Abstract
Pancreaticobiliary maljunction (PBM) is a congenital anomaly defined as a junction of the pancreatic and bile ducts located outside the duodenal wall, usually forming a markedly long common channel. As the action of the sphincter of Oddi does not functionally affect the junction in PBM patients, continuous pancreatobiliary reflux occurs, resulting in a high incidence of biliary cancer. PBM can be divided into PBM with biliary dilatation (congenital choledochal cyst) and PBM without biliary dilatation (maximal diameter of the bile duct ≤ 10 mm). The treatment of choice for PBM is prophylactic surgery before malignant changes can take place. Endoscopic retrograde cholangiopancreatography (ERCP) is the most effective examination method for close observation of the pattern of the junction site. When the communication between the pancreatic and bile ducts is maintained, despite contraction of the sphincter on ERCP, PBM is diagnosed. In these patients, levels of pancreatic enzymes in the bile are generally elevated, due to continuous pancreatobiliary reflux via a long common channel. Magnetic resonance cholangiopancreatography and 3D-computed tomography can diagnose PBM, based on findings of an anomalous union between the common bile duct and the pancreatic duct, in addition to a long common channel. Endoscopic ultrasonography and intraductal ultrasonography can demonstrate the junction outside the duodenal wall, and are useful for the diagnosis of associated biliary cancer. Gallbladder wall thickness on ultrasonography can be a screening test for PBM.
Keywords: Pancreaticobiliary maljunction, Pancreatobiliary reflux, Congenital choledochal cyst, Endoscopic retrograde cholangiopancreatography, Endoscopic ultrasonography, Magnetic resonance cholangiopancreatography
INTRODUCTION
The main pancreatic duct and the common bile duct open into the duodenum, where they frequently form a common channel, the incidence of which is reported to be from 55%[1] to 82%[2]. The length of the common channel ranges from 1 mm to 12 mm, with an average length of 4.4 mm[3]. The sphincter of Oddi is located at the distal end of the pancreatic and bile ducts; it regulates the outflow of bile and pancreatic juice. A common channel can be so long that the sphincter action does not functionally affect the junction, resulting in two-way regurgitation (pancreatobiliary reflux: regurgitation of pancreatic juice into the common bile duct, and biliopancreatic reflux: regurgitation of bile juice into the pancreatic duct). Pancreaticobiliary maljunction (PBM) forms a markedly long common channel, and is divided into PBM with, and without, biliary dilatation[4-7]. Pancreatobiliary reflux has been shown to induce carcinogenesis in the biliary tract; similarly, biliopancreatic reflux can induce pancreatitis[4-6].
PANCREATICOBILIARY MALJUNCTION
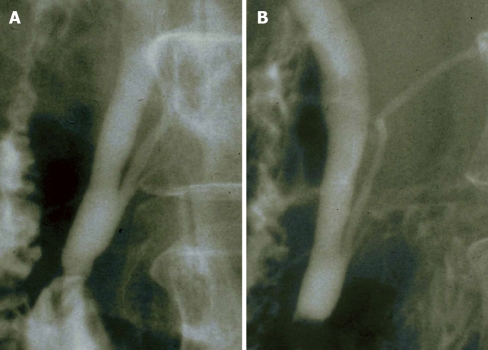
PBM is a congenital anomaly defined as a junction of the pancreatic and bile ducts located outside the duodenal wall, usually forming a markedly long common channel. PBM occurs predominantly in females, and is often found in Asian populations. PBM can be divided into PBM with biliary dilatation (congenital choledochal cyst (CCC) (Figure 1) and PBM without biliary dilatation (Figure 2)[4-7]. CCC is an anomaly in which the extrapancreatic bile duct, or the extra and intrahepatic bile ducts, are dilated in various ways. The Alonso-Lej classification[8] , which is based on the shape of the dilated bile duct, is notable, and includes a cystic type (Type I), a diverticular type (Type II), and a cyst in the duodenum (choledochal cyst, Type III). Type I is almost always associated with PBM, but Type II and III rarely have PBM. Bile duct diameter greater than 10 mm on a cholangiogram is the most commonly used definition of dilatation[7].
Figure 1.
