate pre-existing group similarity in performance. Both groups’ accuracy decreased slightly as the stimuli became smaller in order to shape accurate responses. After initial touch-screen training the intradimensional/extradimensional attentional set-shifting procedure (ID/ED) was performed twice to balance the direction of the dimensional shift; there were no pre-existing group differences on this task (Figure 2B). The animals were then divided into 2 groups with group assignment balanced factors of initial alcohol preference and discrimination learning on the first ID/ED evaluation.
Figure 2.
A) Touch accuracy during the initial touch-screen training, prior to any EtOH exposure, is presented for the eventual Alcohol (N=4) and Control (N=3) groups. B) Performance on the Intradimensional / Extradimensional Attentional Set Shift task, conducted prior to EtOH exposure, is presented for the eventual treatment groups. Data are presented as a square root transformation of errors-to-criterion. Simple Discrimination, SD; Simple Discrimination Reversal, SR; Compound Discrimination, CD; Compound Discrimination Reversal, CR; Intradimensional Shift, IDS; Intradimensional Shift Reversal, IDR; Extradimensional Shift, EDS; Extradimensional Shift Reversal, EDR. A significant difference between pairs of acquisition and reversal stages (SD/SR, CD/CR, IDS/IDR, EDS/EDR) within treatment group is indicated by *. Bars indicate SEM in both panels.
3.3. visuo-spatial Paired Associates Learning
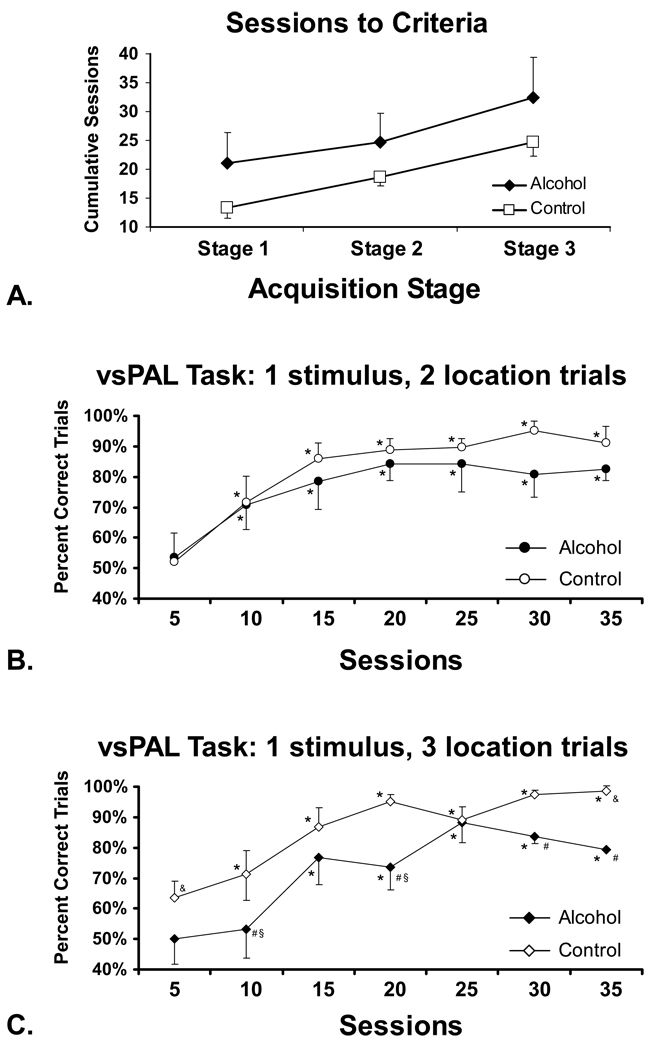
Each group successfully acquired the vsPAL task within a mean of 32.5 (SEM = 5.91) and 24.7 (SEM = 2.96) sessions for the Alcohol and Control groups respectively (Figure 3A). The apparent trend for the Alcohol group to require more sessions than the Control group to meet acquisition criteria overall and at each of the three stages was not statistically reliable.
Figure 3.
A) The mean number of training sessions required to meet acquisition criteria for the visuo-spatial Paired Associates Learning task is presented for the Alcohol (N=4) and Control (N=3) groups. Mean accuracy for B) the 1-stim (2-loc) and C) the 1-stim (3-loc) trials are presented for the Alcohol (N=4) and Control groups (N=3).) A significant difference in performance from the first block of 5 training sessions is indicated by * and the # indicates a significant difference between treatment groups. Other symbols reflect a significant increase (&) or decrease (§) relative to performance on the 1-stim (2-loc) trials for a given block of training sessions. Bars indicate the SEM in all panels.
