Abstract
The objective of this research was to evaluate the efficacy of a recombinant non-viral vector for targeted delivery of a thymidine kinase (TK) suicide gene to xenograft SKOV-3 tumors. The vector was genetically engineered and used to condense TK gene into particles of less than 100nm in size. The nanoparticles were used to transfect and kill SKOV-3 cancer cells in combination with ganciclovir in vitro. The results demonstrated that the vector could effectively kill up to 80% of the SKOV-3 cancer cells. In the next step, the ability of the vector to deliver TK suicide gene to xenograft tumors of SKOV-3 was studied. The results demonstrated that the vector could transfect tumors and result in significant tumor size reduction during the period that ganciclovir was administered. Administration of ganciclovir for at least three weeks post transfection was of paramount importance. These results illustrate the therapeutic efficacy and application of a designed recombinant non-viral vector in cancer gene therapy.
Keywords: Recombinant vector, non-viral, gene delivery, suicide gene, cancer therapy
INTRODUCTION
In the last decade, significant progress has been made in the development of vectors (viral and non-viral) for cancer gene therapy. However, none of these vectors have been able to collectively demonstrated safety, efficiency, tissue specificity, and cost effectiveness. Consequently, the need for research into innovative and novel delivery vehicles remains. Recently, there has been a great deal of interest in the development of vectors based upon biological motifs with potential applications in gene therapy. Unfortunately, none of the peptide motifs of biological origin (e.g., TAT, Mu, SV40-NLS, etc.) have demonstrated the ability to independently overcome the major cellular barriers associated with successful targeted gene delivery [1–3]. In contrast, recombinant multifunctional vectors, which are the fusion of multiple biological motifs, have shown promise in overcoming these obstacles by independently performing several self-guided functions [4–6]. These include, but are not limited to, cell targeting, DNA condensation, endosome disruption, and nuclear localization. Based on this information, a series of genetically engineered biomacromolecules were developed with improved efficiency, biodegradability, ease of production, and cell targetability [4, 7, 8]. This class of vectors (i.e., recombinant fusion vectors) not only can be used as a tool for precise structure/activity relationship studies, but holds the potential to merge the strengths of both viral and non-viral vectors in order to overcome the abovementioned obstacles[9].
We have recently reported the structure of a recombinant multifunctional vector with chimeric architecture composed of a HER2 targeting affibody, four repeating units of Histone H2A, and a pH-responsive fusogenic peptide (GALA) (Figure 1)[8]. We have previously shown that this vector has the ability to condense plasmid DNA encoding green fluorescent protein (pEGFP) into nanosize particles and protect from serum endonucleases, target HER2 positive cancer cells (i.e., SKOV-3) but not HER2 negative ones (e.g., MDA-MB-231), escape from the endosomal compartment into cytosol with the help of the fusogenic peptide, utilize microtubules to transfer genetic material toward the cell nucleus and mediate efficient gene expression.
Figure 1.
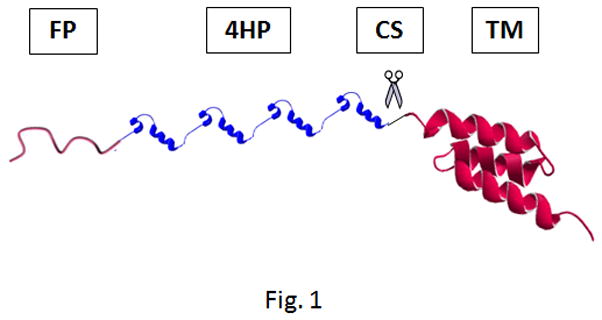
Schematic representation of vector GHT composed of a GALA fusogenic peptide (FP), four repeating units of histone H2A (4HP), a cathepsin D substrate (CS), and a targeting motif (TM). The vector was genetically engineered in E.coli and purified. Reproduced with permission from [8].
The objective of this study was to examine the ability of the vector, namely GHT, to efficiently deliver thymidine kinase (TK) suicide gene and kill SKOV-3 cancer cells in vitro and in vivo. While SKOV-3 cancer cells were used to evaluate the in vitro cell killing efficiency, nude mice bearing xenograft tumors of SKOV-3 and plasmid DNA that encodes a mutant thymidine kinase were used as models for suicide cancer gene therapy. The basis of suicide gene therapy is that a gene, which encodes an enzyme, in this case Herpes Simplex Virus type 1 Thymidine Kinase (HSV-TK), is delivered to tumor cells followed by a non-cytotoxic prodrug such as ganciclovir (GCV) [10]. The encoded enzyme carries the unique capability of converting the prodrug into a cytotoxic compound that subsequently induces apoptosis [11, 12]. In addition, this cytotoxic compound could have a “bystander effect”, meaning that it could increase the number of apoptotic cells compared to the number of cells transduced, minimizing the necessity to transfect 100% of tumor cells [13]. The bystander effect is defined as the situation where more cells die than would be predicted by the number of cells transduced. This bystander effect is especially important because the current status of delivery vehicles used in gene therapy trials is unsatisfactory [14, 15]. Here we used a mutant HSV-TK, namely SR39, which has shown high efficacy in preventing tumor growth in xenograft tumor models and is currently being used in clinical trials (Phase I-III) [16, 17]. The sequence of SR39 mutant has previously been reported [18]. We hypothesized that the vector GHT can complex with pSR39 to form stable nanoparticles with ability to transfect SKOV-3 xenografts efficiently resulting in significant tumor size reduction.
MATERIALS AND METHODS
Production of GHT vector
The details of the methods of vector cloning, expression and purification are reported previously [8]. In brief, the plasmid DNA encoding the GHT was transformed into E.coli BL21 (DE3) pLysS and expressed by IPTG induction. GHT was purified using Ni-NTA column chromatography and used in the subsequent studies.
