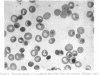
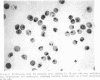
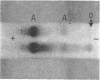
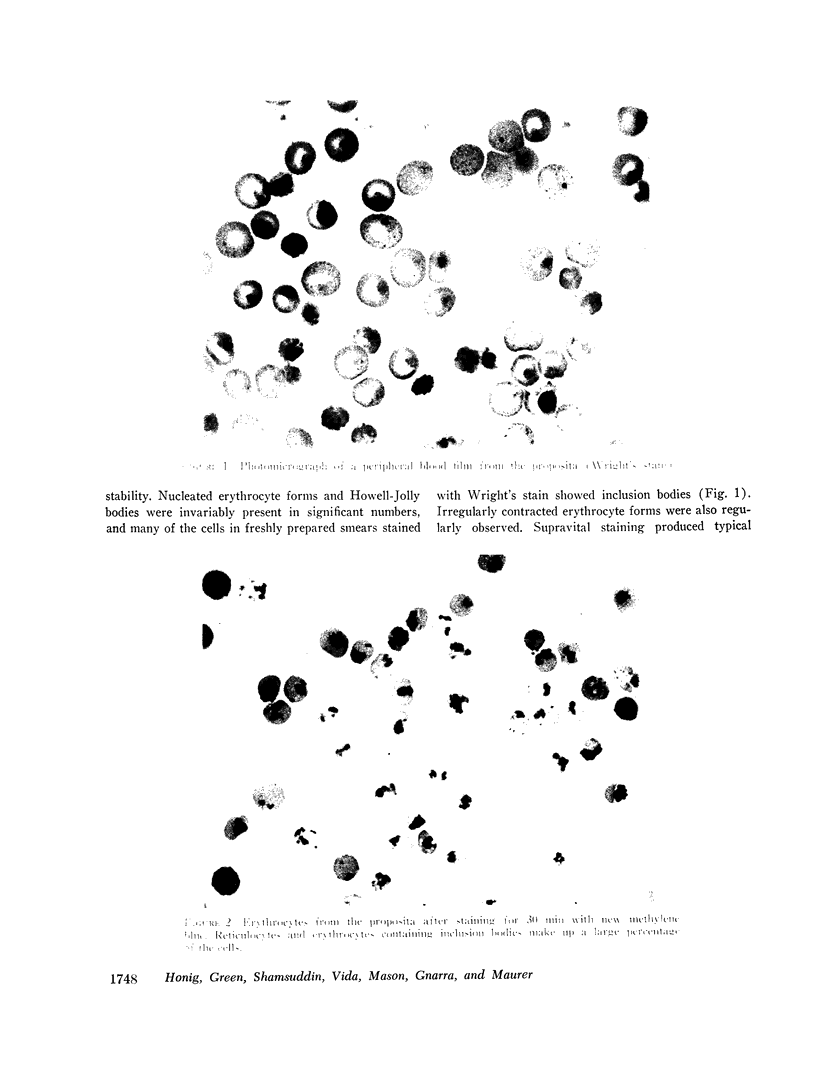
Abstract
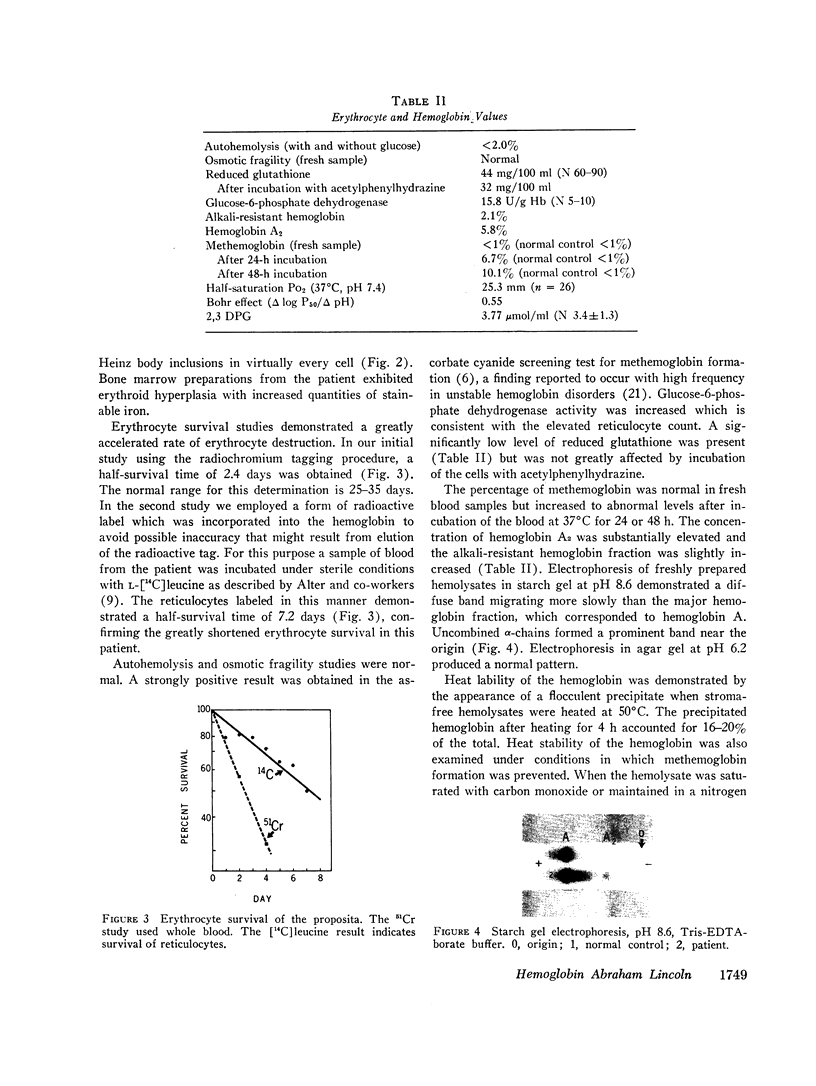
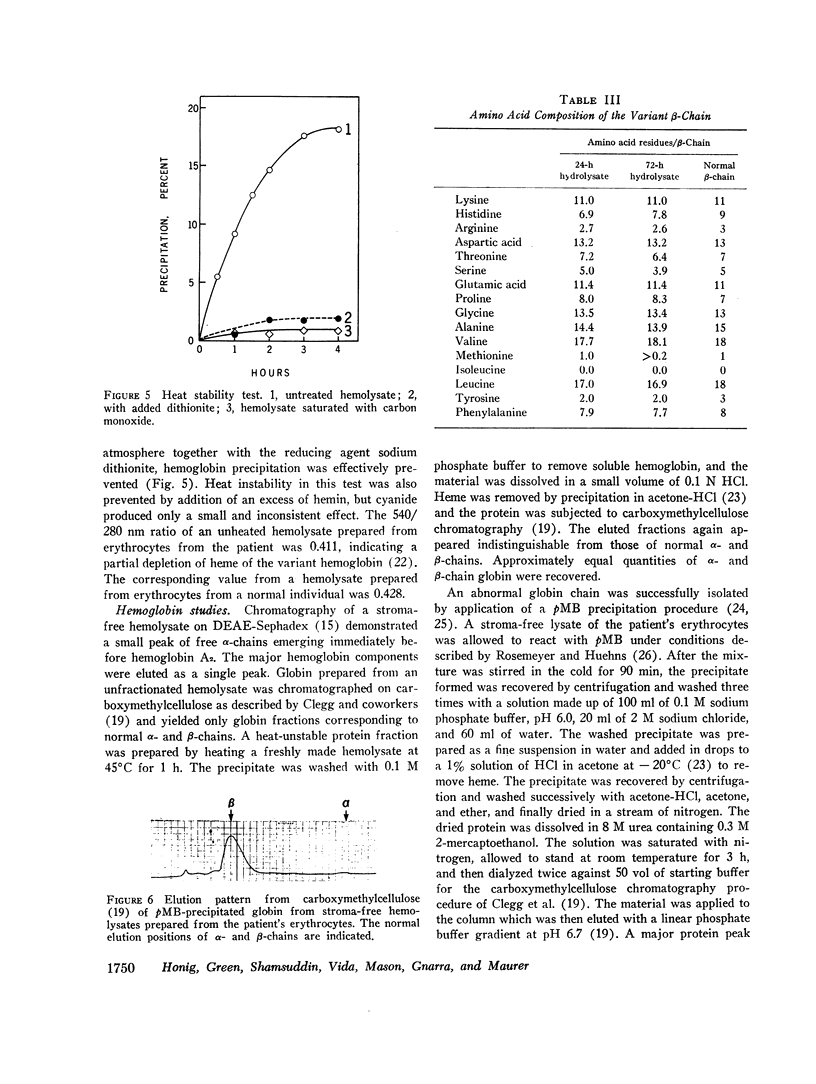
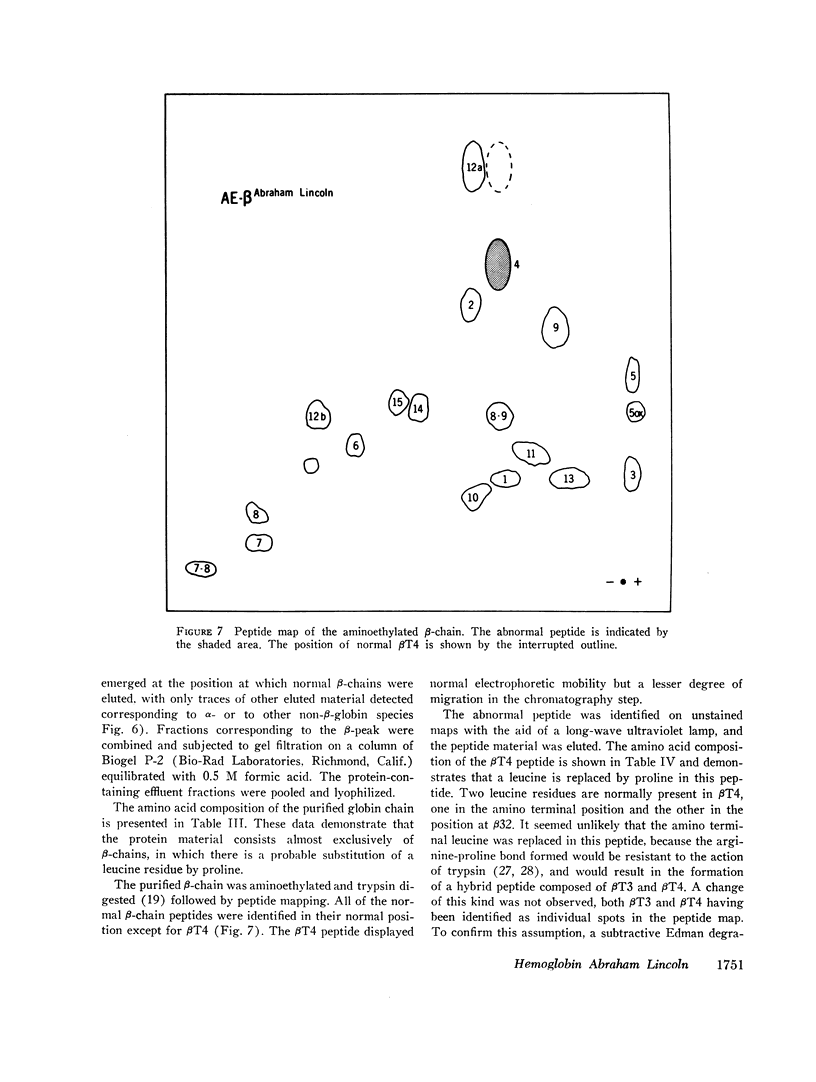
An unstable hemoglobin variant was identified in a Negro woman with hemolytic anemia since infancy. A splenectomy had been performed when the patient was a child. The anemia was accompanied by erythrocyte inclusion bodies and excretion of darkly pigmented urine. Neither parent of the proposita demonstrated any hematologic abnormality, and it appeared that this hemoglobin variant arose as a new mutation. Erythrocyte survival in the patient was greatly reduced: the erythrocyte t½ using radiochromium as a tag was 2.4 days, and a reticulocyte survival study performed after labeling the cells with L-[14C]leucine indicated a t½ of 7.2 days. When stroma-free hemolysates were heated at 50°C, 16-20% of the hemoglobin precipitated. The thermolability was prevented by the addition of hemin, carbon monoxide, or dithionite, suggesting an abnormality of heme binding. An increased rate of methemoglobin formation was also observed after incubation of erythrocytes at 37°C. The abnormal hemoglobin could not be separated from hemoglobin A by electrophoresis or chromatography, but it was possible to isolate the variant β-chain by precipitation with p-hydroxymercuribenzoate. Purification of the β-chain by column chromatography followed by peptide mapping and amino