Abstract
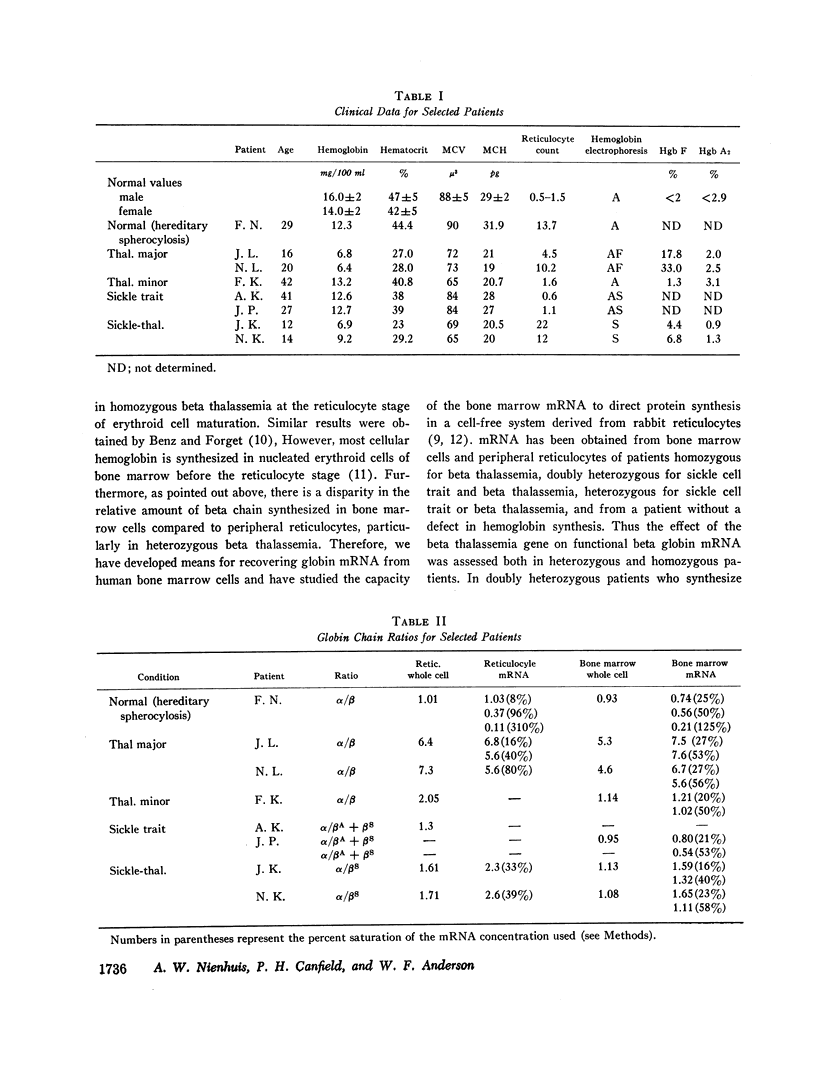
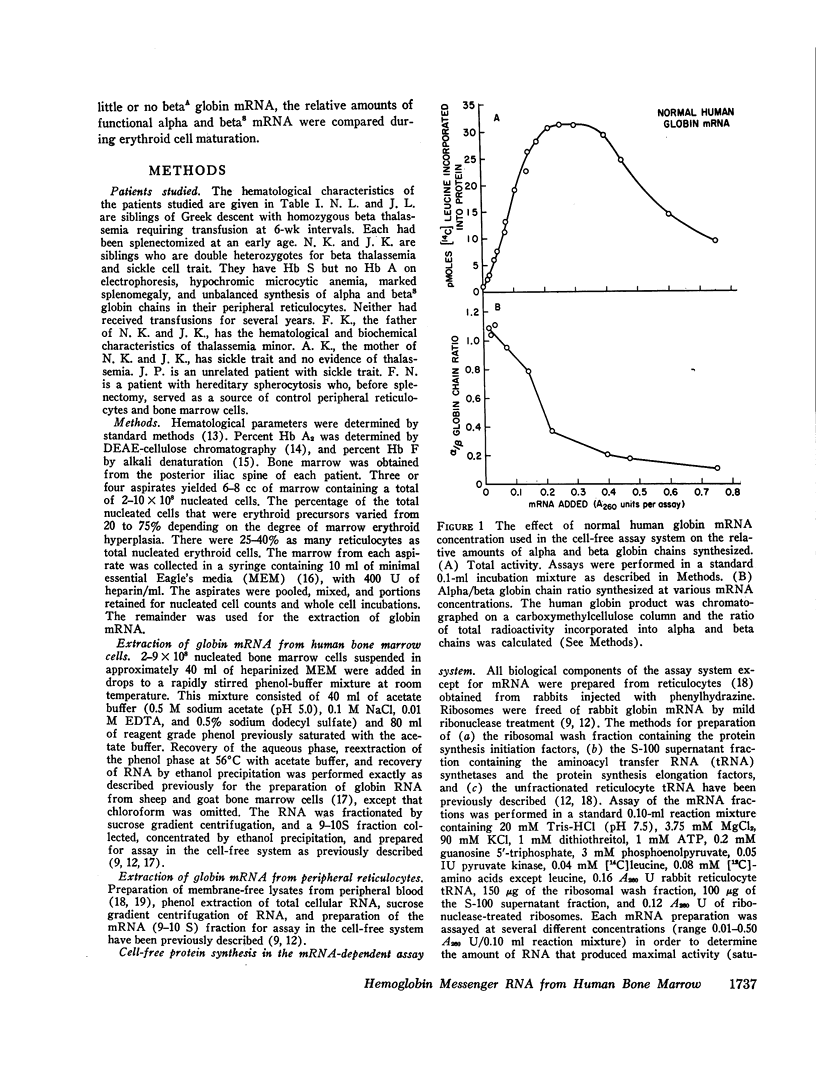
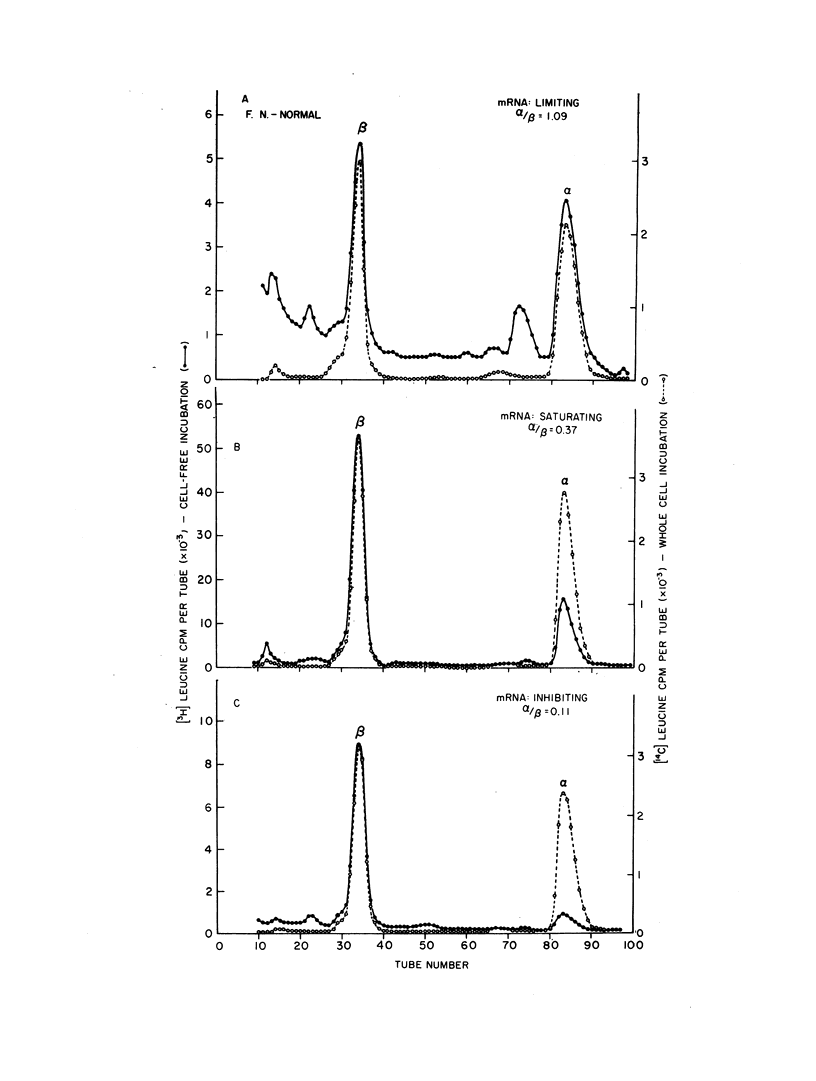
A method for isolating human hemoglobin messenger RNA (mRNA) from bone marrow cells was developed to investigate the molecular basis for the defect in globin synthesis in beta thalassemia. Active mRNA was isolated from the bone marrow cells and peripheral reticulocytes of patients with homozygous beta thalassemia, heterozygous beta thalassemia, sickle cell trait, double heterozygosity for beta thalassemia and sickle cell trait, as well as from a patient with normal hemoglobin synthesis but with an elevated reticulocyte count secondary to hereditary spherocytosis. The mRNA was prepared for assay in an mRNA-dependent rabbit reticulocyte cell-free system and the amount of alpha and beta globin chains synthesized was determined by carboxymethylcellulose column chromatography. The relative synthesis of alpha to beta chains in response to normal hemoglobin mRNA was found to be a function of the amount of mRNA added to the assay system: increasing the amount of mRNA led to a decrease in the alpha-to-beta-chain synthetic ratio. Therefore, assays were carried out at limiting concentrations of mRNA.
