Abstract
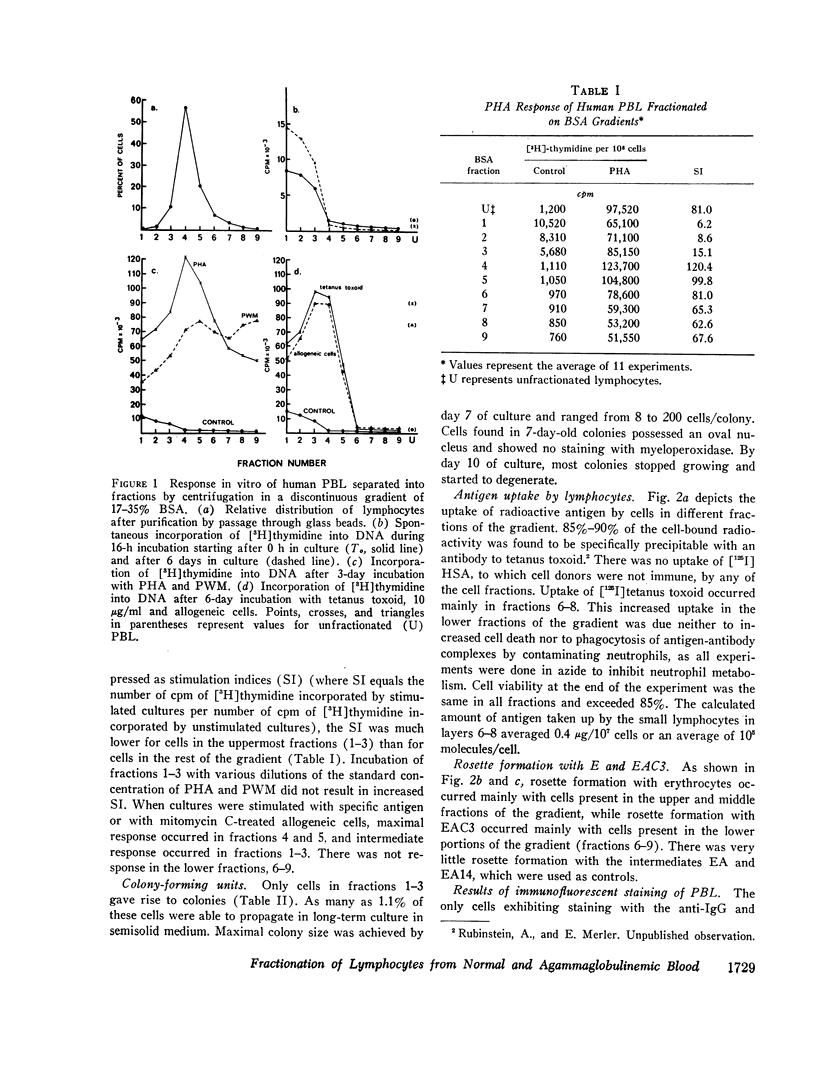
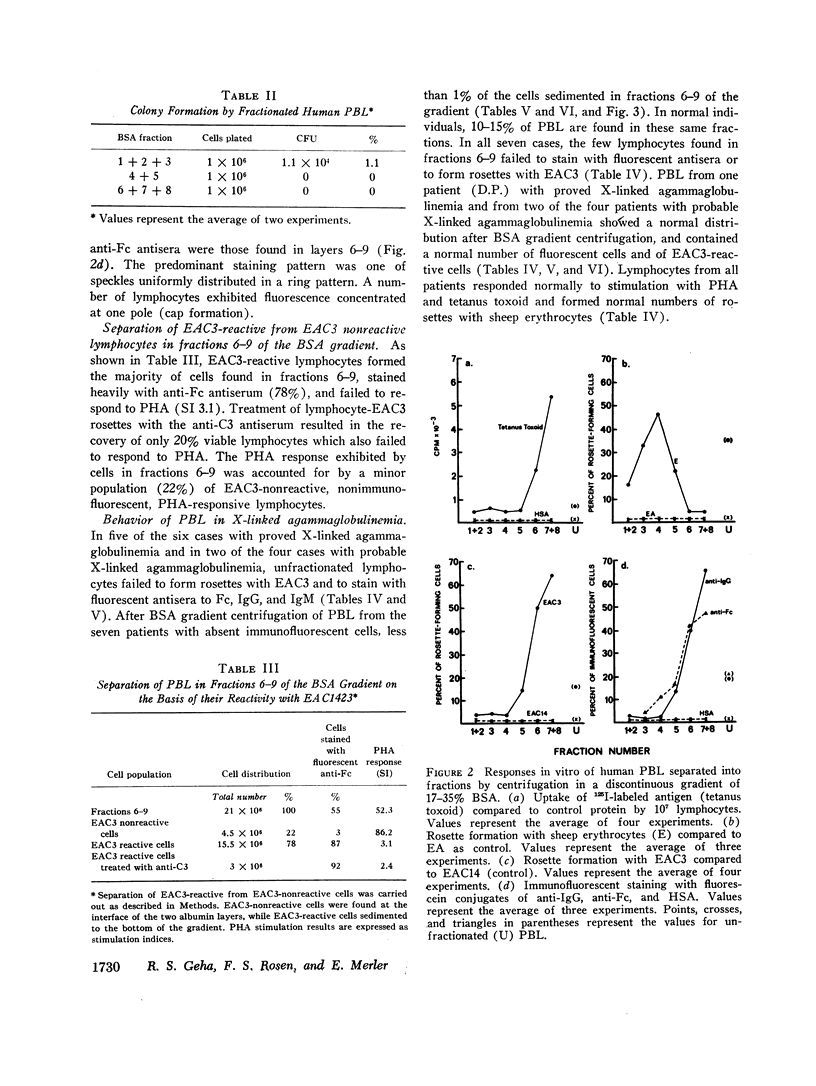
Normal human peripheral blood lymphocytes were separated on discontinuous gradients of 17-35% bovine serum albumin (BSA) into nine fractions. Three subpopulations of lymphocytes were obtained. One occupies the top third of the gradient (fractions 1-3, 17-23% BSA) and is rich in cells characterized by a high spontaneous rate of DNA synthesis and by the ability to give rise to colony-forming units. The middle portion of the gradient (fractions 4 and 5, 23-27% BSA) is rich in thymus-derived (T) lymphocytes identified by their vigorous response to mitogens and by their ability to form rosettes with sheep erythrocytes (E). The third subpopulation at the bottom of the gradient (fractions 6-9, 27-35% BSA) is rich in bone marrow-derived (B) lymphocytes capable of staining with fluorescent antiimmunoglobulin antisera and of forming rosettes with EAC1423.
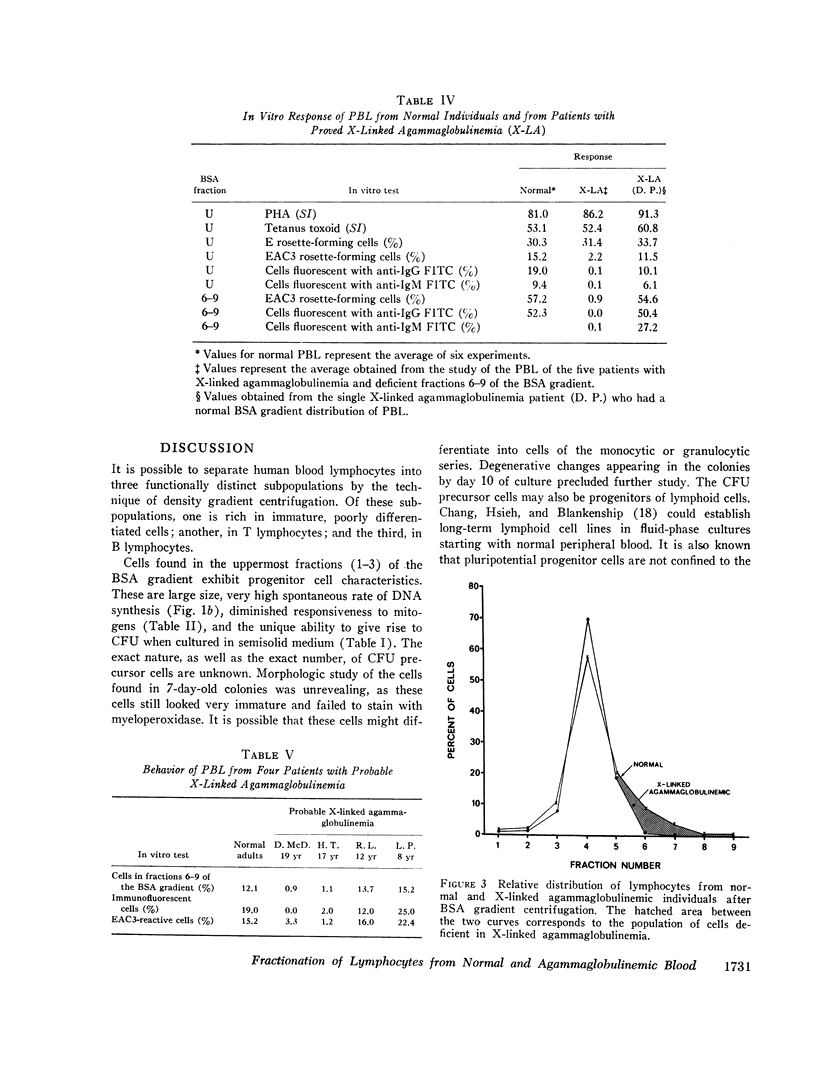
The peripheral blood lymphocytes of five boys with proved X-linked agammaglobulinemia and two with probable X-linked agammaglobulinemia were found to be totally deficient in B lymphocytes (fractions 6-9) and lacked the subpopulation identified by immunofluorescent staining or rosette formation with EAC1423. One boy with proved X-linked agammaglobulinemia and two with probable X-linked agammaglobulinemia possessed a normal amount of circulating B lymphocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August C. S., Merler E., Lucas D. O., Janeway C. A. The response in vitro of human lymphocytes to phytohemagglutinin and to antigens after fractionation on discontinuous density gradients of albumin. Cell Immunol. 1970 Dec;1(6):603–618. doi: 10.1016/0008-8749(70)90026-2. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Voynow N. K. One-way stimulation in mixed leukocyte cultures. Science. 1966 Jul 29;153(3735):545–547. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- Balner H., Dicke K. A., van Putten L. M., van Bekkum D. W. Experimental aspects of bone marrow transplantation in primates. Transplant Proc. 1969 Mar;1(1):25–30. [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Hsieh M. W., Blankenship W. Initiation and establishment of lymphoid cell lines from the blood of healthy persons. J Natl Cancer Inst. 1971 Aug;47(2):469–477. [PubMed] [Google Scholar]
- Cooper M. D., Gabrielsen A. E., Good R. A. Role of the thymus and other central lymphoid tissues in immunological disease. Annu Rev Med. 1967;18:113–138. doi: 10.1146/annurev.me.18.020167.000553. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Dicke K. A., Tridente G., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphocyte cell mixtures. 3. In vitro test for detection of immunocompetent cells in fractionated mouse spleen cell suspensions and primate bone marrow suspensions. Transplantation. 