acid analysis demonstrated a substitution of proline for β32 leucine. It appears likely that a major effect of this substitution is a disruption of the normal orientation of the adjacent leucine residue at β31 to impair heme stabilization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Kan Y. W., Nathan D. G. Reticulocyte survival in sickle cell anemia: effect of cyanate. Blood. 1972 Nov;40(5):733–739. [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobin molecules. Proc Natl Acad Sci U S A. 1966 Sep;56(3):974–978. doi: 10.1073/pnas.56.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Hutchison H. E. Haemoglobin Köln (beta-98 valine--methionine): an unstable protein causing inclusion-body anaemia. Nature. 1966 May 28;210(5039):915–916. doi: 10.1038/210915a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Martin R. J. Mixing technique for the oxygen-hemoglobin equilibrium and Bohr effect. J Appl Physiol. 1966 Nov;21(6):1898–1902. doi: 10.1152/jappl.1966.21.6.1898. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Opfell R. W., Burgert E. O., Jr Three families with unstable hemoglobinopathies (Köln, Olmsted and Santa Ana) causing hemolytic anemia with inclusion bodies and pigmenturia. Am J Med. 1969 Mar;46(3):344–359. doi: 10.1016/0002-9343(69)90037-0. [DOI] [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Hollán S. R., Szelényi J. G., Miltényi M., Charlesworth D., Lorkin P. A., Lehmann H. Unstable haemoglobin disease caused by Hb Santa Ana- 88 (F4) Leu leads to Pro. Haematologia (Budap) 1970;4(2):141–155. [PubMed] [Google Scholar]