Particle size and charge
Various amounts of vector GHT was dissolved in 5% glucose solution and complexed with either 1 μg of pEGFP (plasmid DNA encoding green fluorescent protein) or pSR39 (plasmid DNA encoding mutant thymidine kinase under CMV promoter). The plasmid DNA encoding SR39 gene was generously provided by Dr. Margaret Black in the Department of Pharmaceutical Sciences at Washington State University. Complexes were formed at different N/P ratios (nitrogen groups in vector to phosphate groups in plasmid DNA) and incubated at room temperature for 20 min before measurement. For example, to prepare N:P ratio of 1, 1.4 μg of vector was used to complex with 1μg of pEGFP or pSR39. In calculations of N:P ratio, we considered all positively charged N-atoms in the vector structure versus negatively charged P-atoms in pDNA. The mean hydrodynamic particle size for vector/pDNA complexes was determined by dynamic light scattering and the surface charge of particles was measured by Laser Doppler Velocimetry. All the measurements were performed using Malvern Nano ZS90 instrument and analyzed by DTS software (Malvern Instruments, UK). The particle size and zeta potential of the complexes were measured and reported as mean ± SEM, (n=3). Each mean is the average of 15 measurements and n represents the number of separate batches prepared for the measurements.
Cell transfection
SKOV-3 cells were transfected with GHT/pEGFP complexes using the previously reported method [8]. In brief, SK-OV-3 cells were seeded in 96-well plates at 2 × 104 cells per well and incubated over night at 37°C. Cells were transfected with GHT/pEGFP complexes at various N:P ratios (equivalent of 1 μg pDNA) in McCoy’s 5A media supplemented with insulin, transferrin, selenium, ovalbumin, dexamethasone and fibronectine. The in vitro transfection studies are performed in the absence of serum. This is due to the fact that addition of serum to the media increases the viscosity of the media which could block the sedimentation of small size particles with low density (e.g., viruses or nanoparticles with sizes <200nm). Transfection in the presence of serum may be valid only for large size particle aggregates (>500nm) which have a better chance to sediment uniformly on the surface of the cells and result in transfection. For example, manufacturers of lipofectamine (Invitrogen, Carlsbad, CA) encourage users to transfect cells in the presence of Optimem where lipid particles form large size flocculates. This phenomenon is shown in reference [4]. To overcome this obstacle, we follow the transfection protocols for viral vectors which are targeted nanoparticles with sizes less than 100nm. For more information, please see the standard protocol of cell transfection with adenovirus from MP Biomedicals (Solon, OH).
The green fluorescent protein (GFP) was visualized after 48hrs using an epifluorescent microscope (Zeiss Axio Observer Z1) to evaluate gene expression. Lipofectamine and polyethyleneimine (PEI) 25 kDa (Sigma) were used as positive controls to validate the transfection protocol. To quantify gene expression, total green fluorescence intensity was measured using a flowcytometer (FacsCalibur, Becton Dickinson, San Jose, CA) and the data processed by FlowJo Cytometry Analysis Software. The total fluorescence intensity of GFP positive cells was normalized against the total fluorescence intensity of untransfected cells (background control). The data are presented as mean ± s.d, n=3.
In brief, 5,000 cells in control group (untransfected) were analyzed by flowcytometer to determine the fluorescence intensity of each untransfected cell. Using the 99% gating, the level of fluorescence intensity of the cell in the 99% percentile was identified and used as the basal level of autofluorescence. The fluorescence related to the top 1 percentile was considered debris. In the next step, 5,000 cells in the treatment group (transfected) were analyzed by flowcytometer to determine the fluorescence intensity of each cell. Cells that fluoresced above the basal level (established as autofluorescence) were considered transfected. The cumulative fluorescence intensity of the transfected cells is reported on the Y-axis of graphs as “Total Fluorescence Intensity”. The percentage of transfected cells was calculated by using the following formula:
Detection of TK expression
To determine the expression of thymidine kinase (SR39) in cells that were transfected in vitro, a western blot analysis was performed using polyclonal antibody against thymidine kinase. SKOV-3 cells were seeded in 96-well plates at a seeding density of 2 × 104 cells/well. Cells were transfected with GHT/pSR39 at various N/P ratios (equivalent of 1 μg pSR39). 48 hr post transfection, cells were washed with ice-cold PBS and lysed using lysis buffer. The lysate was spun down at 12,000 g for 20 minutes at 4 °C. The supernatant was removed and protein content was determined by Bradford Assay. 30 μg of sample was loaded onto Tris-Glycine SDS-PAGE and transferred to PVDF membrane. The membrane was then blotted with rabbit anti-TK primary polyclonal antibody (R&R Rabbitry, Stanwood, WA) (1:5000 dilution) and goat anti-rabbit secondary antibody (Abcam, Cambridge MA) (1:10,000). Expression of thymidine kinase protein was then evaluated using Odyssey Infrared Imaging System (LI-COR Technology, NE). Untransfected cells were used as negative control.
To detect the expression of TK in tumors, mice that were used in protocol 9 were euthanized, tumors removed and snap-frozen in liquid nitrogen. Before use, tumor tissues were thawed on ice and dissected into smaller pieces. For each 50mg of tissue, 1.5ml ice-cold lysis buffer (50mM Tris, 150mM NaCl, 1.0% Triton X-100 with protease inhibitor cocktail) was added and homogenized with an electrical homogenizer for 15 seconds. The samples were incubated on ice for 1 hour followed by centrifugation at 12,000 xg for 20 minutes at 4 °C. The supernatant was collected and protein content was quantified using Bradford Assay. Equivalent to 30 μg of protein was loaded onto a 12.5% Tris-glycine SDS-PAGE and transferred onto a PVDF membrane. The membrane was then blotted as mentioned above and expression of thymidine kinase protein visualized. Tumors from mice that were treated with PBS used as negative control. The lysate of SKOV-3 cells that were transfected with pSR39 in vitro was used as positive control.
WST-1 cell toxicity assay
To evaluate the cell killing efficiency, GHT/pSR39 (equivalent of 1 μg pDNA) was administered to 1×104 SKOV-3 cells seeded in 96-well plates. Four hours post transfection, the media was replaced with fresh McCoy’s 5A full medium supplemented with 10% fetal bovine serum and 50 μM ganciclovir (Sigma-Aldrich, MO). Cells were incubated for a week and media supplemented with 50 μM ganciclovir was refreshed every two days. A cell toxicity assay was performed with WST-1 reagent (Roche Applied Science, IN) to determine the survival rate after the treatment. The data is reported as mean±s.d., n=3.