The response accuracy (percent correct trials) for each of the 1-stim (2-loc) and 1-stim (3-loc) difficulty trials (Figure 3B, C) significantly improved in both groups over the ~5 month (the precise duration of training varied by individual) acquisition interval (F6,24=20.26; p < .0001). The ANOVA also confirmed effects of treatment group (F1,4=10.831; p <.01), the interaction of task difficulty with treatment group (F1,4=63.78; p <.001), training block with trial difficulty (F6,24=61.024; p <.05) and the interaction of all three factors (F6,24=3.867; p <.01). The post hoc test confirmed that accuracy for the 1-stim (2-loc) trials was significant higher than the first block of 5 sessions for sessions 10–35 in both treatment groups, which did not reliably differ from each other on these easiest trials. Accuracy for the 1-stim (3-loc) trials was likewise significantly improved over the first five sessions for the Control (sessions 10–35) and Alcohol (sessions 15–35). The Alcohol group performed more poorly than the controls on the 1-stim (3-loc) trials as was confirmed by a significant difference between groups for the 2nd, 4th, 6th and 7th blocks of 5 sessions. Finally, the Control group’s performance on the more difficult trials (i.e. 1-stim (3-loc)) was significantly higher than performance of the less difficult trials (significant difference for the first and last 5-session blocks of sessions) whereas the Alcohol group’s performance on the more difficult trials was lower than their performance on the less difficult trials (significant difference 2nd and 4th group of 5 sessions).
3.4. Incremental Learning Probe
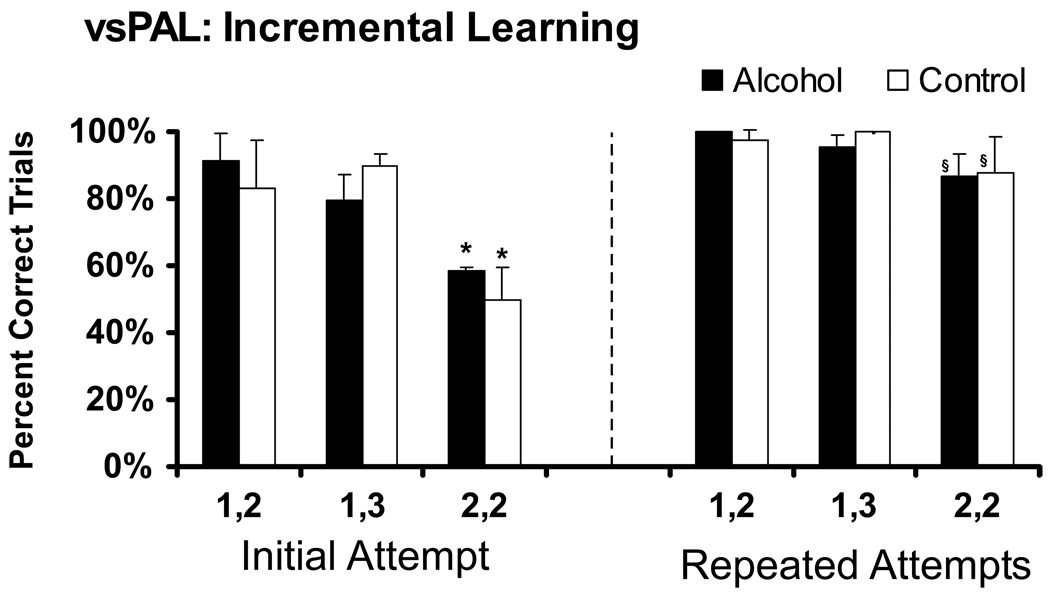
The monkeys’ average performance on the incremental learning version of vsPAL is illustrated in Figure 4. The learning component was introduced when each monkey was averaging greater than 50% correct responses on the 2-stim (2-loc) trials at the end of the initial acquisition interval. Performance was significantly altered by trial difficulty (F2,10= 18.70; p <.001), the opportunity for repeated attempts (F1,5= 68.53; p <.001); there was also a significant interaction between these factors (F2,10= 7.17; p <.05). There were no main effects of, nor interactions with, treatment group. The Fisher’s LSD post hoc test confirmed that the initial percent correct for 2-stim (2-loc) trials was significantly lower than for either other trial type in both treatment groups. In addition, the overall percent correct for the 2-stim (2-loc) trials was significantly better than initial percent correct in both groups. Thus the dependence of accuracy on trial difficulty and the ability to learn with repeated attempts was similar in both Alcohol and Control animals.
Figure 4.
Mean percent correct trials on the initial attempt, as well as after up to 5 additional repeated attempts to complete the trial, are given for the Alcohol (N=4) and Control groups (N=3); bars indicate SEM. A significant difference from performance on the easiest trial type (1, 2: 1 stimulus (2 locations)) is indicated by * and the § indicates significantly improved performance between the initial attempt and after up to 5 additional repeated attempts.