- Huehns E. R. Diseases due to abnormalities of hemoglobin structure. Annu Rev Med. 1970;21:157–178. doi: 10.1146/annurev.me.21.020170.001105. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Brown A. K., Efremov G. D., Wilson J. B., Reynolds C. A., Uy R., Smith L. L. Hemoglobin Savannah (B6(24) beta-glycine is greater than valine): an unstable variant causing anemia with inclusion bodies. J Clin Invest. 1971 Mar;50(3):650–659. doi: 10.1172/JCI106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R. D., Hall M. D., Wiltshire B. G., Lehmann H. Haemoglobin Southampton, 106 (G8) Leu leads to pro: an unstable variant producing severe haemolysis. Lancet. 1972 Dec 2;2(7788):1170–1172. doi: 10.1016/s0140-6736(72)92594-9. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Jandl J. H. A simple visual screening test for glucose-6-phosphate dehydrogenase deficiency employing ascorbate and cyanide. N Engl J Med. 1966 May 26;274(21):1162–1167. doi: 10.1056/NEJM196605262742102. [DOI] [PubMed] [Google Scholar]
- Jacob H. S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970 Jul;7(3):341–354. [PubMed] [Google Scholar]
- Kleihauer E. F., Reynolds C. A., Dozy A. M., Wilson J. B., Moores R. R., Berenson M. P., Wright C. S., Huisman T. H. Hemoglobin-Bibba or alpha-2-136Pro-beta 2, an unstable alpha chain abnormal hemoglobin. Biochim Biophys Acta. 1968 Jan 22;154(1):220–222. doi: 10.1016/0005-2795(68)90274-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Variations in the structure of human haemoglobin. With particular reference to the unstable haemoglobins. Br Med Bull. 1969 Jan;25(1):14–23. doi: 10.1093/oxfordjournals.bmb.a070664. [DOI] [PubMed] [Google Scholar]