1969 Oct;8(4):422–434. doi: 10.1097/00007890-196910000-00014. [DOI] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merler E., Silberschmidt M. Uptake of antigen by human lymphocytes. Immunology. 1972 May;22(5):821–831. [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Naor D., Bentwich Z., Cividalli G. Inability of peripheral lymphoid cells of agammaglobulinaemic patients to bind radioiodinated albumins. Aust J Exp Biol Med Sci. 1969 Dec;47(6):759–761. doi: 10.1038/icb.1969.173. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Paraskevas F., Lee S. T., Orr K. B., Israels L. G. A receptor for Fc on mouse B-lymphocytes. J Immunol. 1972 May;108(5):1319–1327. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Silveira N. P., Mendes N. F., Tolnai M. E. Tissue localization of two populations of human lymphocytes distinguished by membrane receptors. J Immunol. 1972 May;108(5):1456–1460. [PubMed] [Google Scholar]
- Storb R., Epstein R. B., Thomas E. D. Marrow repopulating ability of peripheral blood cells compared to thoracic duct cells. Blood. 1968 Oct;32(4):662–667. [PubMed] [Google Scholar]
- Suter E. R., Probst H., Dukor P. The fine structure of complement-receptor lymphocytes in mice. Eur J Immunol. 1972 Apr;2(2):189–190. doi: 10.1002/eji.1830020218. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- Wioland M., Sabolovic D., Burg C. Electrophoretic mobilities of T and B cells. Nat New Biol. 1972 Jun 28;237(78):274–276. doi: 10.1038/newbio237274a0. [DOI] [PubMed] [Google Scholar]