3.5. Spatial Delayed Response
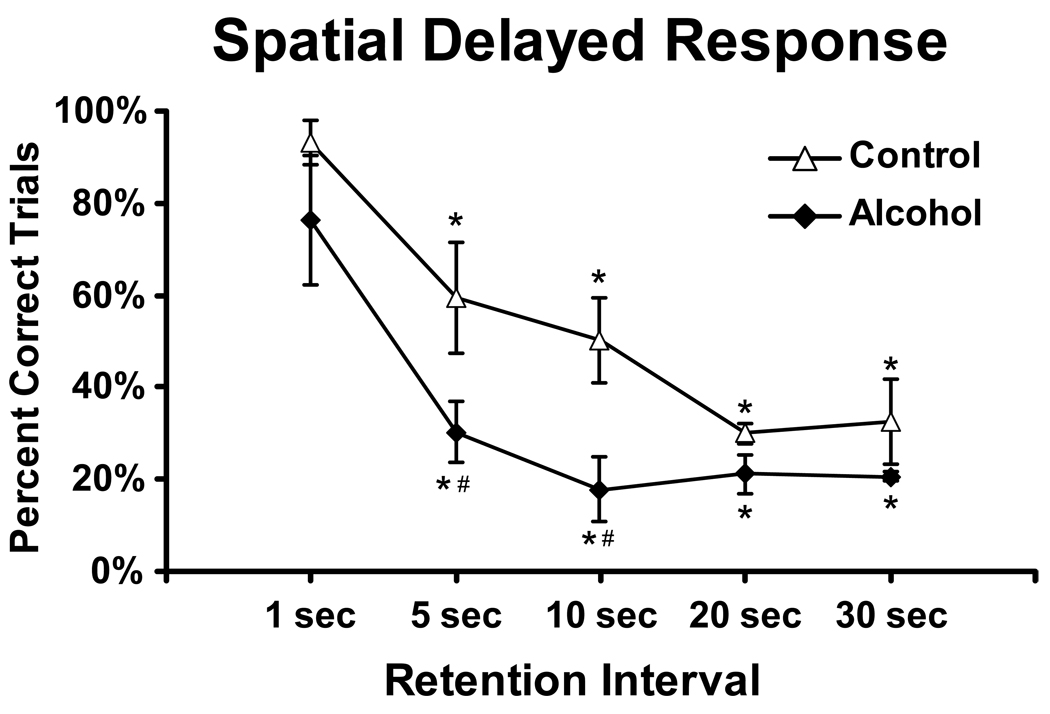
This probe was conducted during months 3–4 of maintenance drinking. Accuracy was lower when the retention interval was increased (F4,20= 20.18; p <.0001); the post hoc analysis confirmed lower accuracy relative to the standard 1-sec condition for all longer delays in each group. The Alcohol group was also less accurate than the Control group (F1,5= 22.30; p <.01) and the post hoc test confirmed significant group differences when memory delays were 5 or 10 seconds.
3.6. Bimanual Motor Skill
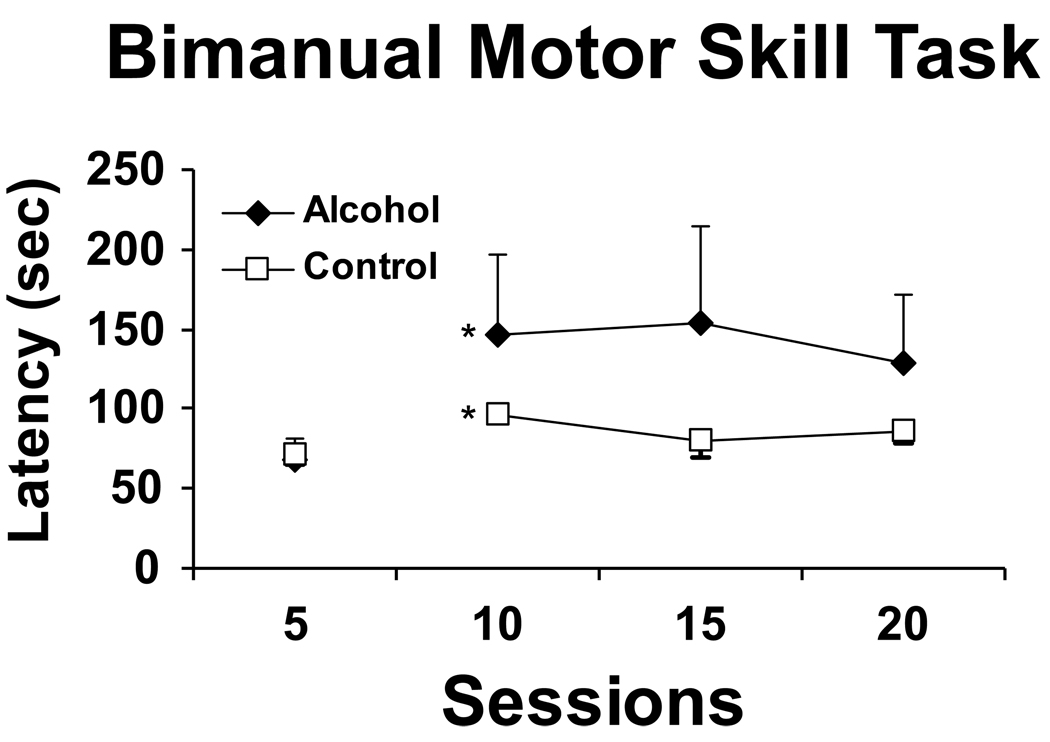
A repeated measures ANOVA on each block (average over 5 sessions) confirmed a significant main effect of increased motor speed over time (F 3,5=945, p<.05), but not by group, nor an interaction of factors (Figure 6). Post-hoc analyses confirmed that speed to retrieve all 15 raisins increased significantly from block 2 (standard conditions with raisins completely in the hole) when compared to block 1 (training conditions when raisins were sticking out of the hole and easier to retrieve).