Clonogenic Assay
The details of the method for clonogenic assay was adapted from the standard protocol reported by others [19]. To determine the cell reproductive death, GHT/pSR39 complexes formed at various N:P ratios (equivalent of 10 μg pSR39) were administered to 1×106 SKOV-3 cells seeded in T25 flasks in serum free medium. 4 hr post transfection, serum free medium was replaced with full media with 10% fetal bovine serum and 50 μM ganciclovir. Cells were incubated in the presence of ganciclovir for 7 days before they were re-seeded at a seeding density of 500 cells/well in the 6-well plates. Cells were incubated at 37 °C for two weeks to form colonies. The colonies were fixed with 0.4% (W/V) crystal violet, 70% (V/V) methanol and counted. The data is reported as mean±s.d., n=3.
Animal Studies
Female athymic nu/nu mice (NCI-Frederick, MD) were housed in a pathogen free environment, treated humanely and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Washington State University. 5×106 SK-OV-3 cells in 50 μl PBS were injected subcutaneously into the right and left hind legs of 4–6 week-old mice. When the tumor size reached ca. 100 mm3, mice were anaesthetized with isoflurane and GHT/pSR39 complexes (equivalent of 2 μg of pDNA encoding thymidine kinase) were injected intratumorally followed by the intraperitoneal injection of GCV (20 mg/kg/D) (n=5). Tumor sizes were measured using a digital caliper every 5 days. Table 1 shows the details of each treatment protocol.
Table 1.
Details of the different treatment protocols designed for the evaluation of the therapeutic efficacy of the GHT/pSR39 in xenograft tumors of SKOV-3.
| Protocol | Treatment method | Abbreviated as |
|---|---|---|
| 1 | PBS intratumoral injection on day 1 (control) | PBS |
| 2 | GCV intraperitoneal injection on day 2 and continued for 7 dyas (control) | GCV |
| 3 | pSR39 intratumoral injection on day 1, plus administration of GCV starting on day 2 and continued for 7 days (control) | pSR39 (2–7) |
| 4 | GHT/pSR39 intratumoral injection on day 1 (control) | GHT/pSR39 |
| 5 | 1 dose of GHT/pSR39 (2ug pDNA) on day 1; plus administration of GCV starting on day 2 and continued for 7 days | 1 × GHT/pSR39 + GCV (2–7) |
| 6 | 2 doses of GHT/pSR39 (4ug pDNA) on days 1 and 3; plus administration of GCV starting on day 2 and continued for 7 days | 2 × GHT/pSR39 + GCV (2–7) |
| 7 | 3 doses of GHT/pSR39 (6ug pDNA) on days 1, 3 and 5; plus administration of GCV starting on day 2 and continued for 7 days | 3 × GHT/pSR39 + GCV (2–7) |
| 8 | 3 doses of GHT/pSR39 (6ug pDNA) on days 1, 3 and 5; plus administration of GCV starting on day 5 and continued for 7 days | 3 × GHT/pSR39 + GCV (5–7) |
| 9 | 3 doses of GHT/pSR39 (6ug pDNA) on days 1, 3, and 5; plus administration of GCV starting on day 2 and continued for 21 days | 3 × GHT/pSR39 + GCV (2–21) |
RESULTS
Determination of vector related toxicity
The details of the methods used to genetically engineer the GHT vector in E.coli, purify to >96% purity and form nanosize particles with pEGFP have been previously reported by our group [8]. Nanoparticles were prepared by adding various concentrations of the vector to pEGFP and used to transfect SKOV-3 cancer cells. The results demonstrated that the vector by itself has no detectable acute toxicity and can efficiently transfect SKOV-3 cancer cells over a wide range of N:P ratios (Figure 2). To be consistent with our previous studies and because we did not observe any significant vector related toxicity at the range tested, we chose nanoparticles formed at N:P 10 for subsequent studies. As positive controls, lipofectamine and PEI 25 kDa were used to deliver 1 μg of pEGFP in order to validate the transfection and toxicity protocols. The maximum transfection efficiency for lipofectamine was observed at 2:1 v/w ratio (lipofectamine/pEGFP) (Supplementary Figure 1a). At this 2:1 ratio, approximately 70% of the transfected cells had lost their viability. Similarly, PEI had maximum efficiency/toxicity ratio at 2:1 w/w ratio (PEI/pEGFP) (Supplementary Figure 1b).
Figure 2.
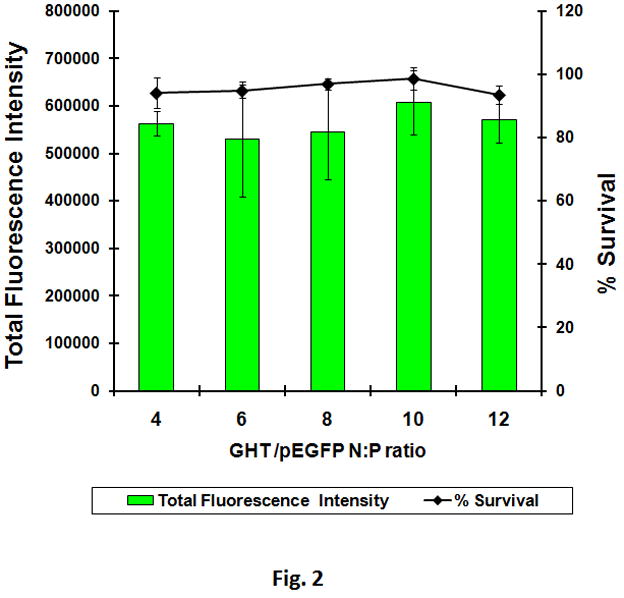
The transfection efficiency and toxicity of the GHT vector in SKOV-3 cancer cells. Various amounts of vector were used to complex with pEGFP to form nanoparticles. At N:P 10, twenty micrograms of the vector was used to complex with 1 μg of pEGFP to form nanoparticles. Nanoparticles were used to transfect SKOV-3 cells. While green fluorescent protein was measured by flowcytometry, the cytotoxicity was measured using WST-1 cell toxicity assay.