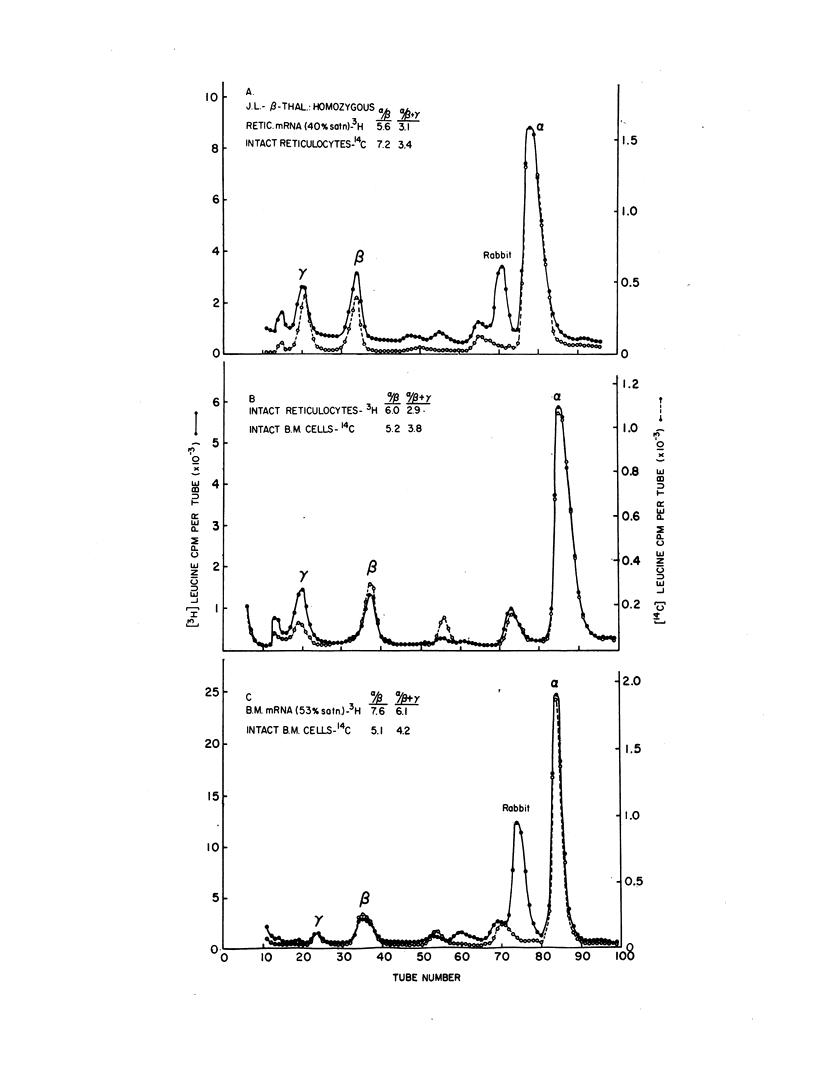
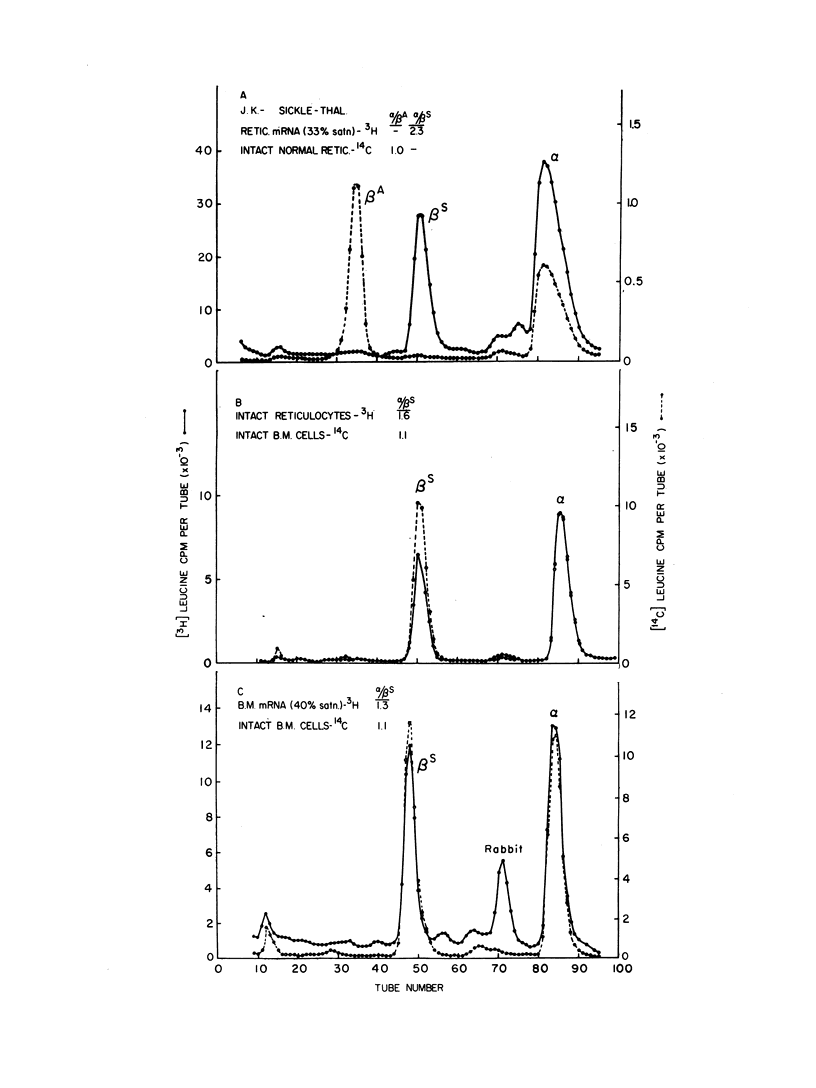
The molecular defect in homozygous beta thalassemia was shown to be carried in the mRNA of bone marrow cells as well as in the mRNA from peripheral reticulocytes, because much less beta than alpha globin was produced in the cell-free system in response to mRNA from either type of cell. In patients doubly heterozygous for beta thalassemia and sickle cell trait, little or no synthesis of betaA globin occurred in the bone marrow cells or the peripheral reticulocytes. The alpha to betaS synthetic ratio of the intact bone marrow cells was approximately 1, while the same ratio in the peripheral reticulocytes was between 1.5 and 2. The virtual absence of translatable beta globin mRNA in the mRNA prepared from the cells of these doubly heterozygous patients further demonstrates that the molecular defect produced by the beta thalassemia gene is in the beta globin mRNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOOK H., LINGREL J. B., SCARO J. L., MILLETTE R. L. Synthesis of haemoglobin in relation to the maturation of erythroid cells. Nature. 1962 Oct 27;196:347–350. doi: 10.1038/196347a0. [DOI] [PubMed] [Google Scholar]
