Abstract
AIM: To investigate the safety of consecutive mini-laparoscopy guided liver biopsies for the diagnosis and staging of liver diseases.
METHODS: In this study we retrospectively analyzed the safety of mini-laparoscopic liver biopsy performed in an endoscopy unit in 1071 patients. We measured the incidence of bleeding and evaluated the management and outcome of bleeding interventions.
RESULTS: The most common etiologies of liver injury were viral hepatitis and autoimmune liver disease. 250 patients had macroscopically and histologically proven cirrhosis. 13 patients had no pathological findings. 33% of all patients had bleeding that required argon plasma coagulation of the puncture site during laparoscopy. Significant bleeding occurred more often in patients with liver cirrhosis compared to non-cirrhotic liver diseases but was effectively treated with laparoscopic coagulation.
CONCLUSION: In conclusion, mini-laparoscopy liver biopsy can be performed safely and effectively in high risk patients with advanced liver disease; mini-laparoscopy with liver biopsy can be done safely in an endoscopy unit.
Keywords: Mini-laparoscopy, Cirrhosis, Argon plasma coagulation
INTRODUCTION
Although noninvasive techniques for the diagnosis and staging of liver disease have been developed in recent years, histological evaluation still remains the most accurate method for the assessment of severity and stage of liver diseases[1,2]. However, in patients with acute or advanced liver disease, there may be an increased risk of bleeding complications with percutaneous liver biopsy, even when done under ultrasound guidance[3,4]. Furthermore, recent studies have shown that liver histology obtained by blind biopsy underestimates the stage of liver disease and misses the diagnosis of cirrhosis in up to 25% of patients, especially in early and incomplete cirrhosis or macronodular cirrhosis[5]. To overcome the limitations of safety and diagnostic accuracy in percutaneous liver biopsy, laparoscopic liver biopsy has been touted as a safe and effective diagnostic tool in patients with liver disease[6,7]. The development of the so-called mini-laparoscopy by using a small diameter laparoscope allows for a minimally invasive procedure for macroscopic evaluation of the peritoneal cavity and safe biopsy of the liver[8,9]. In this study, we retrospectively analyzed the safety of 1071 consecutive mini-laparoscopy guided liver biopsies, all performed in an endoscopy unit.
MATERIAL AND METHODS
Patients
The medical records of 1071 consecutive patients undergoing diagnostic mini-laparoscopy performed in our endoscopy unit at Johannes Gutenberg University Mainz, Germany from January 2000 until April 2006 were reviewed. All patients gave written informed consent before undergoing mini-laparoscopy. Patients with INR values above 1.5 and platelet counts below 50.000/μL received 2 to 4 units of fresh frozen plasma or 1 to 2 units of platelet concentrate immediately before the intervention respectively. Furthermore, every patient received an abdominal ultrasound prior to mini-laparoscopy to exclude ascites. All patients were treated as day cases with post interventional observation. Since this study represents a retrospective analysis, no IRB approval was required.
Technique of mini-laparoscopy
Mini-laparoscopy was performed using intravenous procedural sedation, as described previously[6]. The abdominal wall was cleaned with Betadine solution and covered with sterile drapes. Puncture site of the abdominal wall was performed at the point of Kalk, located 2 cm left and cephalad of the umbilicus. The puncture itself was performed with a Veress needle with 2.3 mm diameter (Richard Wolf GmbH, Knittlingen, Germany) through a trocar of 2.75 mm diameter (Richard Wolf GmbH, Germany) after local anesthesia of the puncture site with 10 mL of mepivacaine 1% (AstraZeneca, London, UK). After forming a pneumoperitoneum by insufflation of approximately 2 liters nitric oxide, the Veress needle was replaced by the optical instrument (Richard Wolf GmbH, Germany). A xenon light source (Richard Wolf GmbH, Germany) was used for illumination of the abdominal cavity. For optimal visibility of the liver, a rotating operation table (Maquet GmbH, Rastatt, Germany) was used and patients were slightly rotated to the left while the upper body was elevated. Besides macroscopic assessment of the liver, the upper abdomen was systematically examined for signs of portal hypertension such as splenomegaly, dilated intra abdominal vessels or peritoneal carcinosis. Liver biopsy was performed under direct laparoscopic visualization with an 18G biopsy needle (Bard Inc., Covington, USA) via a second 3 mm incision in the upper right quadrant of the abdomen. If vigorous bleeding was detected immediately after the liver biopsy or the bleeding did not stop after approximately two minutes, a small trocar with a diameter of 3 mm was inserted at the liver biopsy puncture site of the abdominal wall and the bleeding puncture site was treated with argon plasma coagulation (APC) (Söring GmbH, Quickborn, Germany) under direct visualization. If necessary, in patients with high risk of bleeding, the second trocar was inserted in the right upper abdominal quadrant before performance of the liver biopsy in order to rapidly apply APC immediately after the biopsy. Coagulation was considered successful when no further signs of active bleeding could be observed after 2 min.
