Abstract
Pregnancy impairs baroreflex gain, but the mechanism is incompletely understood. To test the hypothesis that reductions in brain insulin contribute, we determined if pregnant rats exhibit lower cerebrospinal fluid (CSF) insulin concentrations and if intracerebroventricular (icv) infusion of insulin normalizes gain of baroreflex control of heart rate in conscious pregnant rats. CSF insulin was lower in pregnant (68±21 pg/ml) compared to virgin (169±25 pg/ml) rats (P<0.05). Pregnancy reduced baroreflex gain (in bpm/mmHg: 2.4±0.2, pregnant; 4.6±0.3, virgin; P<0.0001) and the maximum heart rate elicited by hypotension (in bpm: 455±15, pregnant; 507±12, virgin; P=0.01). Infusion of insulin icv (100 µU/min) increased baroreflex gain in pregnant (in bpm/mmHg: 2.4±0.4 to 3.9±0.5; P<0.01) but not virgin (in bpm/mmHg: 4.6±0.4 to 4.2±0.4, ns) rats. Maximum heart rate was not altered by icv insulin in either group. Interestingly, while in pregnant rats the baroreflex was unchanged by icv infusion of the artificial CSF vehicle, in virgin rats, vehicle infusion lowered baroreflex gain (in bpm/mmHg: 4.7±0.3 to 3.9±0.3; P<0.05) and the maximum baroreflex heart rate (in bpm: 495±19 to 444±21; P<0.05). These data support the hypothesis that brain insulin is required to support optimal baroreflex function and that a decrease in brain insulin contributes to the fall in baroreflex gain during pregnancy.
Keywords: heart rate, insulin resistance, baroreceptor reflex sensitivity, pregnant, cerebrospinal fluid insulin
INTRODUCTION
Pregnancy impairs function of the baroreceptor reflex [for reviews, see 1–3]. As a consequence, pregnant individuals exhibit an increased incidence of orthostatic hypotension 4 and a reduced ability to maintain arterial pressure during hemorrhage 5–7. Despite the fact that peripartum hemorrhage is a major cause of maternal death 8;9, the mechanisms by which pregnancy causes baroreflex dysfunction remain unclear.
Recent studies suggest that insulin resistance contributes to this impairment. First, in pregnant humans, rabbits and rats, baroreflex sensitivity or gain and insulin sensitivity decrease in parallel as gestation progresses 3;10;11. Second, treatment of pregnant rabbits with the insulin sensitizing drug, rosiglitazone, normalizes baroreflex gain in pregnant rabbits 10. However, the mechanism by which insulin resistance reduces baroreflex gain has not been identified.
Although insulin is present in the brain, it is not made there 12;13. Instead, pancreatic insulin moves from plasma into the brain across the blood-brain barrier via an active, saturable transendothelial transport mechanism. Insulin resistance is associated with reduced blood-brain barrier insulin transport and brain insulin levels in several states, including obesity, aging and Alzheimer’s disease 12;13. Interestingly, pregnancy in rabbits also markedly reduces insulin levels in cerebrospinal fluid (CSF) 10. Because insulin acts centrally to increase baroreflex gain 14;15, pregnancy-induced falls in brain insulin levels could impair baroreflex function. However, whether brain insulin is the link between insulin resistance and a depressed baroreflex has not been directly examined. To test this hypothesis, it was determined, first, if late pregnant rats exhibit reduced CSF insulin levels as in rabbits, and second, if normalization of brain insulin levels, via intracerebroventricular (icv) insulin infusion, improves baroreflex gain in conscious late pregnant rats.
METHODS
Animals
Female Sprague Dawley rats (n=35; Charles River Laboratories), 12 weeks old, were acclimated to the laboratory for at least 1 week prior to any experimentation. Animals were housed with a 12 hour light and 12 hour dark cycle, and food (LabDiet 5001) and water were provided ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Health and Use of Laboratory Animals and were approved by the Oregon Health & Science University animal care and use committee.
CSF collection
Late pregnant (gestational day 20; n=4) and nonpregnant (n=3) rats were anesthetized with pentobarbital (15 mg, i.p.) and positioned in a Kopf stereotaxic instrument, with the head flexed downward. A small midline incision was made through skin and muscle on the back of the neck to expose the cistern magna. A 30 g needle attached to a 1 mL syringe with PE 10 tubing was lowered, and after the dura was pierced, slight suction initiated CSF flow. The syringe was then disconnected, and ~250 µL CSF was collected by gravity over 15–20 min into ice-chilled tubes; 30–45 min elapsed between anesthesia induction and the completion of CSF collection. Insulin was measured in 150 µL aliquots of the CSF using a sensitive rat insulin radioimmunoassay kit (Millipore-Linco, Billerica, MA) and previously published procedures 11.
Animal survival surgery
Several small groups of 2 pregnant and 2 age-matched virgin rats were studied. To induce pregnancy, each female rat was placed in a male rat’s home cage. Vaginal epithelial cytology was examined daily and the presence of sperm indicated pregnancy day zero; the female rat was then placed back in its home cage.