- Bank A., Braverman A. S., O'Donnell J. V., Marks P. A. Absolute rates of globin chain synthesis in thalassemia. Blood. 1968 Feb;31(2):226–233. [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia. J Clin Invest. 1971 Dec;50(12):2755–2760. doi: 10.1172/JCI106778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernini L. F. Rapid estimation of hemoglobin A2 by DEAE chromatography. Biochem Genet. 1969 Jan;2(4):305–310. doi: 10.1007/BF01458491. [DOI] [PubMed] [Google Scholar]
- Braverman A. S., Bank A. Changing rates of globin chain synthesis during erythroid cell maturation in thalassemia. J Mol Biol. 1969 May 28;42(1):57–64. doi: 10.1016/0022-2836(69)90486-0. [DOI] [PubMed] [Google Scholar]
- CHERNOFF A. I., HORTON L. L. Determinations of fetal hemoglobin in whole blood, using a modified alkali denaturation test. Am J Clin Pathol. 1958 Sep;30(3):204–208. doi: 10.1093/ajcp/30.3.204. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J. Haemoglobin synthesis during erythroid maturation in -thalassaemia. Nat New Biol. 1972 Dec 6;240(101):190–192. doi: 10.1038/newbio240190a0. [DOI] [PubMed] [Google Scholar]