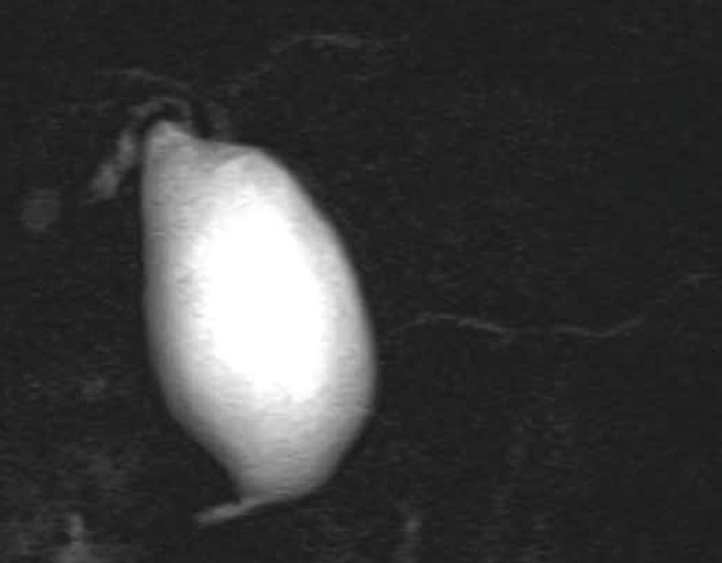
Magnetic resonance cholangiopancreatography showing pancreaticobiliary maljunction with biliary dilatation (congenital choledochal cyst).
Figure 2.
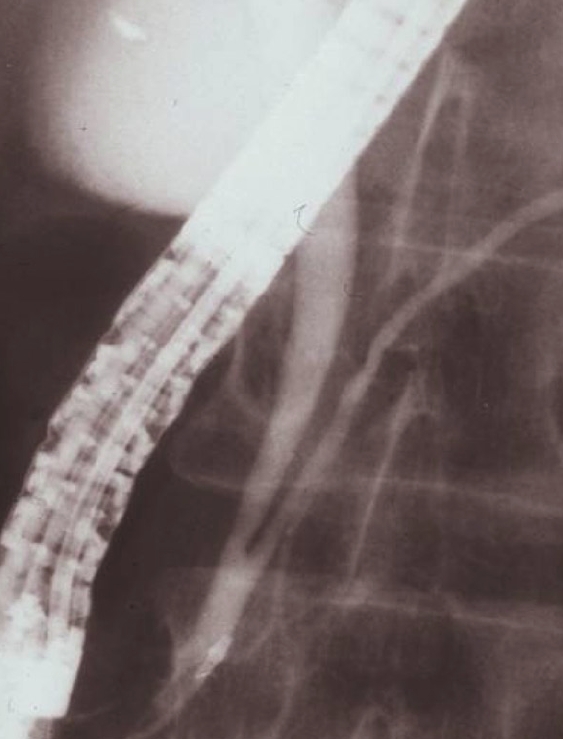
Endoscopic retrograde cholangiopancreatographyshowing pancreaticobiliary maljunction without biliary dilatation.
In PBM patients, since the action of the sphincter of Oddi does not functionally affect the junction, continuous reciprocal reflux between pancreatic juice and bile occurs, resulting in various pathological conditions in the biliary tract and pancreas. As the hydro pressure within the pancreatic duct is usually greater than that in the bile duct, pancreatic juice frequently refluxes into the biliary duct in PBM patients, which results in a high incidence of cancer in the biliary tract[4-7]. In our previous study, bile duct and gallbladder cancers were seen in 14% and 22% of 49 CCC patients, respectively, but, in 70% of 53 PBM patients without biliary dilatation, only gallbladder cancer was detected.
Once PBM is diagnosed, prophylactic flow-diversion surgery (bile duct resection and bilioenteric anastomosis) is performed for CCC. On the other hand, any treatment of PBM without biliary dilatation and without cancer is controversial. Prophylactic cholecystectomy is performed in many institutes, as most biliary cancers that develop in PBM patients without biliary dilatation are gallbladder cancers. However, some surgeons propose excision of the extrahepatic bile duct, together with the gallbladder, for PBM patients without biliary dilatation, because of the risk of bile duct cancer[9,10].
ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY
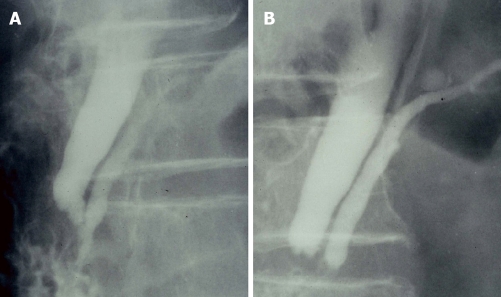
Endoscopic retrograde cholangiopancreatography (ERCP) is the most effective examination method for close observation of the pattern of the junction site. When the communication between the pancreatic and bile ducts is maintained despite contraction of the sphincter on ERCP, PBM is diagnosed (Figure 3A and B). The finding can also be assessed with cholangiography, via the biliary drainage tube or during operation[4-7].
Figure 3.
Endoscopic retrograde cholangiopancreatography of a pancreaticobiliary maljunction patient. A: Showing a long common channel; B: The communication between pancreatic and bile ducts was maintained despite contraction of the sphincter.
Pancreatography via the minor duodenal papilla can also demonstrate pancreatobiliary reflux in PBM patients. When injected endoscopically via the minor duodenal papilla, the contrast medium is refluxed into the bile duct through a long common channel without outflow into the duodenum[5].
Biliary levels of pancreatic enzymes, especially amylase, are generally elevated due to continuous pancreatobiliary reflux via a long common channel in PBM patients. There are some cases with a relatively long common channel that are not classified as PBM because the sphincter of Oddi includes the pancreaticobiliary ductal junction. We defined a high confluence of pancreaticobiliary ducts (HCPBD) as a common channel length ≥ 6 mm, in which the communication was occluded when the sphincter was contracted (Figure 4A and B), and investigated the clinical significance of a relatively long common channel[5-7]. In HCPBD patients, the amylase level in the bile was frequently elevated, and hyperplastic change of the gallbladder epithelium was frequently observed. Gallbladder cancer occurred in 11% of the 84 HCPBD patients. These findings suggest that pathophysiological changes similar to PBM occur in HCPBD. However, in HCPBD patients, there was no difference between the sexes, whereas in PBM there was (male:female ratio, 1 : 0.7 in HCPBD and 1 : 3.4 in PBM), and the average age at the time of diagnosis was significantly older in HCPBD patients than in PBM patients without biliary dilatation (average age, 62.0 years vs 56.7 years, respectively). The elevated amylase level in the bile in HCPBD patients was lower than that of PBM patients (average 48 665 IU/L vs 250 025 IU/L, respectively) and the rate of associated gallbladder cancer was lower in HCPBD patients than in PBM patients. These differences in sex, age at diagnosis, bile amylase level, and rate of associated gallbladder cancer between HCPBD and PBM patients appear to be related to whether pancreatobiliary reflux occurs consistently or intermittently[5-7].
Figure 4.
Endoscopic retrograde cholangiopancreatography of a patient with high confluence of pancreaticobiliary ducts. A: A common channel of 9 mm in length; B: The communication between pancreatic and bile ducts was destroyed by sphincter contraction.
MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY AND 3D-COMPUTED TOMOGRAPHY
Magnetic resonance cholangiopancreatography (MRCP) has become a common non-invasive method for obtaining high quality images of the pancreaticobiliary tree.
Reconstruction images on 3D-computed tomography (CT) can also show pancreaticobiliary images. MRCP and 3D-CT can diagnose PBM, based on findings of an anomalous union between the common bile duct and the pancreatic duct, in addition to a long common channel. However, in some cases in which a common channel is not so long and cannot be depicted on MRCP, the MRCP diagnosis of PBM is not possible[11]. For a definite diagnosis of PBM, ERCP is necessary in order to exclude various false positive or negative results on MRCP or 3D-CT. Diagnostic accuracy can be increased with dynamic MRCP with secretin stimulation or three-dimensional MRCP. Although a large amount of contrast must be injected to evaluate the whole image of CCC on ERCP, it can be achieved with MRCP and 3D-CT.