All surgeries were performed using aseptic technique. Rats received a single intramuscular injection of 30,000 U Penicillin G (Hanford’s United States Veterinary Products) 10–15 minutes prior to incision and codeine (1mg/100mL) in their drinking water for 2–3 days post surgery.
Icv cannulae insertion
The first surgery was performed when the pregnant rats were at 7–9 days gestation. Anesthesia was induced with 5% isoflurane in 100% oxygen and was maintained with 1.5–2% isoflurane. The rats were then placed in a Kopf stereotaxic apparatus with the skull flattened between bregma and lambda. After making a midline skin incision, clearing tissue on top of the skull, and drilling a hole through the skull, the tip of the icv guide cannula (23 g, Plastics One, Roanoke, VA) was positioned using the following coordinates (in mm relative to bregma): 1 caudal, 1–1.4 lateral, and 3.8–4.1 ventral. The cannula was secured to the skull using dental acrylic and 3 small screws. When not in use, the guide cannula was plugged with an obturator. For experiments, the obturator was replaced with a 30 g inner cannula that protruded 0.5 mm beyond the tip of the guide cannula.
Femoral catheter implantation
Seven to nine days following the first surgery, the rats were again anesthetized, and an arterial catheter (PE10 or PE50) was inserted through a small inguinal incision into the femoral artery and advanced into the distal abdominal aorta. In addition, two venous catheters (PE10) were inserted into the femoral vein and advanced into the distal inferior vena cava. The catheters were tunneled subcutaneously and were exteriorized between the scapulae. Catheter patency was maintained by flushing with sterile heparin saline (100 U/mL) 3 times per week. At least 3 days of recovery were allowed before experiments were conducted.
Experimental Protocol
Experiments were performed in pregnant rats on gestational days 19–21 and in aged-matched virgins while the rats remained in their home cage. Femoral and icv cannulae were attached to infusion pumps and the pressure transducer using sterile PE tubing. After a 1–2 hour equilibration period, a control baroreflex curve was produced using well-established, previously published methodology 16;17. Briefly, arterial pressure was gradually and smoothly increased and decreased using slow iv infusions of increasing doses of phenylephrine (1mg/ml; 0.7 to 27 µl/min) and nitroprusside (1 mg/ml; 1.35 to 68 µL/min), respectively, with each ramp in pressure completed in ~3–5 min. Blood pressure and HR were allowed to return to basal levels before another ramp was initiated. After completion of the first curve, an icv infusion of insulin (100 µU/min; Regular Human Insulin, Novo Nordisk) or the artificial CSF (aCSF) vehicle (0.6 µL/min) commenced. In mM/L, the aCSF contained: 128 NaCl, 2.6 KCl, 1.3 CaCl2, 0.9 MgCl2, 20 NaHCO3, and 1.3 Na2HPO4 (pH = 7.4; passed through a 0.2 µm syringe filter immediately before use). After 1 hr of infusion, a second baroreflex curve was generated.
Data were collected using a Biopac MP100 data acquisition and analysis system sampling at 1000 Hz. The sigmoidal baroreflex relationships between mean arterial pressure (MAP) and HR generated in each experiment were fitted and compared (see Figure 4) using the Boltzman equation: HR=A1 – A2/[1 + e(MAP−A3)/A4)] + A2, where A1 equals the maximum HR, A2 equals the minimum HR, A3 equals the MAP at the midpoint between the minimum and maximum HR, and A4 is the width or operating range. Maximum baroreflex gain was calculated by dividing the HR range (A1 – A2) by four times the width.
Figure 4.
Representative experiment showing that icv insulin infusion increases baroreflex gain in a rat in late gestation.
At the end of the experiment, the rats were anesthetized, and Alcian Blue dye (Sigma) was infused via the icv cannula. After the rats were euthanized, the brain was removed and sectioned to confirm correct cannula placement via visualization of dye in the ventricular system. In addition, in pregnant rats, the abdomen was opened to count the number of viable fetuses (range, 10–17 pups).
Statistical Analysis
A t-test was used to compare CSF insulin levels and baroreflex parameters between pregnant and nonpregnant rats. Three way ANOVA for repeated measures was used to determine if icv insulin infusion normalizes baroreflex gain in pregnant rats [factors were group (pregnant, virgin), icv infusate (insulin, aCSF) and time (before and after icv infusion)]. If a significant interaction was revealed, two 2-way ANOVAs [factors were group (pregnant, virgin) and time (before and after insulin or aCSF)] and the post hoc Neuman Keuls test were used to identify specific within and between group differences. Results of the 3-way ANOVA are provided in the figure or table legends. Baroreflex gain data were log transformed before analysis to normalize variability. Data are expressed as mean ± SEM. P < 0.05 was considered statistically significant.
RESULTS
Effects of pregnancy on CSF insulin
CSF insulin was lower in pregnant (68±21 pg/ml) compared to virgin (169±25 pg/ml) rats (P<0.05).