Condensation of pSR39 into nanosize particles and characterization
The ability of the GHT vector to efficiently condense pSR39 into nanosize particles suitable for cellular uptake was measured. It was observed that the vector can condense pSR39 into nanoparticles of less than 100nm in size over a wide range of N:P ratios. For example, at N:P 10 the size of the nanoparticles was measured to be 60±2 nm (Figure 3). The surface charge of the nanoparticles was also characterized because highly positively charged particles (> 40 mV) could cause cell toxicity. At N:P 10, the nanoparticles were slightly positively charged with surface charge of 20±1 mV.
Figure 3.
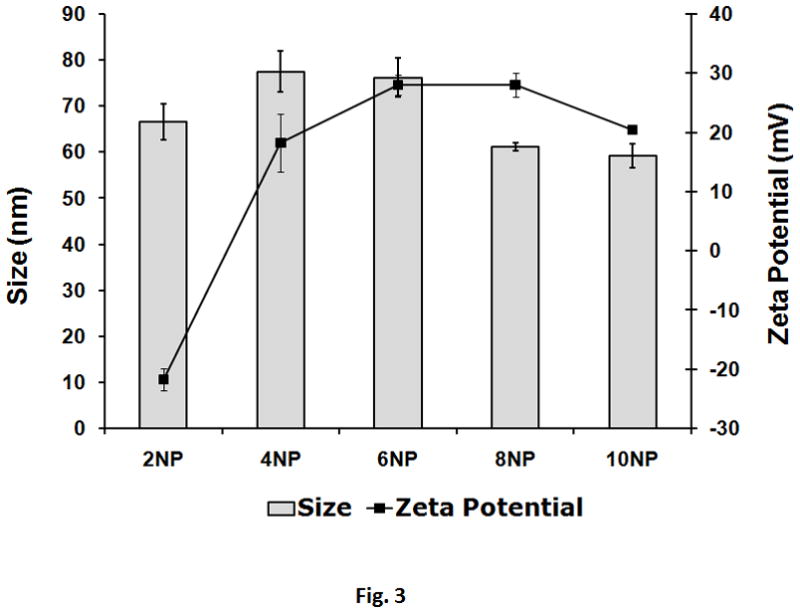
Particle size and charge of analysis of the nanoparticles formed with GHT and 1 μg of pSR39. Various amounts of vector were used to complex with pSR39 to form complexes. The size and charge of the nanoparticles were measured by a zetasizer. The data are reported as mean±SEM (n=3).
In vitro cancer cell killing efficiency
The ability of the GHT vector to efficiently deliver pSR39 to SKOV-3 cancer cells and induce cell death was evaluated. A WST-1 cell toxicity assay was performed and the results of this assay illustrated that the GHT/pSR39 complexes formed at N:P ratio of 10 can kill up to 80% of the SKOV-3 cells when used in combination with 50 μM GCV (Figure 4a). None of the other control groups showed any significant signs of cell toxicity (p>0.05). A western blot analysis was also performed to detect and verify expression of the TK in transfected SKOV-3 cells. The results showed significant expression of the TK in transfected cells in comparison to untransfected ones (control) (Figure 4b).
Figure 4.
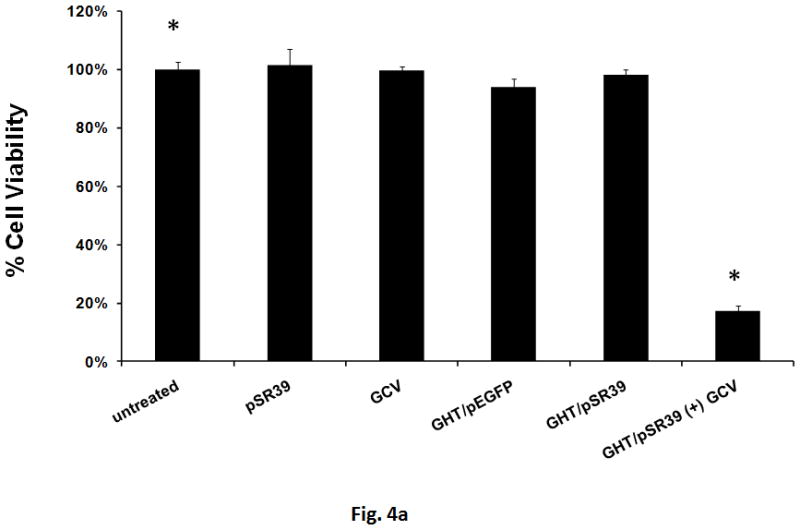
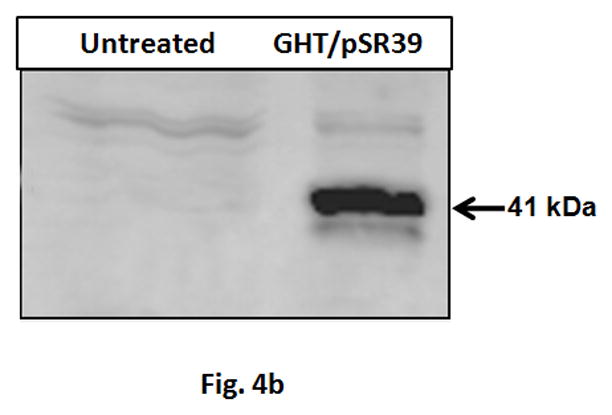
a) Measurement of cell toxicity to SKOV-3 cells transfected with GHT in complex with 1 μg of pSR39. The control groups are: pSR39 (1 μg), GCV (50 μM), GHT/pEGFP nanoparticles (1 μg pEGFP), and GHT/pSR39 nanoparticles without GCV. The data is normalized against the untreated SKOV-3 cells which were considered as 100% viable. The data is shown as mean±SD (n=3) (*t-test, p<0.05). b) Westernblot analysis of the SKOV-3 cells transfected with 1ug GHT/pSR39. Primary polyclonal antibody against thymidine kinase was used for the detection of the protein expression.