Pancreaticobiliary reflux in PBM patients can be visualized radiologically using secretin-stimulated dynamic MRCP. In normal pancreaticobiliary dynamics, the extrahepatic and intrahepatic bile ducts show no change following secretin injection. On the other hand, in PBM patients, the volume of the extrahepatic bile duct and the gallbladder increases, due to the regurgitation of pancreatic fluid secreted after the injection of secretin into the bile duct[12].
ENDOSCOPIC ULTRASONOGRAPHY
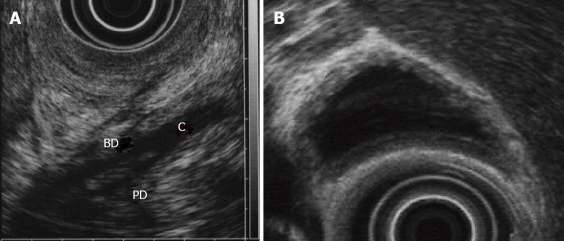
In PBM, endoscopic ultrasonography (EUS) can detect the confluence of pancreatic duct and bile duct in the proximal portion of the duodenal wall, the so-called common channel (Figure 5A). Therefore, EUS allows definite diagnosis of PBM. When PBM is known about in advance, it may be relatively easy to depict the confluence. PBM often shows a thickness of the inner low echoic layer of the gallbladder (Figure 5B), which means histologically mucosal hyperplasia. Therefore, when we find that sonographic finding by means of EUS, we have to keep the presence of PBM in mind. In fact, several investigators have reported that EUS could confirm the PBM in 4 (2.9%) of 137 patients who underwent screening US[13].
Figure 5.
Endoscopic ultrasonography of a pancreaticobiliary maljunction patient. A:The confluence of pancreatic duct and bile duct in the proximal portion of the duodenal wall; B: thickness of inner low echoic layer of the gallbladder. BD: bile duct; PD: pancreatic duct; C: common channel.
Bile duct and gallbladder cancers are often seen in PBM. The efficacy of EUS for the diagnosis of gallbladder cancer is well known[14]. However, it is often difficult, even when using EUS, to distinguish mucosal hyperplasia from early gallbladder cancer to indicate whether the depth of invasion of the cancer is mucosa or muscularis propria of the gallbladder.
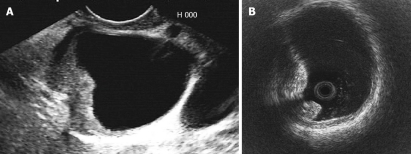
EUS is also useful for the diagnosis of bile duct cancer. Since, in particular, congenital bile duct dilation is well-known as a high risk condition for bile duct cancer in the dilated bile duct (Figure 6A), in these cases, EUS should be preoperatively performed for the diagnosis of tumor spreading and staging.
Figure 6.
Ultrasonography of a congenital choledochal cyst patient with bile duct cancer in the dilated bile duct. A: Endoscopic ultrasonography; B: Intraductal ultrasonography.
INTRADUCTAL ULTRASONOGRAPHY
Intraductal ultrasonography (IDUS) is performed over-the-guidewire during the ERCP, and is useful for the depiction of the confluence of pancreatic duct and bile duct.
IDUS is also useful for the diagnosis of bile duct cancer (Figure 6B)[15,16]. However, IDUS has limitations for the diagnosis of bile duct and gallbladder lesions because of shallow US penetration (< 2.0 cm) and maneuverability of passage of probe in case of bile duct stricture or a narrow cystic duct.
Direct choledochoscopy with or without biopsy is useful to confirm the diagnosis of bile duct cancer.
ULTRASONOGRAPHY
Mark dilatation of the common bile duct on ultrasonography (US) suggests CCC. The epithelium of the gallbladder frequently becomes hyperplastic due to exposure to refluxed pancreatic juice. Gallbladder wall thickness on US during medical checkups may serve as an indication of PBM without the need for biliary dilatation, and can serve as a screening test for PBM[17].
CONCLUSION
When the communication between the pancreatic and bile ducts is maintained despite contraction of the sphincter on ERCP, PBM is diagnosed. MRCP and 3D-CT can be used to diagnose PBM, based on the discovery of an anomalous union between the common bile duct and the pancreatic duct, in addition to a long common channel. EUS and IDUS can demonstrate the junction outside the duodenal wall, and they are useful for the diagnosis of associated biliary cancer. Gallbladder wall thickness on US can be a screening test for PBM.