Effects of pregnancy on baroreflex function
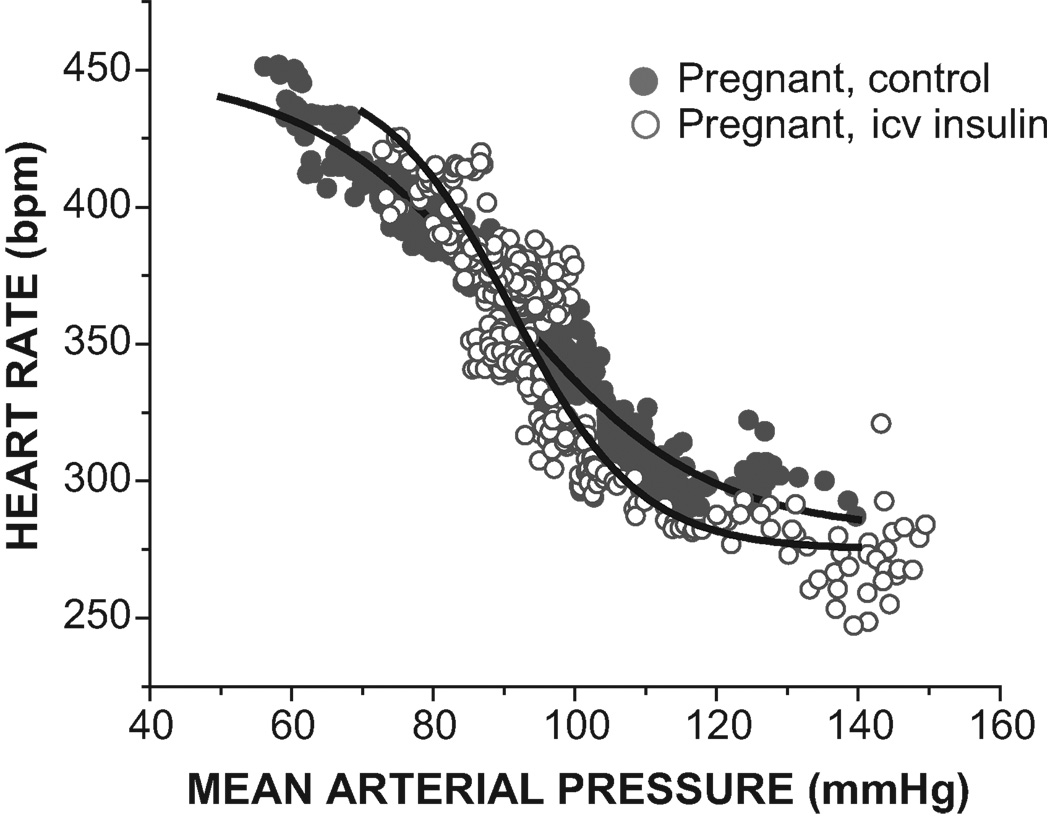
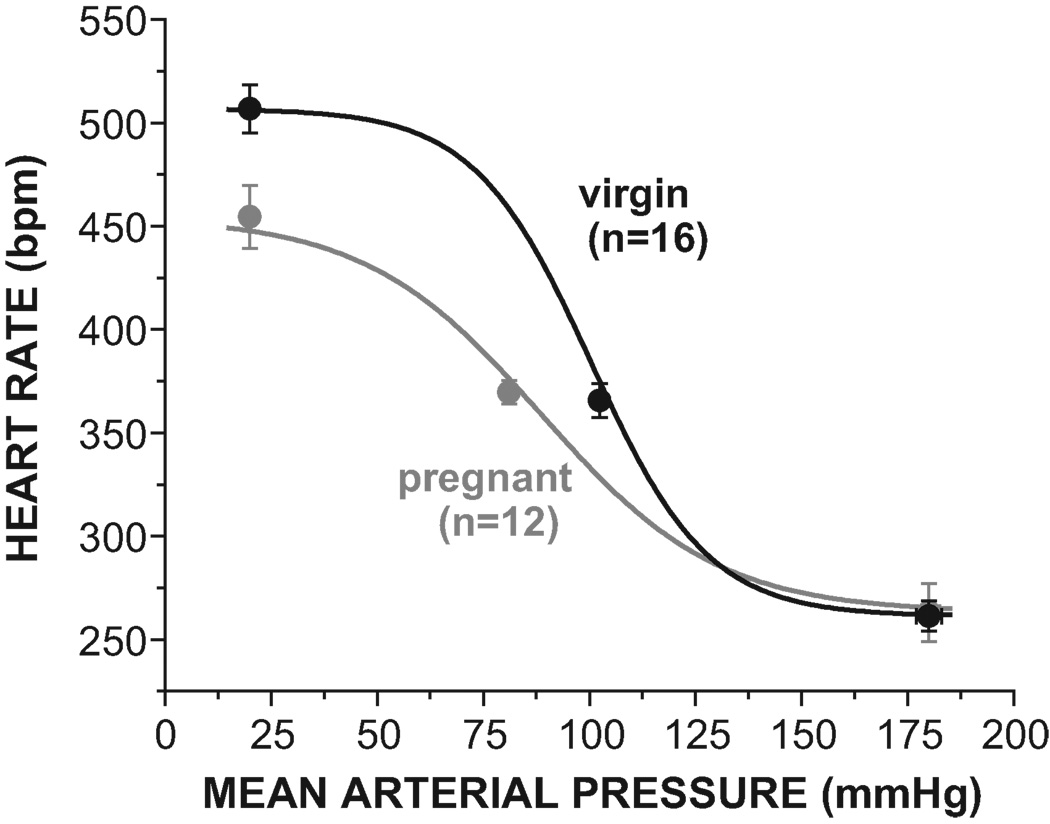
As expected, pregnancy markedly impaired baroreflex gain, due both to a reduction in HR range and an increase in width (Figures 1–2 and Table 1). The maximal level of HR achieved during hypotension was suppressed, but the minimum HR was not altered (Figure 1 and Table 1). In addition, MAP and MAP50 were reduced, but basal HR did not differ significantly between pregnant and virgin animals (Table 1).