Clonogenic assay
To examine the chronic toxicity of the GHT/pSR39 to SKOV-3 cells, a clonogenic assay was performed. A clonogenic assay or colony formation assay is an in vitro cell survival assay that evaluates the ability of a single cancer cell to grow into a colony. The results of this study revealed that ca. 90% of the transfected cells were not viable and unable to re-grow giving rise to the formation of new cancer cell colonies (Figures 5a and b).
Figure 5.
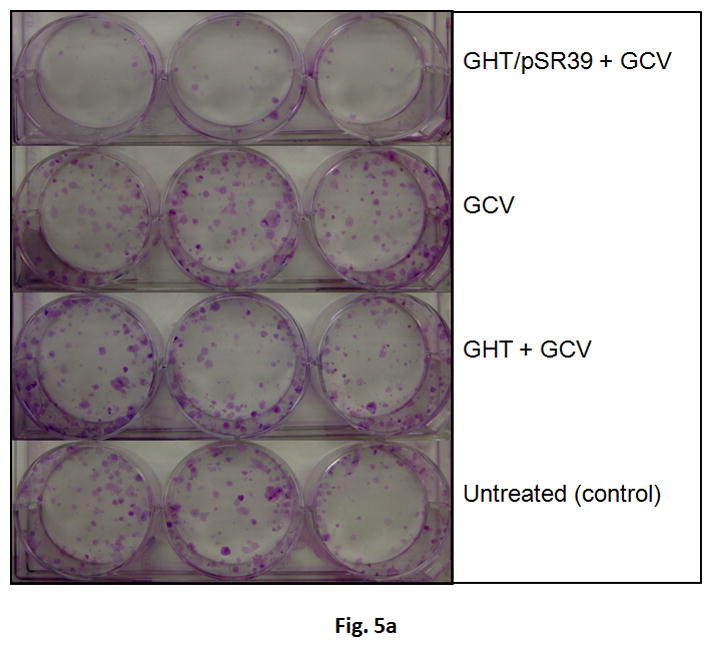
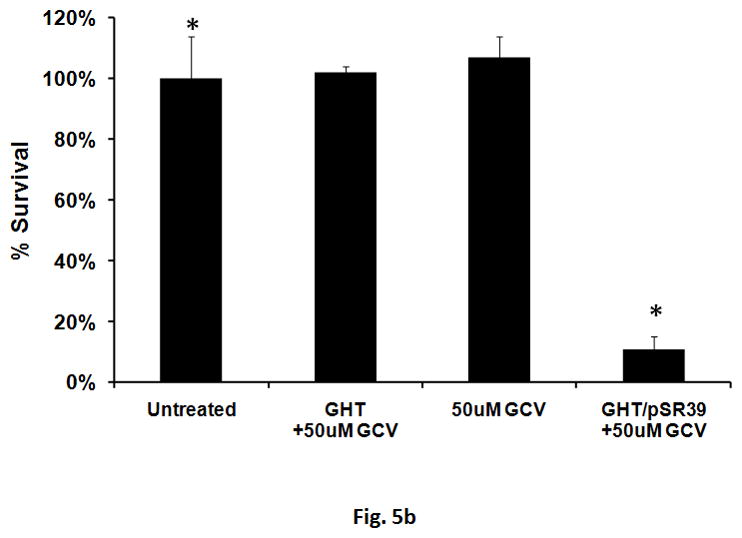
Clonogenic assay for the SKOV-3 cells transfected with GHT in complex with 10 μg of pSR39. a) The pictures of the six-well plates (3 replicates) with produced SKOV-3 colonies. b) Quantification of the percent survival of the SKOV-3 cells as represented by a bar chart. The data is shown as mean±SD (n=3) (*t-test, p<0.05).
Evaluation of the in vivo therapeutic efficacy
The results of the in vitro characterization studies justified further investigation into potential application of the GHT vector in delivering pSR39 to SKOV-3 xenograft tumors in nude mice. Nine different treatment protocols were designed out of which four were controls (Table 1). The GHT/pSR39 nanoparticles (1 dose, equivalent of 2 μg pSR39) were delivered intratumorally to transfect SKOV-3 tumors followed by GCV administration. We did not observe any significant tumor size reduction among tumors that were treated with control groups (protocols 1 to 4) (*ANOVA, p<0.05). However, when we compared the size of the tumors that were treated with protocol 5 (1× GHT/pSR39 + GCV (2–7)) with tumors in the other four control groups, we found significant tumor size reduction at all time points (*ANOVA followed by Tukey Post Hoc test, p<0.05). The tumor size reduction was especially conspicuous during the first seven days when GCV was administered (Figure 6a). Once the administration of the GCV was stopped, the tumor size gradually started to increase. Concurrently, a second set of animal studies was performed using various doses of pSR39 followed by GCV administration as delineated in Table 1 (Protocols 5 to 9). The results of this study showed that there is no significant difference between the size of the tumors treated with 1, 2 and 3 doses of GHT/pSR39 when GCV is delivered for a short period of time (i.e., 7 days) (Figure 6b). Among the protocols tested, the most effective one appeared to be when 3 doses of GHT/pSR39 (equivalent of 6 μg pSR39) were delivered to transfect tumors followed by administration of GCV starting on day 2 and continuing for 21 days (protocol 9) (*ANOVA, followed by Tukey Post Hoc test, p<0.05). The expression of TK at the end point in tumors that were transfected with 3 doses of GHT/pSR39 followed by GCV administration for 21 days (protocol 9) was determined by a westernblot analysis. The results confirmed the low levels of expression of TK in mice tumors after 21 days (Figure 6c).
Figure 6.