Statistical analysis
Statistical significance levels were calculated by chi square test.
RESULTS
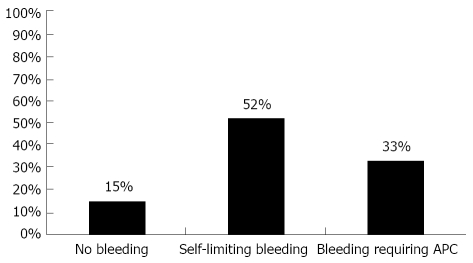
1071 patients had mini-laparoscopy with the intent of liver biopsy. Indication for mini-laparoscopy and liver biopsy was for diagnosis of acute or chronic liver disease of unknown origin and for staging of cirrhosis. Patients with a history of abdominal surgery were not considered for mini-laparoscopy due to potential intra abdominal adhesions. Gender distribution was 55% males versus 45% females and the median age was 48 (± 14) years and 50 (± 14) years respectively. The etiology of the underlying liver disease is shown in Tables 1 and 2. Five hundred and fifty seven (52%) patients displayed minor self-limiting bleeding after the puncture of the liver and 161 (15%) showed no bleeding at all. In 353 cases (33%) of all mini-laparoscopic liver biopsies, prolonged bleeding occurred that required APC of the puncture site (Figure 1).
Table 1.
Etiology of liver disease in non-cirrhotic patients
| Viral hepatitis | Autoimmune liver disease | Liver disease of other origins |
| n = 319 | n = 141 | n = 349 |
| Chronic hepatitis C n = 263 (82%) | Autoimmune hepatitis n = 49 (35%) | Non-alcoholic fatty liver disease n = 99 (28%) |
| Chronic hepatitis B n = 56 (18%) | Primary biliary cirrhosis n = 48 (34%) | Alcoholic liver disease n = 18 (5%) |
| Primary sclerosing cholangitis n = 32 (23%) | Primary/secondary hepatobiliary neoplasia n = 53 (15%) | |
| Overlap syndrome n = 13 (9%) | Toxic liver injury n = 59 (17%) | |
| Others n = 120 (34%) |
Table 2.
Etiology of liver disease in cirrhotic patients
| Viral hepatitis | Autoimmune liver disease | Liver disease of other origins |
| n = 119 | n = 20 | n = 111 |
| Chronic hepatitis C n = 96 (85%) | Autoimmune hepatitis n = 8 (40%) | Alcoholic liver disease n = 52 (47%) |
| Chronic hepatitis B n = 23 (15%) | Primary biliary cirrhosis n = 6 (30%) | Others n = 59 (53%) |
| Primary sclerosing cholangitis n = 6 (30%) |
Figure 1.
Overall occurrence of bleeding in mini-laparoscopic liver biopsies. APC: argon plasma coagulation.
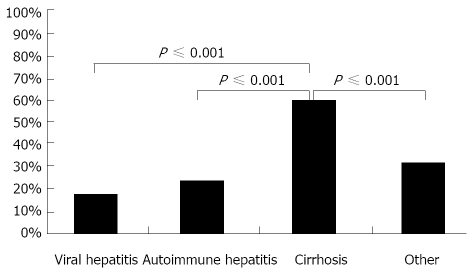
In order to determine whether the underlying liver injury influenced the risk and severity of bleeding, patients were stratified into four groups by type of liver disease: viral and autoimmune hepatitis, the most frequent disease entities; liver cirrhosis, the category with the highest expected risk of bleeding; and liver diseases of other origins. There were no significant differences in the severity of the bleeding between patients with viral and autoimmune hepatitis. Both groups displayed either no bleeding in 14% or self-limiting bleeding in 69% and 62% respectively. APC was used to treat bleeding in 17% of patients with viral hepatitis and in 24% of patients with autoimmune mediated liver disease (Figure 2A and B). In patients with liver cirrhosis, 5% of cases showed no signs of bleeding and 35% had only minor bleeding after liver biopsy (Figure 2C); APC was used in 60% of all cases. Patients with liver diseases of different origins than the ones mentioned above had no or mild bleeding in 17% and 51% respectively. APC was required in 32% of all cases (Figure 2D). Significant bleeding with the need for APC occurred with greater frequency in patients with liver cirrhosis compared to patients without advanced liver disease (P ≤ 0.001, Figures 3 and 4).