Figure 1.
Pregnancy impairs baroreflex control of heart rate by decreasing maximal gain and the maximal heart rate (see Table 1 for statistical comparison of baroreflex sigmoidal parameters).
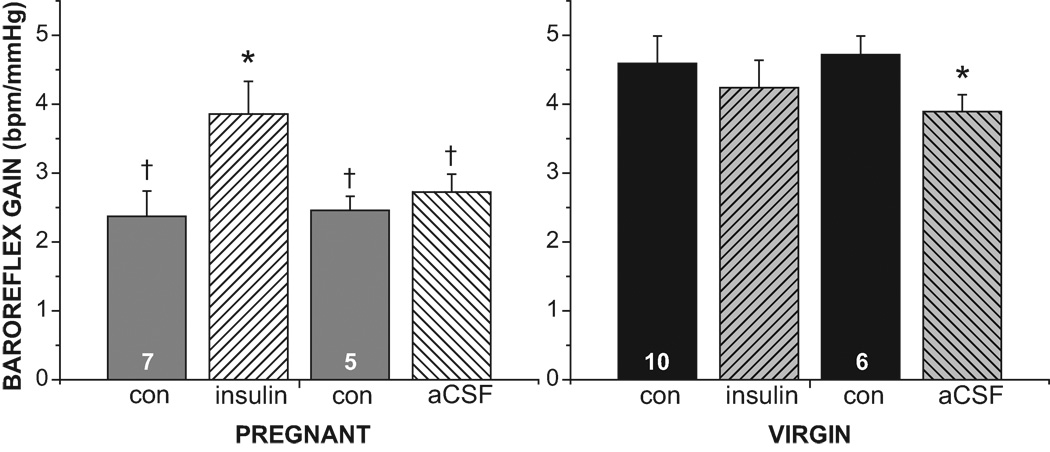
Figure 2.
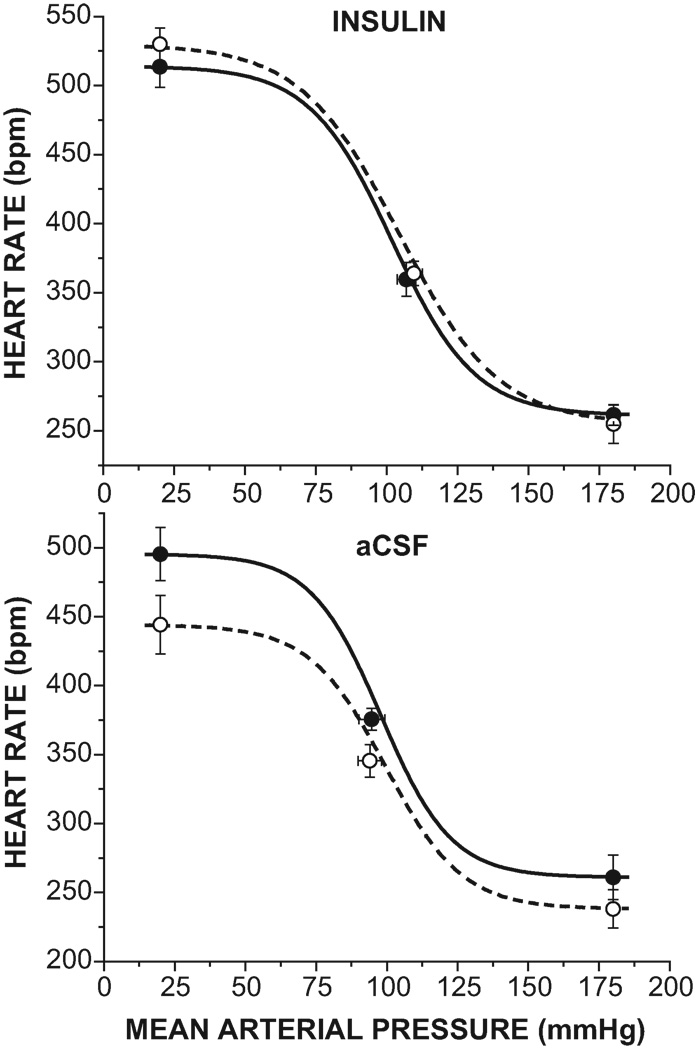
Intracerebroventricular (icv) infusion of insulin increases baroreflex gain in pregnant but not virgin rats. Results of 3-way ANOVA revealed significant (P<0.05) group and time effects, as well as group by time, treatment by time, and group by treatment by time interactions. Con, control; insulin, following 1–2 hr icv insulin infusion; aCSF, following 1–2 hr icv aCSF infusion. N are numbers in the control bars. †, P<0.05 compared to virgin. *, P<0.05, within group effect of insulin or aCSF.