- MARDER V. J., CONLEY C. L. Electrophoresis of hemoglobin on agar gels; frequency of hemoglobin D in a Negro population. Bull Johns Hopkins Hosp. 1959 Aug;105(2):77–88. [PubMed] [Google Scholar]
- Maurer H. S., Behrman R. E., Honig G. R. Dependence of the oxygen affinity of blood on the presence of foetal or adult haemoglobin. Nature. 1970 Jul 25;227(5256):388–390. doi: 10.1038/227388a0. [DOI] [PubMed] [Google Scholar]
- Opfell R. W., Lorkin P. A., Lehmann H. Hereditary non-spherocytic haemolytic anaemia with post-splenectomy inclusion bodies and pigmenturia caused by an unstable haemoglobin Santa Ana-beta-88 (F4) leucine--proline. J Med Genet. 1968 Dec;5(4):292–297. doi: 10.1136/jmg.5.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Pure native globin from human hemoglobin: preparation and some physico-chemical properties. Biochim Biophys Acta. 1958 Apr;28(1):221–221. doi: 10.1016/0006-3002(58)90462-1. [DOI] [PubMed] [Google Scholar]
- Ranney H. M., Jacobs A. S., Udem L., Zalusky R. Hemoglobin Riverdale-Bronx an unstable hemoglobin resulting from the substitution of arginine for glycine at helical residue B6 of the B beta polypeptide chain. Biochem Biophys Res Commun. 1968 Dec 30;33(6):1004–1005. doi: 10.1016/0006-291x(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Rieder R. F., Oski F. A., Clegg J. B. Hemoglobin Philly (beta 35 tyrosine phenylalanine): studies in the molecular pathology of hemoglobin. J Clin Invest. 