Figure 2.
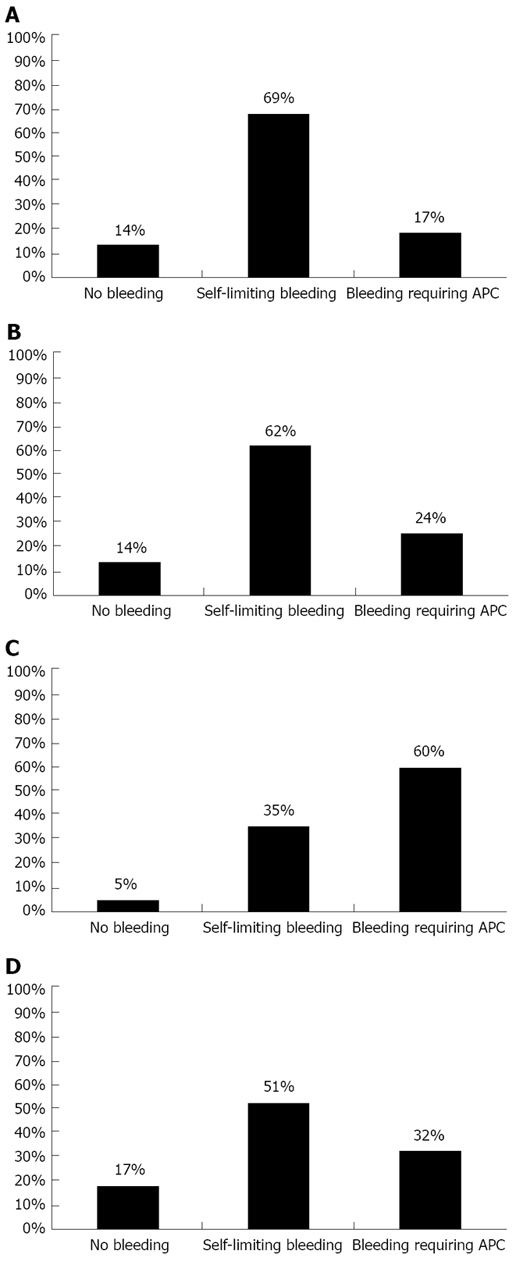
Occurrence of bleeding in mini-laparoscopic liver biopsies in regard to the underlying disease. A: Viral hepatitis; B: Autoimmune hepatitis; C: Cirrhosis; D: Other; APC: argon plasma coagulation.
Figure 3.
Occurrence of bleeding requiring argon plasma coagulation in regard to underlying disease.
Figure 4.
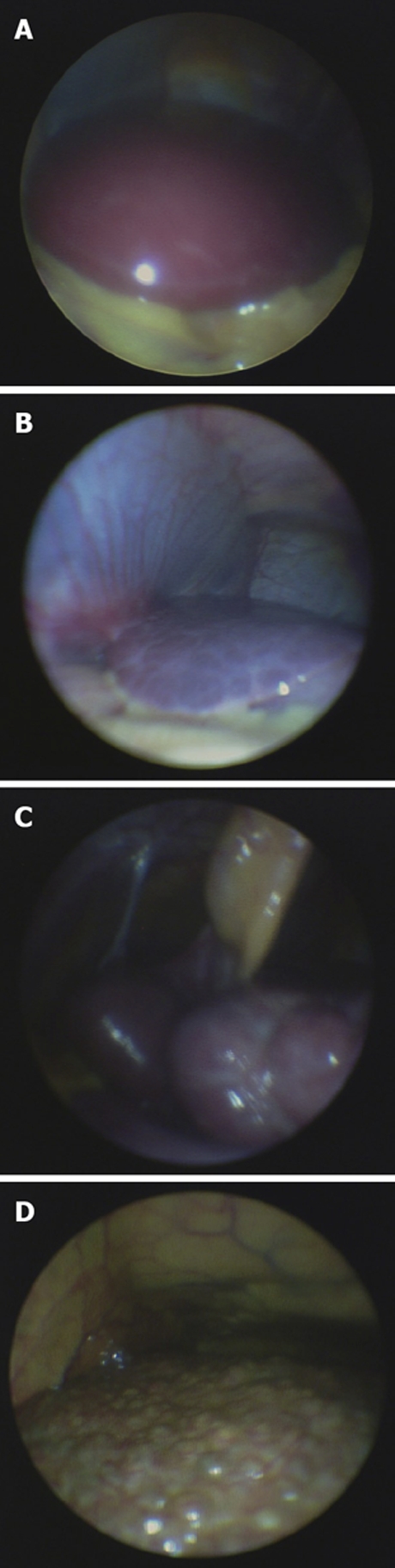
Laparoscopc images of different stages of liver disease. A: Healthy liver; B: Fibrotic liver; C: Macronodular cirrhosis; D: Micronodular cirrhosis.