Table 1.
Effect of pregnancy on baroreflex parameters, mean arterial pressure (MAP), and heart rate (HR).
| Variable | Pregnant (n=12) | Virgin (n=16) | P |
|---|---|---|---|
| Maximal Gain (bpm/mmHg) | 2.41±0.23 | 4.64±0.27 | <0.0001 |
| Maximum (bpm) | 455±15 | 507±12 | 0.01 |
| Minimum (bpm) | 263±14 | 261±7 | ns |
| Range (bpm) | 192±19 | 246±9 | <0.01 |
| Width (mmHg) | 20.8±1.6 | 13.8±0.8 | <0.001 |
| MAP50 (mmHg) | 89±5 | 100±3 | 0.05 |
| MAP (mmHg) | 81±3 | 102±3 | <0.0001 |
| HR (bpm) | 370±6 | 366±8 | ns |
Effects of icv insulin infusion: pregnant rats
Infusion of insulin icv in pregnant rats increased baroreflex gain by reducing width, but not by increasing maximum HR and therefore HR range (Figure 2–4 and Table 2). MAP, HR and other baroreflex parameters were not significantly altered (Table 2). On the other hand, icv aCSF infusion did not alter baroreflex gain (Figures 2–3, Table 2). As a result, baroreflex gain was elevated in pregnant rats receiving insulin compared to those treated with the aCSF vehicle (Figure 2, P<0.05).
Table 2.
Effect of intracerebroventricular (icv) infusion of insulin or artificial cerebrospinal fluid (aCSF) on baroreflex parameters, mean arterial pressure (MAP), and heart rate (HR) in pregnant and virgin rats.
| PREGNANT RATS | ||||
|---|---|---|---|---|
| Variable | icv insulin infusion (n=7) | icv aCSF infusion (n=5) | ||
| pre-insulin | post-insulin | pre-aCSF | post-aCSF | |
| Maximum HR (bpm) | 455±24† | 437±26† | 455±19† | 436±18 |
| Minimum HR (bpm) | 275±6 | 267±5 | 247±33 | 254±24 |
| Range (bpm) | 180±28† | 170±21† | 208±25 | 182±17 |
| Width (mmHg) | 20.7±2.6† | 11.8±1.3*† | 21.0±1.4† | 16.9±1.2*† |
| MAP50 (mmHg) | 84±6† | 89±7† | 95±9 | 90±8 |
| MAP (mmHg) | 80±4† | 78±3† | 83±4† | 83±4† |
| HR (bpm) | 364±7 | 362±12 | 378±10 | 360±9 |
|
VIRGIN RATS | ||||
| icv insulin infusion (n=10) | icv aCSF infusion (n=6) | |||
| pre-insulin | post-insulin | pre-aCSF | post-aCSF | |
| Maximum HR (bpm) | 514±15 | 530±12 | 495±19 | 444±21* |
| Minimum HR (bpm) | 261±8 | 255±14 | 261±16 | 238±14 |
| Range (bpm) | 252±10 | 268±10 | 234±18 | 206±14 |
| Width (mmHg) | 14.6±1.2 | 17.4±1.8 | 12.4±0.7 | 13.5±0.9 |
| MAP50 (mmHg) | 102±5 | 105±4 | 98±4 | 99±4 |
| MAP (mmHg) | 107±3 | 110±3 | 95±5 | 94±4 |
| HR (bpm) | 360±12 | 364±9 | 376±8 | 345±12 |
The results of the three-way ANOVA are as follows: Maximum HR significant (P < 0.05) group, treatment, and time effects, as well as significant group by treatment, treatment by time and group by treatment by time interactions; Minimum HR no significant effects; Range significant group and time effects, as well as significant group by treatment and treatment by time interactions; Width significant group and time effects, as well as significant group by treatment, group by time, and group by treatment by time interactions; MAP50 significant group effect, as well as a significant treatment by time interaction; MAP significant group and group by treatment interaction; HR: significant time effect and time by treatment interaction. See text for definition of baroreflex parameters.
P < 0.05 within group comparison, pre- versus post-insulin or aCSF.
P < 0.05 compared to virgin rats.