Figure 6.
The mean time required to retrieve 15 raisins in the bimanual motor skill task is presented for the Alcohol group (N=4) and Control groups (N=3) under initial training (the raisins are partially extended for the first 5 sessions) and standard (sessions 10–20) conditions.
3.7. Brain Stem Auditory Evoked Potentials
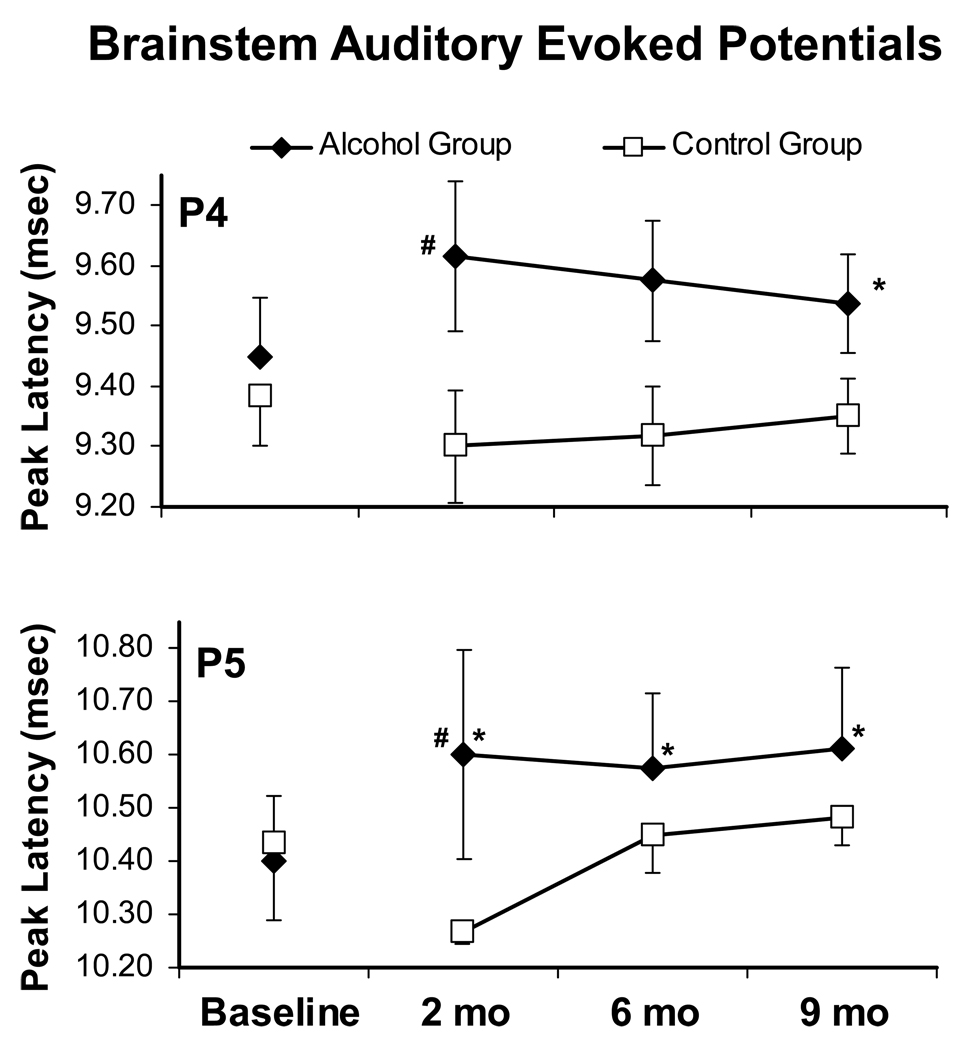
Alcohol significantly slowed the latency of later components of the BSAEPs and these alterations continued for at least 9 months as is illustrated in Figure 7. The post hoc exploration confirmed that the significant difference in P4 (F 1,5=8.938, p<.05) and P5 (F 1,5=12.571, p<.05) latencies observed between groups was attributable to a significant difference after 2 months of drinking. In addition the post hoc test confirmed that P4 (Month 9) and P5 (Months 2, 6 and 9) latencies were significantly increased over the baseline in the Alcohol group.