1969 Sep;48(9):1627–1642. doi: 10.1172/JCI106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. CHARACTERIZATION OF GENETIC VARIANTS OF BLOOD PROTEINS. Vox Sang. 1965 May-Jun;10:359–362. [PubMed] [Google Scholar]
- Sansone G., Carrell R. W., Lehmann H. Haemoglobin Genova: beta-28 (B10) leucine replaced by proline. Nature. 1967 May 27;214(5091):877–879. doi: 10.1038/214877a0. [DOI] [PubMed] [Google Scholar]
- Sansone G., Pik C. Familial haemolytic anaemia with erythrocyte inclusion bodies, bilifuscinuria and abnormal haemoglobin (haemoglobin Galliera Genova). Br J Haematol. 1965 Sep;11(5):511–517. doi: 10.1111/j.1365-2141.1965.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- Steadman J. H., Yates A., Huehns E. R. Idiopathic Heinz body anaemia: Hb-Bristol (beta67 (E11) Val to Asp). Br J Haematol. 1970 Apr;18(4):435–446. doi: 10.1111/j.1365-2141.1970.tb01457.x. [DOI] [PubMed] [Google Scholar]
- White J. M., Brain M. C., Lorkin P. A., Lehmann H., Smith M. Mild "unstable haemoglobin haemolytic anaemia" caused by haemoglobin Shepherds Bush(B74(E18) gly--asp). Nature. 1970 Mar 7;225(5236):939–941. doi: 10.1038/225939a0. [DOI] [PubMed] [Google Scholar]
- White J. M., Dacie J. V. The unstable hemoglobins--molecular and clinical features. Prog Hematol. 1971;7(0):69–109. [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Characterization of Heinz bodies in unstable haemoglobin haemolytic anaemia. Nature. 1972 Nov 17;240(5377):150–152. doi: 10.1038/240150b0. [DOI] [PubMed] [Google Scholar]