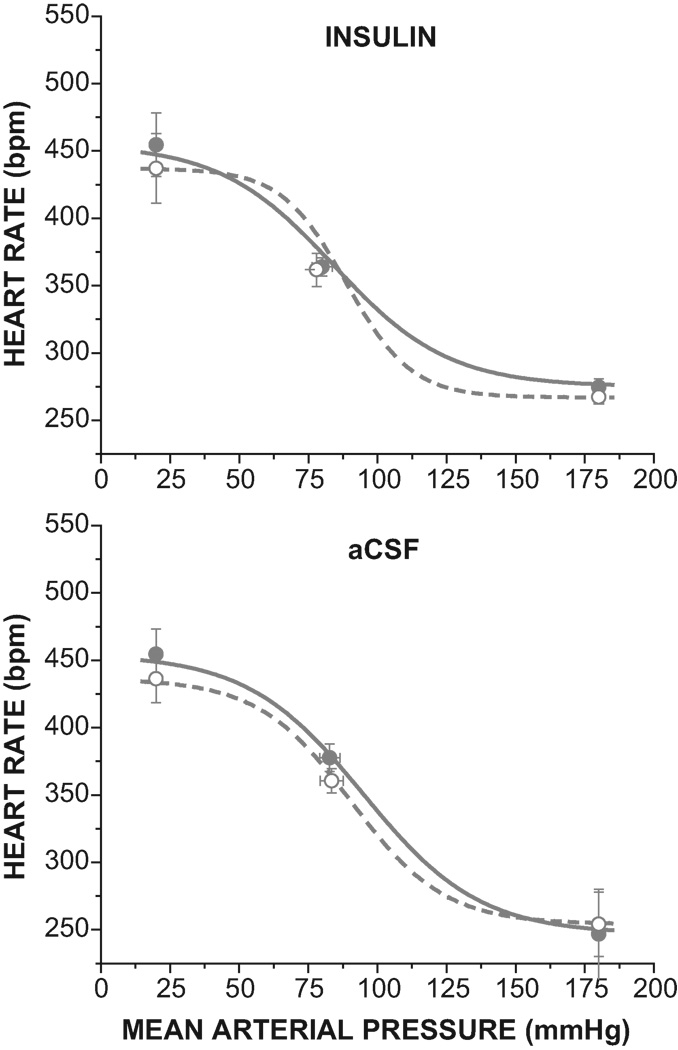
Figure 3.
Intracerebroventricular infusion of insulin (top), but not aCSF (bottom), improves baroreflex gain in pregnant rats (see Table 2 and Figure 2 for statistical comparison of baroreflex sigmoidal parameters).
Effects of icv insulin infusion: virgin rats
In contrast to pregnant rats, icv insulin infusion did not alter baroreflex control of HR in virgin rats (Figure 5 and Table 2). Interestingly, however, icv infusion of the aCSF vehicle shifted the curve downward on the y axis, as reflected by significant decreases in the HR maximum (Figure 5 and Table 2) and in baroreflex gain (Figures 2, 5).
Figure 5.
Intracerebroventricular infusion of insulin (top) had no effect on the baroreflex in virgin rats, but aCSF (bottom) reduced the maximal heart rate and maximal baroreflex gain (see Table 2 and Figure 2 for statistical comparison of baroreflex sigmoidal parameters).
DISCUSSION
The purpose of the present study was to test whether low brain insulin contributes to baroreflex dysfunction during pregnancy. The important new findings are (1) pregnancy decreases CSF insulin in rats; (2) icv infusion of insulin normalizes baroreflex gain in conscious pregnant rats without significantly improving other baroreflex parameters; (3) icv infusion of insulin in conscious virgin rats does not significantly alter baroreflex function; (4) icv infusion of aCSF has no effect in pregnant rats, but in virgin rats, it decreases baroreflex gain and the maximum HR elicited by severe hypotension. Collectively these data support the hypothesis that pregnancy decreases baroreflex gain in part by attenuating the action of insulin in the brain to support baroreflex function.
While the effect of pregnancy to impair baroreflex function has been known for several decades, it is only recently that insulin resistance has been recognized as a potential mediator. Evidence to support this mechanistic link include the following: several other insulin resistant states besides pregnancy, such as obesity 18 and aging 19;20, are also associated with dysfunctional baroreflexes; decreases in baroreflex gain and insulin sensitivity are temporally correlated during gestation 3;10;11; and treatment of pregnant rabbits with the insulin-sensitizing drug, rosiglitazone, improves insulin sensitivity and, to a similar extent, the baroreflex 10.