Figure 7.
The mean (±SEM) latencies for the P4 and P5 peaks of the brainstem auditory evoked potentials (BSAEP) are presented for the Alcohol (N=4) and Control (N=3) monkeys. Recordings were conducted before alcohol exposure (baseline) and periodically in the months during chronic alcohol exposure. A significant difference from baseline within a treatment group is indicated by * and the # indicates a significant difference between treatment groups.
4. Discussion
This study found that chronic high-level alcohol exposure in adolescent monkeys impairs several critical aspects of higher level cognitive function. The affected domains of visuo-spatial associative memory and spatial working memory suggest effects of chronic alcohol on circuitry of the dorsolateral prefrontal and temporal cortical regions. The fact that alcohol-related impairment was related to increased task difficulty provided evidence that the effects were indeed specific to the cognitive domain assessed and not a generalized behavioral or motivational impairment. In addition an observed alteration of brainstem auditory evoked potential peak latency showed that additional brain systems are also at risk from exposure to chronic alcohol.
The primary cognitive findings were that poorer performance in the acquisition of the vsPAL task and impaired retention of spatial information in the spatial delayed-response probe were associated with chronic alcohol drinking. There was a trend for the Alcohol group to require more sessions to meet acquisition criteria on the vsPAL task of visuo-spatial learning but more importantly the Alcohol group was less accurate than the Control group throughout the acquisition training. This impairment only reached statistical reliability on the more complex trials and the performance for both groups improved with training. This difficulty-dependence of the effect, and the preserved ability of the treated animals to improve with sustained training, suggests a specific cognitive impairment rather than a global performance deficit. The Spatial Delayed-Response probe illustrated a potential source of the deficit since the Alcohol group’s performance was more sensitive to retention interval in comparison with the Control group. These data suggest that the Alcohol group was less able to maintain spatial information in short term memory. Finally, the Incremental Learning probe test suggests that within-trial learning was essentially unimpaired in the Alcohol group.
A body of prior investigation has clearly associated dorsolateral prefrontal cortex with the maintenance of spatial memories across retention intervals (Bauer and Fuster 1976; Ichihara-Takeda and Funahashi 2007; Watanabe 1981; Watanabe et al. 2005). Nevertheless widespread neural circuitry is clearly involved in such tasks. For example a conjoint lesion of hippocampus and amygdala impairs spatial delayed-response in a manner dependent on retention interval (Zola-Morgan and Squire 1985) and a delayed match-to-sample task activates dorsolateral prefrontal cortical regions in addition to medial temporal lobe structures in monkeys (Porrino et al. 2005). Similarly tasks thought to assess working memory appear to activate fronto-parietal (Schweinsburg et al. 2005) as well as fronto-hippocampal (Karlsgodt et al. 2005) circuitry and also enhance dopamine release in both frontal and temporal regions (Aalto et al. 2005) in humans. Thus although the behavioral deficits point to disruption of frontal cortical functions it is likely that other areas such as the temporal lobe memory system are also being affected. For example, we have reported in a recent study (Taffe et al. 2010) that the Alcohol animals from this study had reduced hippocampal neurogenesis and increased nonapoptotic neural degeneration compared with the Control monkeys (see below).
One critical feature of this study is the fact that the groups of monkeys performed equivalently on a complex task of visual discrimination, learning and attentional flexibility ( the ID/ED attentional shift procedure) prior to any exposure to alcohol. This task depends on intact function of orbital frontal (reversal learning) and dorsolateral frontal (extradimensional shift) cortical regions (Dias et al. 1997) and is thus a reasonably good test for relevant pre-existing differences in cognitive abilities associated with frontal cortical function. An inherent inability to definitively assess pre-existing differences in human studies (Begleiter et al. 1984; Harden and Pihl 1995; Hill et al. 1990; Ozkaragoz et al. 1997; Polich et al. 1994; Whipple et al. 1991) has made it difficult to fully establish a causative role of alcohol in cases of impaired cognitive function. The present results demonstrate using an animal model that even when pre-existing behavioral parameters are controlled, alcohol has an impairing effect on neuropsychological functioning.