Footnotes
Peer reviewers: Omar Javed Shah, Professor, Head, Department of Surgical Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India; Sanjiv Mahadeva, MBBS, MRCP, CCST, MD, Associate Professor, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia
S- Editor Zhang HN L- Editor Herholdt A E- Editor Liu N
References
- 1.Sterling JA. The common channel for bile and pancreatic ducts. Surg Gynecol Obstet. 1954;98:420–424. [PubMed] [Google Scholar]
- 2.Suda K, Miyano T, Konuma I, Matsumoto M. An abnormal pancreatico-choledocho-ductal junction in cases of biliary tract carcinoma. Cancer. 1983;52:2086–2088. doi: 10.1002/1097-0142(19831201)52:11<2086::aid-cncr2820521120>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Dowdy GS Jr, Waldron GW, Brown WG. Surgical anatomy of the pancreatobiliary ductal system. Observations. Arch Surg. 1962;84:229–246. doi: 10.1001/archsurg.1962.01300200077006. [DOI] [PubMed] [Google Scholar]
- 4.The Japanese Study Group on Pancreaticobiliary Maljunction. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219–221. [Google Scholar]
- 5.Kamisawa T, Okamoto A. Biliopancreatic and pancreatobiliary refluxes in cases with and without pancreaticobiliary maljunction: diagnosis and clinical implications. Digestion. 2006;73:228–236. doi: 10.1159/000095424. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K. Diagnosis and clinical implications of pancreatobiliary reflux. World J Gastroenterol. 2008;14:6622–6626. doi: 10.3748/wjg.14.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K, Sasaki T. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84–S88. doi: 10.1016/j.cgh.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Lej F, Rever EBJr, Pessagno DJ. Congenital choledochal cyst. Int Abstr Surg. 1959;108:1–30. [PubMed] [Google Scholar]
- 9.Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, Shimada H, Takamatsu H, Miyake H, Todani T. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345–351. doi: 10.1007/s00534-002-0741-7. [DOI] [PubMed] [Google Scholar]
- 10.Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;394:159–169. doi: 10.1007/s00423-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129–33. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 12.Hosoki T, Hasuike Y, Takeda Y, Michita T, Watanabe Y, Sakamori R, Tokuda Y, Yutani K, Sai C, Mitomo M. Visualization of pancreaticobiliary reflux in anomalous pancreaticobiliary junction by secretin-stimulated dynamic magnetic resonance cholangiopancreatography. Acta Radiol. 2004;45:375–382. doi: 10.1080/02841850410005462. [DOI] [PubMed] [Google Scholar]
- 13.Yamao K, Mizutani S, Nakazawa S, Inui K, Kanemaki N, Miyoshi H, Segawa K, Zenda H, Kato T. Prospective study of the detection of anomalous connections of pancreatobiliary ducts during routine medical examinations. Hepatogastroenterology. 1996;43:1238–1245. [PubMed] [Google Scholar]
- 14.Fujita N, Noda Y, Kobayashi G, Kimura K, Yago A. Diagnosis of the depth of invasion of gallbladder carcinoma by EUS. Gastrointest Endosc. 1999;50:659–663. doi: 10.1016/s0016-5107(99)80015-7. [DOI] [PubMed] [Google Scholar]
- 15.Tamada K, Ido K, Ueno N, Kimura K, Ichiyama M, Tomiyama T. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am J Gastroenterol. 1995;90:239–246. [PubMed] [Google Scholar]
- 16.Noda Y, Fujita N, Kobayashi G, Ito K, Horaguchi J, Takazawa O, Obana T, Nakahara K, Ishida K, Suzuki T, et al. Intraductal ultrasonography before biliary drainage and transpapillary biopsy in assessment of the longitudinal extent of bile duct cancer. Dig Endosc. 2008;20:73–78. [Google Scholar]
- 17.Sugai M, Ishido K, Endoh M, Hada R, Munakata H. Sonographic demonstration of wall thickness of the gallbladder in pediatric patients with pancreatico-biliary maljunction. J Hepatobiliary Pancreat Sci. 2010;17:345–348. doi: 10.1007/s00534-009-0252-x. [DOI] [PubMed] [Google Scholar]