Further information indirectly suggests that insulin resistance may decrease baroreflex gain by reducing insulin actions in brain. First, the site at which pregnancy impairs the baroreflex within the baroreflex neuronal circuitry is the brain, rather than afferent pathways 2;3. Second, pregnancy decreases insulin concentration in CSF in rabbits 10, and in the present study, in rats. The mechanism that mediates the reductions in CSF insulin levels is currently unidentified, but possibilities include reduced transport of insulin into the brain and CSF, as has been documented in other insulin resistant states, or increased brain degradation of insulin 12;13. Interestingly, in other insulin resistant states such as obesity, the brain insulin receptor also becomes resistant to insulin 21, suggesting an additional pathway by which pregnancy could impair the actions of insulin in brain. Third, insulin acts centrally to increase gain of baroreflex control of HR and sympathetic nerve activity 14;15;22. Despite this evidence, no experiments have been performed to directly establish that reduced brain insulin mediates baroreflex impairment during pregnancy, or any other insulin resistant condition.
The major finding of the present study is that icv insulin infusion normalized maximal gain of baroreflex control of HR in pregnant rats, without altering the baroreflex in virgin animals. This result suggests two key conclusions: First, reductions in brain levels and/or actions of insulin contribute to baroreflex impairment during pregnancy, and second, insulin levels must be sufficiently high in normal virgin rats to maximally support baroreflex control of HR. Interestingly, insulin did not reverse the depressed maximal baroreflex HR observed in pregnant rats, despite the fact that icv insulin infusion can increase the HR maximum 14. One explanation for this finding is that other mechanisms underlie this effect of pregnancy. In support of this idea, Heesch and colleagues have suggested that, during pregnancy, actions of the neurosteroid progesterone metabolite, allopregnanolone, in the rostral ventrolateral medulla (RVLM) decrease maximal levels of renal sympathetic nerve activity during hypotension 2;3. While insulin initiates its action in the forebrain 14, a glutamatergic synapse in the RVLM is a link in the pathway by which insulin increases sympathetic nerve activity 23, and the RVLM is a synaptic relay in brainstem sympathetic baroreflex pathways 3. Pregnancy decreases gain of baroreflex control of HR primarily by impairing hypotension-induced increases in cardiac sympathetic activity 24. Therefore, we propose that the effects of acute increases in forebrain insulin to increase maximal responses to baroreceptor unloading are negated during pregnancy by the inhibitory actions of allopregnanolone in the RVLM.
Another interesting finding of the present study was that icv aCSF infusion in virgin, but not pregnant, rats depressed HR baroreflex responses. This result suggests that a CSF/periventricular brain factor in female animals normally supports baroreflex function and that this factor was diluted by the aCSF infusion. Further, it appears that the actions of this factor are muted during pregnancy such that a further reduction in its levels is without effect. The identity of this factor is unknown, but it is tempting to speculate that it may be insulin. In support of this hypothesis, while infusion of aCSF alone attenuated the baroreflex, infusion of aCSF with insulin had no effect in virgin animals. Moreover, addition of insulin to aCSF in pregnant animals improved the baroreflex, while aCSF alone had no effect.
The present results are the first to suggest that brain insulin normally supports baroreflex function and that a decrease in insulin’s central actions, as during pregnancy, can contribute to baroreflex dysfunction; however, some limitations must be acknowledged. First, while insulin was infused icv to localize its actions to the brain, the concentrations of insulin in CSF produced by the infusion likely are well above the physiological range. Indeed, CSF insulin levels are considerably lower than levels in plasma 12. However, it is important to emphasize that insulin normally enters the brain via transport across the blood-brain barrier from the plasma, and only a small fraction reaches the CSF 25. Moreover, insulin in CSF only slowly penetrates brain tissue across the periventricular border 26; therefore, high levels are required to drive sufficient insulin movement from CSF into distant brain regions. Indeed, it takes ~60 min for icv insulin infusion at the present dose to significantly improve baroreflex function 14. Thus, whether considering endogenous insulin that enters the brain via the plasma or exogenous insulin that enters the brain via the CSF, the CSF insulin levels do not reflect the concentration of insulin at its receptors. Second, the present results do not identify the site of action of insulin in brain to improve baroreflex function, though previously we demonstrated that insulin acts proximal to the fourth ventricle 14. Several forebrain sites that influence cardiovascular autonomic function, in particular in the hypothalamus, are enriched with insulin receptors 27;28. Given that insulin improves baroreflex function within a relatively short period and its slow rate of penetration, we speculate that the site of action is periventricular, such as the paraventricular nucleus (PVN) or the arcuate nucleus. Indeed, recent work suggests that the PVN is involved 3. Finally, while our studies indicate that normal brain insulin levels are required for optimal regulation of baroreflex control of HR, whether a similar role for insulin can be documented in the baroreflex control of sympathetic outflow remains to be investigated.