It was likewise important that the groups were balanced for alcohol preference. Prior study of monkeys exposed to voluntary alcohol indicates a stability of individual preference that appears to be trait-like (Grant et al. 2008a; Katner et al. 2004; Katner et al. 2007; Vivian et al. 2001). The present approach therefore avoided unknowingly assigning disproportionate numbers of high- or low-alcohol preferring phenotypes, which might be associated with cognitive differences as is found in Family History Positive/Negative children (Begleiter et al. 1984; Harden and Pihl 1995; Hill et al. 1990; Ozkaragoz et al. 1997; Polich et al. 1994; Whipple et al. 1991). As with the balancing on pre-existing behavioral capability, this strengthens the association of the alcohol exposure regimen with the observed cognitive deficits.
The observed impairment of cognitive performance in the Alcohol group is consistent with prior work in animal (Hiller-Sturmhofel and Swartzwelder 2004) and human studies (Tapert et al. 2004). Markwiese and colleagues (Markwiese et al. 1998) found that adolescent rats exposed to either low or high doses of alcohol showed impairment on a memory and learning task when compared with control rodents. Furthermore, chronic alcohol exposure may interfere with spatial working memory (but not reference memory) in rats (Santin et al. 2000); a deficit in spatial memory reversal may reflect similar processes (Obernier et al. 2002). Although there have been some failures to observe spatial memory deficits in rats after chronic alcohol exposure (Blokland et al. 1993; Fadda et al. 1999; Lukoyanov et al. 1999), similar spatial impairments may appear transiently (Steigerwald and Miller 1997) or it may be necessary to administer alcohol early (such as post-natal days 5–10) in the rat (Girard et al. 2000; Pauli et al. 1995).
Human studies also show that alcohol use in adolescence is associated with neuropsychological deficits. Brown and colleagues (Brown et al. 2000) found that the alcohol-dependent adolescents performed worse on tests of verbal and nonverbal memory than the control adolescents, findings which were supported by a longitudinal study of adolescents with alcohol-use disorders (Tapert and Brown 1999). Those studies reported that alcohol users perform poorly on tasks of attention and visuospatial abilities, again consistent with the present results. The ability to control both cognitive training/testing and alcohol exposure in this monkey model was able to show that these deficits very likely began during the acquisition of cognitive tasks.
In addition to the effects of chronic alcohol exposure on spatial memory, the Alcohol group was initially impaired on the test of bimanual motor coordination. The effect was seen relatively early in the training of this task, long before asymptotic performance is typically reached (Weed et al. 1999), and only when the raisins were first fully inserted into the holes. The retrieval latencies of the two groups rapidly became indistinguishable with additional training on the task (not shown). This suggests that the effect of chronic alcohol drinking was on the procedural learning of the task rather than on psychomotor speed. Among other things, these results show that fine motor coordination was not impaired a day after a prior drinking session throughout the course of this study.
Chronic alcohol drinking also significantly altered a relatively simple measure of brain function since the latencies of the P4 and P5 brainstem auditory evoked potential peaks were slowed in the Alcohol group; the two groups did not differ in their P4 and P5 latencies prior to any alcohol exposure. These findings are consistent with prior observations that human alcoholics exhibit slowed peak latencies of the brainstem auditory evoked response (Smith and Riechelmann 2004; Verma et al. 2006). The current data support an observation that such electrophysiological indices of brain changes induced by alcohol exposure may occur early in the course of abuse (Maurage et al. 2009). P4 originates in the lateral lemniscus and P5 originates in the inferior colliculus (Jewett 1970) thus the selectivity of the effect of chronic drinking to these peaks might indicate pontine-midbrain regions that are particularly sensitive to chronic alcohol exposure. We have previously shown that these peak latencies are decreased by a repeated high-dose regimen of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) that produces lasting serotonin reductions (Taffe et al. 2002a; Taffe et al. 2003; Taffe et al. 2001) and increased with the progression of disease in the Simian Immunodeficiency Virus model of HIV/AIDS (Horn et al. 1998), sometimes when other measures of brain function are less sensitive or consistently affected.