- Conconi F., Bargellesi A., Pontremoli S., Vigi V., Volpato S., Gaburro D. Absence of beta-globin synthesis and excess of gamma-globin synthesis in homozygous beta-thalassaemic subjects from the Ferrara region. Nature. 1968 Jan 20;217(5125):259–260. doi: 10.1038/217259a0. [DOI] [PubMed] [Google Scholar]
- Conconi F., Rowley P. T., Del Senno L., Pontremoli S., Volpato S. Induction of -globin synthesis in the -thalassaemia of Ferrara. Nat New Biol. 1972 Jul 19;238(81):83–87. doi: 10.1038/newbio238083a0. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Nienhuis A. W., Elson N. A., Anderson W. F. Initiation of globin synthesis. Preparation and use of reticulocyte ribosomes retaining initiation region messenger ribonucleic acid fragments. J Biol Chem. 1972 Sep 10;247(17):5357–5368. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fuhr J. E., Natta C. Translational control of and globin chain synthesis. Nat New Biol. 1972 Dec 27;240(104):274–276. doi: 10.1038/newbio240274a0. [DOI] [PubMed] [Google Scholar]
- Fuhr J., Natta C., Marks P. A., Bank A. Protein synthesis in cell-free systems from reticulocytes of thalassaemic patients. Nature. 1969 Dec 27;224(5226):1305–1307. doi: 10.1038/2241305a0. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Thornton A. G., Nienhuis A. W., Anderson W. F. Cell-free hemoglobin synthesis in beta-thalassemia. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1854–1861. doi: 10.1073/pnas.67.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill F., Atwater J., Schwartz E. Hemoglobin Lepore trait: globin synthesis in bone marrow and peripheral blood. Science. 1972 Nov 10;178(4061):623–625. doi: 10.1126/science.178.4061.623. [DOI] [PubMed] [Google Scholar]
- HEYWOOD J. D., KARON M., WEISSMAN S. AMINO ACIDS: INCORPORATION INTO ALPHA- AND BETA-CHAINS OF HEMOGLOBIN BY NORMAL AND THALASSEMIC RETICULOCYTES. Science. 1964 Oct 23;146(3643):530–531. doi: 10.1126/science.146.3643.530. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Nathan D. G., Lodish H. F. Equal synthesis of - and -globin chains in erythroid precursors in heterozygous -thalassemia. J Clin Invest. 1972 Jul;51(7):1906–1909. doi: 10.1172/JCI106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Nathan D. G. Mild thalassemia: the result of interactions of alpha and beta thalassemia genes. J Clin Invest. 1970 Apr;49(4):635–642. doi: 10.1172/JCI106274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F., Nathan D. G. Regulation of hemoglobin synthesis. Preferential inhibition of and globin synthesis. J Biol Chem. 1972 Dec 10;247(23):7822–7829. [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Hemoglobin switching in sheep and goats: change in functional globin messenger RNA in reticulocytes and bone marrow cells. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2184–2188. doi: 10.1073/pnas.69.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Isolation and translation of hemoglobin messenger RNA from thalassemia, sickle cell anemia, and normal human reticulocytes. J Clin Invest. 1971 Nov;50(11):2458–2460. doi: 10.1172/JCI106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley P. T., Kosciolek B. Distinction between two types of beta-thalassaemia by inducibility of the cell-free synthesis of beta-chains by nonthalassaemic soluble fraction. Nat New Biol. 1972 Oct 25;239(95):234–235. doi: 10.1038/newbio239234a0. [DOI] [PubMed] [Google Scholar]
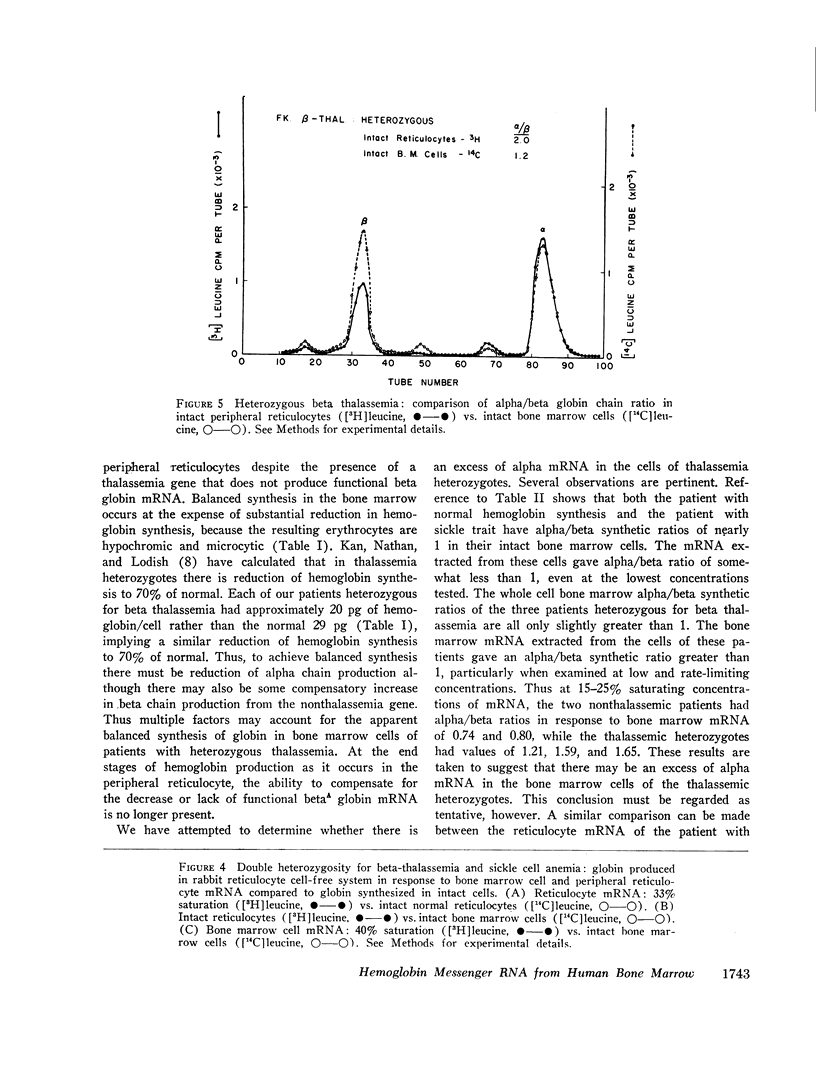
- Schwartz E. Heterozygous Beta thalassemia: balanced globin synthesis in bone marrow cells. Science. 1970 Mar 13;167(3924):1513–1514. doi: 10.1126/science.167.3924.1513. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Naughton M. A. Globin synthesis in thalassaemia: an in vitro study. Nature. 1965 Dec 11;208(5015):1061–1065. doi: 10.1038/2081061a0. [DOI] [PubMed] [Google Scholar]