Perspectives
The major conclusion of the present study is that insulin resistance contributes to baroreflex impairment during pregnancy by reducing brain insulin. Insulin resistance and reduced baroreflex gain are associated in several other pathophysiological states, such as obesity, type 2 diabetes, hypertension, heart failure, and aging. In women with preeclampsia, further reductions in both insulin sensitivity and baroreflex function have been observed 3. Moreover, a recent study by Young et al 22 demonstrated for the first time in healthy humans that (1) physiological increments in plasma insulin produce nearly a doubling of gain of arterial baroreflex control of muscle sympathetic nerve activity, and (2) in these male subjects, the ability of insulin to enhance the baroreflex was greatest in individuals with higher insulin sensitivity; baroreflex gain and insulin sensitivity were highly correlated. These timely findings in humans coupled with evidence that reduced baroreflex sensitivity or decreased heart rate variability are risk factors for subsequent serious cardiovascular events 29–31, increases the need for determining whether insulin resistance, via reductions in brain insulin, contribute to baroreflex dysfunction in these several other diseases as well.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance provided by Korrina Freeman.
SOURCES OF FUNDING.
This work was supported in part by NIH grant HL088552, a Grant-in-Aid from the American Heart Association, Pacific Mountain Affiliate, and from the Medical Research Foundation of Oregon. ASA was supported in part by a fellowship from the Murdock Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST.
None.
REFERENCES
- 1.Brooks VL, Quesnell RR, Cumbee SR, Bishop VS. Pregnancy attenuates activity of the baroreceptor reflex. Clin Exp Pharmacol Physiol. 1995;22:152–156. doi: 10.1111/j.1440-1681.1995.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 2.Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann N Y Acad Sci. 2001;940:348–360. doi: 10.1111/j.1749-6632.2001.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 3.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2010;299:R439–R451. doi: 10.1152/ajpregu.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Easterling TR, Schmucker BC, Benedetti TJ. The hemodynamic effects of orthostatic stress during pregnancy. Obstet Gynecol. 1988;72:550–552. [PubMed] [Google Scholar]
- 5.Humphreys PW, Joels N. Arterial pressure maintenance after hemorrhage in the pregnant rabbit. J Physiol. 1985;366:17–25. doi: 10.1113/jphysiol.1985.sp015782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson K, Anden NE, Johansson K, Thornstrom U. Effects of acute haemorrhagic hypotension during pregnancy and lactation in conscious goats. Acta Physiol Scand. 1987;129:479–487. doi: 10.1111/j.1365-201x.1987.tb10621.x. [DOI] [PubMed] [Google Scholar]
- 7.Clow KA, Giraud GD, Ogden BE, Brooks VL. Pregnancy alters hemodynamic responses to hemorrhage in conscious rabbits. Am J Physiol Heart Circ Physiol. 2003;284:H1110–H1118. doi: 10.1152/ajpheart.00626.2002. [DOI] [PubMed] [Google Scholar]
- 8.Panchal S, Arria AM, Labhsetwar SA. Maternal mortality during hospital admission for delivery: a retrospective analysis using a state-maintained database. Anesth Analg. 2001;93:134–141. doi: 10.1097/00000539-200107000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Rochat RW, Koonin LM, Atrash HK, Jewett JF. Maternal mortality in the United States: report from the Maternal Mortality Collaborative. Obstet Gynecol. 1988;72:91–97. [PubMed] [Google Scholar]
- 10.Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- 11.Brooks VL, Mulvaney JM, Azar AS, Zhao D, Goldman RK. Pregnancy impairs baroreflex control of heart rate in rats: role of insulin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2010;298:R419–R426. doi: 10.1152/ajpregu.00441.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Bunag RD. Insulin acts centrally to enhance reflex tachycardia in conscious rats. Am J Physiol. 1994;266:R481–R486. doi: 10.1152/ajpregu.1994.266.2.R481. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Brooks VL. Sodium intake, angiotensin II receptor blockade and baroreflex function in conscious rats. Hypertension. 1997;29:450–457. doi: 10.1161/01.hyp.29.1.450. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Collister JP, Osborn JW, Brooks VL. Endogenous angiotensin II supports lumbar sympathetic activity in conscious, sodium deprived rats: role of area postrema. Am J Physiol. 1998;275:R46–R55. doi: 10.1152/ajpregu.1998.275.1.R46. [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 21.Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- 22.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks VL, Kane CM, Van Winkle DM. Altered heart rate baroreflex during pregnancy: role of sympathetic and parasympathetic nervous systems. Am J Physiol. 1997;273:R960–R966. doi: 10.1152/ajpregu.1997.273.3.R960. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 26.Baskin DG, Woods SC, West DB, Van Houten M, Posner BI, DORSA DM, Porte D., Jr Immunocytochemical detection of insulin in rat hypothalamus and its possible uptake from cerebrospinal fluid. Endocrinology. 1983;113:1818–1825. doi: 10.1210/endo-113-5-1818. [DOI] [PubMed] [Google Scholar]
- 27.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 28.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 29.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, Yoshimatsu H. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74:1379–1383. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 30.Head GA. Spontaneous baroreflex sensitivity: towards an ideal index of cardiovascular risk. J Hypertens. 2002;20:829–831. doi: 10.1097/00004872-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Parati G, Lantelme P. Mechanical and neural components of the cardiac baroreflex: new insights into complex physiology. J Hypertens. 2005;23:717–720. doi: 10.1097/01.hjh.0000163137.59341.c3. [DOI] [PubMed] [Google Scholar]