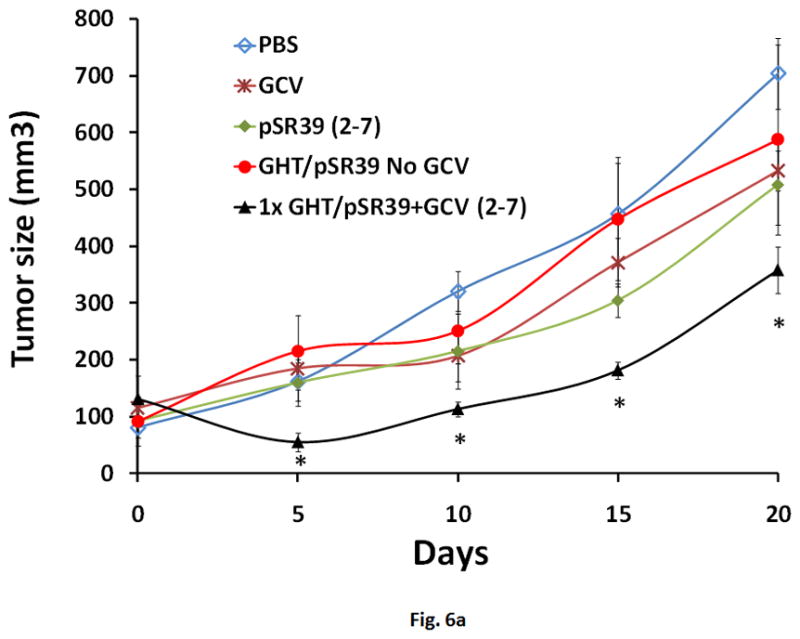
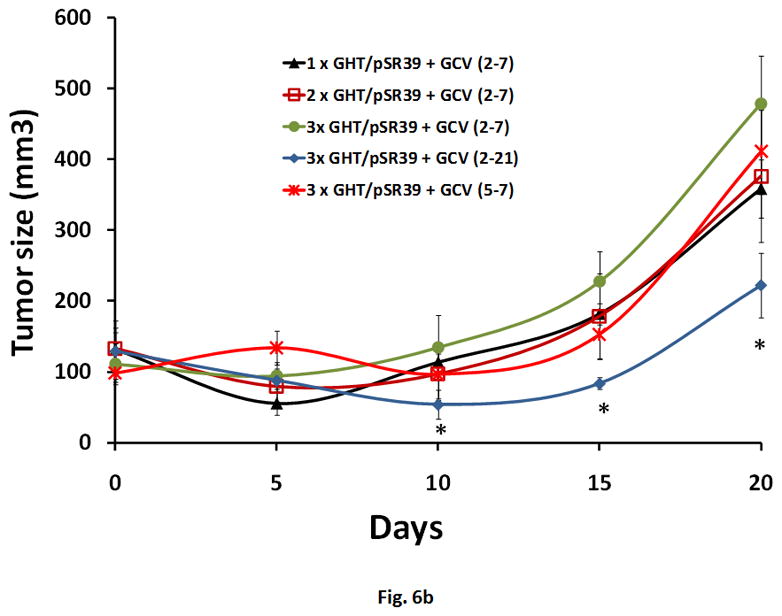
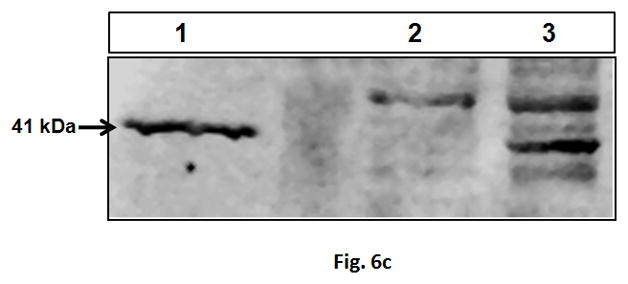
a) Evaluation of the therapeutic efficacy of the GHT/pSR39 nanoparticles in xenograft tumors of SKOV-3 in nude mice. PBS is phosphate buffer saline injected intratumorally on day 1 (control).GCV refers to intraperitoneal injection of ganciclovir on day 1 and continued for seven days (control). pSR39 refers to intratumoral injection of pSR39 alone on day 1, plus administration of GCV starting on day 2 and continued for 7 days (control). GHT/pSR39 No GCV refers to intratumoral injection of nanoparticles on day 1 (control) without administration of GCV. 1× GHT/pSR39 + GCV (2–7) stands for 1 dose of GHT/pSR39 (2 μg pDNA) on day 1; plus administration of GCV starting on day 2 and continued for 7 days. b) Evaluation of the therapeutic efficacy of various treatment protocols after administration of GHT/pSR39 nanoparticles in xenograft tumors of SKOV-3 in nude mice. 1× GHT/pSR39 + GCV (2–7) stands for 1 dose of GHT/pSR39 (2 μg pDNA) on day 1; plus administration of GCV starting on day 2 and continued for 7 days. 2 ×GHT/pSR39 + GCV (2–7) stands for 2 doses of GHT/pSR39 (4 μg pDNA) on days 1 and 3; plus administration of GCV starting on day 2 and continued for 7 days. 3 × GHT/pSR39 + GCV (2–7) stands for 3 doses of GHT/pSR39 (6 μg pDNA) on days 1, 3 and 5; plus administration of GCV starting on day 2 and continued for 7 days. 3 × GHT/pSR39 + GCV (5–7) stands for 3 doses of GHT/pSR39 (6 μg pDNA) on days 1, 3 and 5; plus administration of GCV starting on day 5 and continued for 7 days. 3 × GHT/pSR39 + GCV (2–7) stands for 3 doses of GHT/pSR39 (6 μg pDNA) on days 1, 3 and 5; plus administration of GCV starting on day 2 and continued for 21 days. Statistical significance is shown based on comparison of data points between protocol 5 and 9 (*t-test, p<0.05). c) Detection of TK expression in transfected tumors by westernblot analysis. Lane 1: expressed TK in SKOV-3 cells transfected in vitro with pSR39 (positive control); Lane 2: lysate from tumors treated with PBS (negative control); and Lane 3: lysate from tumors treated with protocol 9.