DISCUSSION
Diagnostic laparoscopy is a valuable tool in the diagnosis of a variety of gastrointestinal illnesses. It is useful in the staging of upper GI-tract malignancies such as gastric and pancreatic cancer and sensitive for the diagnosis of peritoneal carcinosis[10-13]. When used for the evaluation of liver disease, it allows for a macroscopic inspection of the liver and the ability to perform targeted biopsies of focal lesions on the liver surface. Splenic biopsies can also be performed via a diagnostic laparoscopy[14]. For these diagnostic non-surgical procedures, the minimally invasive technique of mini-laparoscopy which requires smaller insertions to the abdominal wall than conventional laparoscopy has been shown to be useful[15].
In our study, we evaluated the occurrence and immediate management of bleeding after liver biopsy during mini-laparoscopy performed in an endoscopy unit. The primary reason for choosing mini-laparoscopy over percutaneous biopsy was to have the ability to treat potential bleeding complications and perform macroscopic evaluation of the liver since the severity of liver injury can be underestimated if based only on the histological result of the biopsy material[16]. Besides the additional macroscopic information, mini-laparoscopic liver biopsy enables the histological assessment of liver injury in patients with advanced cirrhosis where percutaneous biopsy would be contraindicated due to the high risk of a bleeding complication[17]. In this study, we demonstrate that bleeding from liver biopsy occurred significantly more frequently in patients with cirrhosis than non-cirrhotic patients, resulting in an increased need for APC of the biopsy site. Presumably as a result of APC, there were no major post-interventional bleeding complications in any patients. Liver biopsy was performed safely even in patients with decompensated Child C cirrhosis with portal hypertension and marked coagulopathy after administration of fresh frozen plasma and/or platelets.
Some recently published studies describe a lower risk than that of previous reports, indicating that the safety of this procedure has improved[18,19].
These findings confirm previously published data[20,21] where mini-laparoscopy showed similar safety data compared to percutaneous biopsy but mini-laparoscopy was more sensitive in assessing the severity of the liver disease.
In conclusion, mini-laparoscopy guided liver biopsy performed in an endoscopy unit is a safe and effective technique for the evaluation of patients with liver diseases. It enables the macroscopic assessment of the liver and the ability to perform liver biopsy in patients with high risk of bleeding, allowing for the management of complications and enabling histological diagnosis in patients with advanced liver disease or cirrhosis.
COMMENTS
Background
Histological assessment of liver injury still represents the most important diagnostic tool in the evaluation of liver diseases. However, percutaneous liver biopsy may be associated with bleeding complications in patients with advanced liver disease. Thus, a safe method for obtaining liver histology in this high risk patient population is crucial.
Research frontiers
Due to non-invasive assessment of liver fibrosis with fibroscan® technology or radiographic imaging, liver histology may not be necessary in some patients. However, to date, liver biopsy seems crucial in most cases. Whether non-invasive techniques will replace liver biopsy in the future remains to be determined by further research.
Innovations and breakthroughs
The authors demonstrated that the assessment of liver histology is possible even in critically ill patients with compromised liver function.
Applications
Mini-laparoscopy, not only a valuable tool for liver biopsy in patients with advanced liver disease, can also be used for tumor staging in patients with intra abdominal malignancies. Especially, peritoneal carcinosis can be detected in an early stage before visualization with radiological techniques is possible.
Peer reviews
Overall, this is an interesting and well written retrospective study about mini-laparoscopy and liver biopsy in a large series of 1071 consecutive patients. A prospective randomized control study of mini-laparoscopy versus percutaneous biopsy was recommended. However, patients with advanced liver disease or compromised liver function or blood coagulation should not be included in such a study as it is known that these patients have a higher risk of bleeding complications after percutaneous biopsy.