The animals that were in this study were ultimately maintained on ethanol / vehicle for a total of eleven months during the chronic phase and euthanized after 2–2.5 months of abstinence. A recently published study found that hippocampal cellular proliferation and neurogenesis was decreased in the Alcohol exposed group relative to the Control group (Taffe et al. 2010). Furthermore, nonapoptotic neuronal degeneration was increased. Spatial-object or spatial-pattern associative memory appears to depend on intact function of the medial temporal lobe memory system in nonhuman primates (Gaffan 1994; Gaffan and Parker 1996; Malkova and Mishkin 2003; Malkova et al. 1995). These studies, combined with findings in questionably demented and probable Alzheimer’s patients, suggest that the vsPAL task depends at least in part on hippocampal memory systems as has been briefly reviewed (Taffe et al. 2002b; 2004). The present behavioral results were observed in the first 3 months of chronic alcohol and the hippocampal disturbances were observed after months of additional chronic exposure. Nevertheless it is at least possible that the two sets of observations from the same monkeys point to a common sensitivity of hippocampal function to chronic exposure to alcohol. As such this study supports additional study of the role of chronic alcohol in disrupting hippocampal neurogenesis and cell survival and how this may relate to the disturbance of complex memory functions.
The alcohol consumption in the chronic phase of this model was stable, ultimately for about 11 months; see Taffe et al, 2010. Consumption was also consistent with heavy drinking. In 2004, the Advisory Council of the US National Institute on Alcohol Abuse and Alcoholism formally defined binge drinking as “..a pattern of drinking alcohol that brings blood alcohol concentration [BAC] to 0.08 grams percent or above. For the typical adult, this pattern corresponds to consuming five or more drinks for men, or four or more drinks for women, in about 2 hours.”
http://www.niaaa.nih.gov/AboutNIAAA/NIAAASponsoredPrograms/underage.htm. Three of our Alcohol subjects clearly qualified as chronic binge drinkers by this definition- a standard drink represents 0.2 to 0.25 g/kg (definitions vary slightly) thus 1.0–1.5 g/kg in an hour. The blood levels presented in Figure 1 confirm that even ~60 min after drinking 1.5 g/kg or more our animals are well above 0.08 grams percent and those who consume about 1.0–1.5 have blood ethanol levels of about this threshold. These results are nearly identical to a recent report in bonnet macaques (Weed et al. 2008) and are indeed typical of many nonhuman primate studies of alcohol drinking, as has been reviewed extensively by Grant and colleagues (Grant and Bennett 2003; Grant et al. 2008b). Thus, the outcome of this study was consistent with the intent to model human adolescent drinking patterns which entail intoxication past the legal limit to operate an automobile in most US jurisdictions.
In summary, the present study demonstrated that monkeys exposed to chronic alcohol exhibit impairments in the acquisition and performance of a complex cognitive task which places demands on spatial associative memory. Effects on procedural motor learning and brainstem auditory evoked potentials were also noted. The most striking differences on cognitive performance were observed when the tasks became more complex and/or memory delays were increased, thus the deficit was specific to the task demands and unlikely to reflect more generalized behavioral impairment. These findings have important implications for the public health, in that they demonstrate that alcohol has significant effects of the brain’s ability to acquire new tasks, learn new information and form new memories, skills that are essential to adolescent’s success in the educational system and as productive adults in society.
Figure 5.
Mean choice accuracy in the spatial delayed-response probe is presented for Alcohol (N=4; ±SEM) and Control (N=3; ±SEM) groups. A significant decrease relative to the 1 second retention interval condition is indicated by * and a # indicates a significant difference between the groups.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J. Comp. Physiol. Psychol. 1976;90:293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Blokland A, Prickaerts J, Raaijmakers W. Absence of impairments in spatial and temporal discrimination learning in Lewis rats after chronic ethanol consumption. Pharmacol Biochem Behav. 1993;46:27–34. doi: 10.1016/0091-3057(93)90312-h. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. Ilar J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ., 3rd Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from "on-line" processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Hulshoff PolHE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Fadda F, Cocco S, Stancampiano R, Rossetti ZL. Long-term voluntary ethanol consumption affects neither spatial nor passive avoidance learning, nor hippocampal acetylcholine release in alcohol-preferring rats. Behav Brain Res. 1999;103:71–76. doi: 10.1016/s0166-4328(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A. Interaction of perirhinal cortex with the fornix-fimbria: memory for objects and "object-in-place" memory. J Neurosci. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24:300–306. [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008a;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Stafford J, Thiede A, Kiley C, Odagiri M, Ferguson B. Who Is at Risk? Population Characterization of Alcohol SelfAdministration in Nonhuman Primates Helps Identify Pathways to Dependence. Alcohol Research & Health. 2008b;31:289–297. [PMC free article] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J Abnorm Psychol. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hiller-Sturmhofel S, Swartzwelder HS. Alcohol’s Effects on the Adolescent Brain-What can be learned from animal models. Alcohol: Research and Health. 2004;28:213–221. [Google Scholar]
- Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982;16–17:144–154. [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: task-related activity during an oculomotor delayed-response task. Exp Brain Res. 2007 doi: 10.1007/s00221-007-0941-0. [DOI] [PubMed] [Google Scholar]
- Jewett DL. Volume-conducted potentials in response to auditory stimuli as detected by averaging in the cat. Electroencephalogr Clin Neurophysiol. 1970;28:609–618. doi: 10.1016/0013-4694(70)90203-8. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Scheulenberg JE. Secondary school students. Volume I. Bethesda, MD: National Institute on Drug Abuse; 2006. Monitoring the Future national survey results on drug use, 1975–2005; p. 715. (NIH Publication No. 06-5883) [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28:873–883. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Von Huben SN, Davis SA, Lay CC, Crean RD, Roberts AJ, Fox HS, Taffe MA. Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug Alcohol Depend. 2007;91:236–243. doi: 10.1016/j.drugalcdep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JE, Champion G. Prediction of auditory brainstem reflex screening referrals in high-risk infants. Laryngoscope. 2006;116:261–267. doi: 10.1097/01.mlg.0000204312.59452.86. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Madeira MD, Paula-Barbosa MM. Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiol Behav. 1999;66:337–346. doi: 10.1016/s0031-9384(98)00301-1. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Bachevalier J. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behavioral Neuroscience. 1995;109:212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Maurage P, Pesenti M, Philippot P, Joassin F, Campanella S. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J Psychiatry Neurosci. 2009;34:111–118. [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Fung SJ, Rothmond DA, Richards B, Ward S, Noble PL, Woodward RA, Weickert CS, Winslow JT. The effect of gonadectomy on prepulse inhibition and fear-potentiated startle in adolescent rhesus macaques. Psychoneuroendocrinology. 2010;35:896–905. doi: 10.1016/j.psyneuen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- O’Mahony JF, Doherty B. Intellectual impairment among recently abstinent alcohol abusers. Br J Clin Psychol. 1996;35:77–83. doi: 10.1111/j.2044-8260.1996.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Pauli J, Wilce P, Bedi KS. Spatial learning ability of rats following acute exposure to alcohol during early postnatal life. Physiol Behav. 1995;58:1013–1020. doi: 10.1016/0031-9384(95)00120-8. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. Effects of chronic alcohol consumption on spatial reference and working memory tasks. Alcohol. 2000;20:149–159. doi: 10.1016/s0741-8329(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Riechelmann H. Cumulative lifelong alcohol consumption alters auditory brainstem potentials. Alcohol Clin Exp Res. 2004;28:508–515. doi: 10.1097/01.alc.0000117870.11317.92. [DOI] [PubMed] [Google Scholar]
- Steigerwald ES, Miller MW. Performance by adult rats in sensory-mediated radial arm maze tasks is not impaired and may be transiently enhanced by chronic exposure to ethanol. Alcohol Clin Exp Res. 1997;21:1553–1559. [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995a;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995b;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH, Ricaurte GA, Gold LH. Cognitive performance of MDMA-treated rhesus monkeys: Sensitivity to serotonergic challenge. Neuropsychopharmacology. 2002a;27(6):994–1006. doi: 10.1016/S0893-133X(02)00380-9. 27:994–1006. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav. 2003;76:141–152. doi: 10.1016/s0091-3057(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Davis S, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. Functional consequences of repeated (+/−)3,4-methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neuropsychopharmacology. 2001;24:230–239. doi: 10.1016/S0893-133X(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002b;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Caldwell L, Burke C. Alcohol and the Adolescent Brain—Human Studies. Alcohol Res Health. 2004;28:205–212. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Verma RK, Panda NK, Basu D, Raghunathan M. Audiovestibular dysfunction in alcohol dependence Are we worried? Am J Otolaryngol. 2006;27:225–228. doi: 10.1016/j.amjoto.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Watanabe M. Prefrontal unit activity during delayed conditional discriminations in the monkey. Brain Res. 1981;225:51–65. doi: 10.1016/0006-8993(81)90317-6. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hikosaka K, Sakagami M, Shirakawa S. Functional significance of delay-period activity of primate prefrontal neurons in relation to spatial working memory and reward/omission-of-reward expectancy. Exp Brain Res. 2005;166:263–276. doi: 10.1007/s00221-005-2358-y. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Weed MR, Wilcox KM, Ator NA, Hienz RD. Consistent, high-level ethanol consumption in pig-tailed macaques via a multiple-session, limited-intake, oral self-dosing procedure. Alcohol Clin Exp Res. 2008;32:942–951. doi: 10.1111/j.1530-0277.2008.00652.x. [DOI] [PubMed] [Google Scholar]
- Whipple SC, Berman SM, Noble EP. Event-related potentials in alcoholic fathers and their sons. Alcohol. 1991;8:321–327. doi: 10.1016/0741-8329(91)90497-k. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]