DISCUSSION
The model therapeutic agent that we have selected for drug delivery is a mutant of HSV1-TK, pSR39, in combination with GCV. The applicability of the HSV-TK/GCV approach in cancer therapy was first demonstrated in 1992 by Culver et al. using a brain tumor model in rats [20]. Based on encouraging preclinical data with this system, clinical trials were designed to treat brain tumors by using retroviral HSV-TK producer cells. The results of the clinical studies showed that this approach was safe, but had limited efficacy due to the limitations of retroviral vectors in in vivo settings [21]. In another approach, gene transfer experiments using HSV-TK encoded by adenoviral vectors (Ad.HSV-TK) were examined. In phase I clinical trials, Ad.HSV-TK showed significant transduction of cancer cells and expression of the gene, but still were limited by their clinical responses [22]. There are a number of approaches that can be undertaken to improve the efficiency of transgene delivery and clinical efficacy. These include: a) improving vector efficiency, b) enhance the vector’s cell targeting capability, and c) development of transgenes with enhanced tumor killing characteristics. While others have worked to improve the tumor killing efficiency of the therapeutic genes [23], our lab has focused on the development of novel targeted vectors with enhanced efficiency. To date, several groups have demonstrated the ability of the recombinant non-viral vectors to transfect cells with reporter genes, such as GFP or luciferase in vitro [9]. However, the ability of such vectors to efficiently transfect cancer cells in vivo and result in significant tumor size reduction has not been demonstrated. This is majorly attributed to the inability of such vectors to form stable nanoparticles under complex physiological conditions. Here we present data that demonstrate the potential application of GHT in cancer suicide gene therapy.
As a first step towards achieving our objective, a formulation study was performed in order to identify the N:P ratio at which the vector has the maximum transfection efficiency, while maintaining low toxicity. At N:P 10, where efficiency appears on average to be the highest, approximately 14 μg of vector is used complex with and deliver 1 μg of pEGFP (Figure 2). At this N:P ratio, we did not observe any significant vector related toxicity to SKOV-3 cells. Given that at its maximum efficiency the GHT vector by itself did not show toxicity to SKOV-3 cells, its ability to deliver pSR39 and induce cell death was evaluated. Various amounts of the GHT were used to complex with pSR39 in order to prepare nanoparticles of less than 150 nm size (Figure 3). This size limit is critical as particles with sizes bigger than 150 nm may not fit into clathrin-coated vesicles which is essential for receptor mediated endocytosis [24]. Then, GHT/pSR39 nanoparticles were used to transfect SKOV-3 cancer cells in order to evaluate their ability to induce cell death. A cell toxicity assay was performed to evaluate cancer cell killing efficiency along with a westernblot analysis to confirm TK expression. The combination of the results shown in Figures 4a and b indicated that the vector could significantly transfect SKOV-3 cells, express significant amounts of TK, and result in cell death only in the presence of GCV. Beside acute toxicity which results in sudden cell death, evaluation of the chronic toxicity is also of paramount importance. Therefore, we performed a clonogenic assay to examine the potential of transfected SKOV-3 cells to re-grow and form colonies. The results of the clonogenic assay also demonstrated that the majority of the transfected cells that did survive were unable to re-grow and form new colonies (Figures 5a and b). These results encouraged us to examine the ability of the vector to transfect cancer cells in the complex tumor environment. Various treatment protocols were designed to obtain a better understanding of the most appropriate approach toward pSR39 delivery and reducing tumor size. The results of the in vivo studies showed that the vector is efficacious and could potentially be used for the treatment of metastatic HER2 positive cancer cells (Figures 6a, b). Comparison of the therapeutic outcome of protocol 4 with 5 (Figure 6a) indicates that administration of GCV is necessary for tumor size reduction and the host immune system response against the vector could not have been a contributing factor to tumor size reduction. This was expected as nude mouse does not have a robust immune system. Another observation that is worth discussing is that the TK expression was detectable in tumors even after 21 days (Figure 6c). This was somewhat expected as the immune system of nude mouse is significantly compromised which restricts its ability to potentially eliminate the transfected TK-expressing cells. In addition, some of the transfected cells may have been located at the inner layers of the tumors where GCV could not have reached in sufficient amounts to induce cell death or perhaps they were expressing TK below therapeutic levels. The efficacy of the developed GHT vector in terms of controlling tumor growth is similar to what has been reported in literature for adenoviral vectors delivering SR39 gene (i.e., Ad.SR39) [25]. Further in vivo studies in humans (Phase IIA) with various doses of GCV at different time points, along with higher doses of GHT/pSR39 are the subject of our future studies. At this stage, we have delivered nanoparticles intratumorally, which could be applicable to only those tumors that are close to the body surface or endoscopically accessible. The results of this study demonstrate that designed recombinant non-viral vectors can deliver suicide genes efficiently to cancer cells in vitro and in vivo with significant therapeutic outcome. Therefore, it can be concluded that the GHT vector has the potential to be used as an effective tool for cancer suicide gene therapy.
Supplementary Material
a) The transfection efficiency and toxicity of the lipofectamine in SKOV-3 cancer cells. At volume/weight ratio of 2, 2 μl of lipofectamine was used to complex with 1 μg of pEGFP. b) The transfection efficiency and toxicity of the PEI in SKOV-3 cancer cells. At weight/weight ratio of 2, two micrograms of PEI was used to complex with 1 μg of pEGFP.
Acknowledgments
Research Support: This work was supported by the American Cancer Society Institutional 8Research Grant (ACS-IRG-77-003-26) and startup funds from the Washington State University 9to Hatefi. This work was also supported in part by the NIH biotechnology training fellowship (T32-GM008336) to B.F. Canine.