Footnotes
Peer reviewers: Oliver Pech, MD, PhD, Attending Physician of Gastroenterology, Vice Director of the Endoscopy Unit, Department of Internal Medicine 2, HSK Wiesbaden, Wiesbaden, Germany; Takashi Shida, MD, PhD, Department of General Surgery, Chiba University Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK, et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027–1035. doi: 10.1016/j.cgh.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 3.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:IV1–IV11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley A, Petrunia D. Practice guidelines for liver biopsy. Canadian Association of Gastroenterology. Can J Gastroenterol. 2000;14:481–482. doi: 10.1155/2000/872560. [DOI] [PubMed] [Google Scholar]
- 5.Helmreich-Becker I, Schirmacher P, Denzer U, Hensel A, Meyer zum Büschenfelde KH, Lohse AW. Minilaparoscopy in the diagnosis of cirrhosis: superiority in patients with Child-Pugh A and macronodular disease. Endoscopy. 2003;35:55–60. doi: 10.1055/s-2003-36419. [DOI] [PubMed] [Google Scholar]
- 6.Helmreich-Becker I, Meyer zum Büschenfelde KH, Lohse AW. Safety and feasibility of a new minimally invasive diagnostic laparoscopy technique. Endoscopy. 1998;30:756–762. doi: 10.1055/s-2007-1001417. [DOI] [PubMed] [Google Scholar]
- 7.Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 8.Hulscher JB, Nieveen van Dijkum EJ, de Wit LT, van Delden OM, van Lanschot JJ, Obertop H, Gouma DJ. Laparoscopy and laparoscopic ultrasonography in staging carcinoma of the gastric cardia. Eur J Surg. 2000;166:862–865. doi: 10.1080/110241500447245. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KR, Levi J, Livingstone A, Jeffers L, Molina E, Kligerman S, Bernstein D, Kodali VP, Schiff ER. Experience with staging laparoscopy in pancreatic malignancy. Gastrointest Endosc. 1999;49:498–503. doi: 10.1016/s0016-5107(99)70050-7. [DOI] [PubMed] [Google Scholar]
- 10.Conlon KC. Staging laparoscopy for gastric cancer. Ann Ital Chir. 2001;72:33–37. [PubMed] [Google Scholar]
- 11.Arnold JC, Schneider AR, Zöpf T, Neubauer HJ, Jakobs R, Benz C, Riemann JF. [Laparoscopic tumor staging in gastrointestinal carcinomas: significance of internal medicine laparoscopy] Z Gastroenterol. 2001;39:19–23. [PubMed] [Google Scholar]
- 12.Barreiro CJ, Lillemoe KD, Koniaris LG, Sohn TA, Yeo CJ, Coleman J, Fishman EK, Cameron JL. Diagnostic laparoscopy for periampullary and pancreatic cancer: what is the true benefit? J Gastrointest Surg. 2002;6:75–81. doi: 10.1016/s1091-255x(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 13.Denzer U, Hoffmann S, Helmreich-Becker I, Kauczor HU, Thelen M, Kanzler S, Galle PR, Lohse AW. Minilaparoscopy in the diagnosis of peritoneal tumor spread: prospective controlled comparison with computed tomography. Surg Endosc. 2004;18:1067–1070. doi: 10.1007/s00464-003-9139-0. [DOI] [PubMed] [Google Scholar]
- 14.Denzer U, Helmreich-Becker I, Galle PR, Lohse AW. Minilaparoscopy-guided spleen biopsy in systemic disease with splenomegaly of unknown origin. Endoscopy. 2002;34:495–498. doi: 10.1055/s-2002-32005. [DOI] [PubMed] [Google Scholar]
- 15.Weickert U, Jakobs R, Siegel E, Eickhoff A, Schilling D, Riemann JF. [Complications of diagnostic laparoscopy] Dtsch Med Wochenschr. 2005;130:16–20. doi: 10.1055/s-2005-837367. [DOI] [PubMed] [Google Scholar]
- 16.Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161–S172. doi: 10.1053/jhep.2002.36989. [DOI] [PubMed] [Google Scholar]
- 17.Denzer U, Helmreich-Becker I, Galle PR, Lohse AW. Liver assessment and biopsy in patients with marked coagulopathy: value of mini-laparoscopy and control of bleeding. Am J Gastroenterol. 2003;98:893–900. doi: 10.1111/j.1572-0241.2003.07342.x. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J, Card TR. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139:1230–1237. doi: 10.1053/j.gastro.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Inabnet WB, Deziel DJ. Laparoscopic liver biopsy in patients with coagulopathy, portal hypertension, and ascites. Am Surg. 1995;61:603–606. [PubMed] [Google Scholar]
- 21.Denzer U, Arnoldy A, Kanzler S, Galle PR, Dienes HP, Lohse AW. Prospective randomized comparison of minilaparoscopy and percutaneous liver biopsy: diagnosis of cirrhosis and complications. J Clin Gastroenterol. 2007;41:103–110. doi: 10.1097/01.mcg.0000225612.86846.82. [DOI] [PubMed] [Google Scholar]