Footnotes
Declaration of Interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goparaju GN, Satishchandran C, Gupta PK. The effect of the structure of small cationic peptides on the characteristics of peptide-DNA complexes. Int J Pharm. 2009 Mar 18;369(1–2):162–9. doi: 10.1016/j.ijpharm.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Keller M, Harbottle RP, Perouzel E, Colin M, Shah I, Rahim A, et al. Nuclear localisation sequence templated nonviral gene delivery vectors: investigation of intracellular trafficking events of LMD and LD vector systems. Chembiochem. 2003 Apr 4;4(4):286–98. doi: 10.1002/cbic.200390049. [DOI] [PubMed] [Google Scholar]
- 3.Ludtke JJ, Zhang G, Sebestyen MG, Wolff JA. A nuclear localization signal can enhance both the nuclear transport and expression of 1 kb DNA. J Cell Sci. 1999 Jun;112( Pt 12):2033–41. doi: 10.1242/jcs.112.12.2033. [DOI] [PubMed] [Google Scholar]
- 4.Mangipudi SS, Canine BF, Wang Y, Hatefi A. Development of a genetically engineered biomimetic vector for targeted gene transfer to breast cancer cells. Mol Pharm. 2009 Jul-Aug;6(4):1100–9. doi: 10.1021/mp800251x. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopalan R, Xavier J, Rangaraj N, Rao NM, Gopal V. Recombinant fusion proteins TAT-Mu, Mu and Mu-Mu mediate efficient non-viral gene delivery. J Gene Med. 2007 Apr;9(4):275–86. doi: 10.1002/jgm.1014. [DOI] [PubMed] [Google Scholar]
- 6.Xavier J, Singh S, Dean DA, Rao NM, Gopal V. Designed multi-domain protein as a carrier of nucleic acids into cells. J Control Release. 2009 Jan 19;133(2):154–60. doi: 10.1016/j.jconrel.2008.09.090. [DOI] [PubMed] [Google Scholar]
- 7.Canine BF, Wang Y, Hatefi A. Biosynthesis and characterization of a novel genetically engineered polymer for targeted gene transfer to cancer cells. J Control Release. 2009 Sep 15;138(3):188–96. doi: 10.1016/j.jconrel.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Mangipudi SS, Canine BF, Hatefi A. A designer biomimetic vector with achimeric architecture for targeted gene transfer. J Control Release. 2009 Mar 18;137:46–53. doi: 10.1016/j.jconrel.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy HO, Wang Y, Mangipudi SS, Hatefi A. Advances with the use of bio-inspired vectors towards creation of artificial viruses. Expert Opin Drug Deliv. 2010 Feb 13;7(4):1–16. doi: 10.1517/17425240903579989. [DOI] [PubMed] [Google Scholar]
- 10.Mahan SD, Ireton GC, Knoeber C, Stoddard BL, Black ME. Random mutagenesis and selection of Escherichia coli cytosine deaminase for cancer gene therapy. Protein Eng Des Sel. 2004 Aug;17(8):625–33. doi: 10.1093/protein/gzh074. [DOI] [PubMed] [Google Scholar]
- 11.Chu QD, Sun L, Li J, Byrnes K, Chervenak D, DeBenedetti A, et al. Rat adenocarcinoma cell line infected with an adenovirus carrying a novel herpes-simplex virus-thymidine kinase suicide gene construct dies by apoptosis upon treatment with ganciclovir. J Surg Res. 2007 Nov;143(1):189–94. doi: 10.1016/j.jss.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Fischer U, Steffens S, Frank S, Rainov NG, Schulze-Osthoff K, Kramm CM. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005 Feb 10;24(7):1231–43. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- 13.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001 Apr 15;61(8):3330–8. [PubMed] [Google Scholar]
- 14.Norris JS, Hyer ML, Voelkel-Johnson C, Lowe SL, Rubinchik S, Dong JY. The use of Fas Ligand, TRAIL and Bax in gene therapy of prostate cancer. Curr Gene Ther. 2001 May;1(1):123–36. doi: 10.2174/1566523013348977. [DOI] [PubMed] [Google Scholar]
- 15.Qiao J, Black ME, Caruso M. Enhanced ganciclovir killing and bystander effect of human tumor cells transduced with a retroviral vector carrying a herpes simplex virus thymidine kinase gene mutant. Hum Gene Ther. 2000 Jul 20;11(11):1569–76. doi: 10.1089/10430340050083298. [DOI] [PubMed] [Google Scholar]
- 16.Freytag SO, Stricker H, Movsas B, Kim JH. Prostate cancer gene therapy clinical trials. Mol Ther. 2007 Jun;15(6):1042–52. doi: 10.1038/sj.mt.6300162. [DOI] [PubMed] [Google Scholar]
- 17.Black ME, Kokoris MS, Sabo P. Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve prodrug-mediated tumor cell killing. Cancer Res. 2001 Apr 1;61(7):3022–6. [PubMed] [Google Scholar]
- 18.Kokoris MS, Black ME. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. 2002 Sep;11(9):2267–72. doi: 10.1110/ps.2460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 20.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–2. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 21.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000 Nov 20;11(17):2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 22.Sterman DH, Treat J, Litzky LA, Amin KM, Coonrod L, Molnar-Kimber K, et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998 May 1;9(7):1083–92. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 23.Fuchita M, Ardiani A, Zhao L, Serve K, Stoddard BL, Black ME. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer Res. 2009 Jun 1;69(11):4791–9. doi: 10.1158/0008-5472.CAN-09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004 Jan 1;377(Pt 1):159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiewrodt R, Amin K, Kiefer M, Jovanovic VP, Kapoor V, Force S, et al. Adenovirus-mediated gene transfer of enhanced Herpes simplex virus thymidine kinase mutants improves prodrug-mediated tumor cell killing. Cancer Gene Ther. 2003 May;10(5):353–64. doi: 10.1038/sj.cgt.7700589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) The transfection efficiency and toxicity of the lipofectamine in SKOV-3 cancer cells. At volume/weight ratio of 2, 2 μl of lipofectamine was used to complex with 1 μg of pEGFP. b) The transfection efficiency and toxicity of the PEI in SKOV-3 cancer cells. At weight/weight ratio of 2, two micrograms of PEI was used to complex with 1 μg of pEGFP.


